Commercial manufacturers and products
mentioned in this report are for descriptive use only and do not
constitute endorsements by USDOL-OSHA.
The author would like to express appreciation to Robert
Douglas for providing analytical support during the validation. This
support included preparation of standards, sample spikes, and operation of
the ARL 3560. In Addition, the effort that he put forth in evaluating the
sample digestion procedure is discussed in Section 8 of this Backup Data
Report.
1. Introduction
The purpose of this work was to validate a simultaneous
Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES) - an
Applied Research Laboratories (ARL) Model 3560 (Fisons, Sunland, CA) for
use in the analysis of industrial hygiene brazing and soldering fume
samples. Previously, the OSHA Salt Lake Technical Center (SLTC) had been
analyzing solder and brazing fume samples using a Jobin Yvon Model JY 70
ICP (Instruments SA, Edison, NJ) (11.1).
1.1 Evaluation of the
Analytical Method for Measuring Occupational
Exposures during Soldering and Brazing Operations
The main concern of this evaluation is to determine whether this
instrument is able to detect, correctly identify, accurately and
precisely measure exposures near the respective PELs. The general
procedure for the analysis of samples by an ARL ICP has been previously
described (11.2)
and evaluated (11.3).
This validation examines the analytical response of an ARL spectrometer
to eight elements commonly found in soldering and brazing operations.
The eight elements (Ag, Be, Cd, Cu, Pb, Sb, Sn, and Zn) have been
validated using concentration levels of approximately 0.5, 1, and 2
times the Transitional or Final Rule Permissible Exposure Limits (PELs)
(11.4)
as Time Weighted Averages (TWAs).
[Note: After
the evaluation, the regulation for exposure to cadmium was changed to
0.005 mg/m³ as a TWA. Please see the Code of Federal Regulations (29 CFR
1910.1027 or 29 CFR 1926.63) for further information.]
In
addition, this method is sensitive enough to determine the Short-Term
Exposure Limit (STEL) and Ceiling concentrations for Be. This procedure
does not differentiate between dusts and fumes.
Spiked samples
were used to evaluate the method. Each spike was calculated to represent
the amount of analyte found at a concentration near its respective PEL
when taking a 400- to 500-L sample. This air volume range is
approximately equivalent to taking 4-h samples when collected at a flow
rate of 2 L/min.
1.2 Spectrometer
Description
The ARL spectrometer includes a 1.0-m path
length with 1,080 grooves/mm grating and a solid-state radio frequency
(RF) generator. Standard operating conditions for this instrument were
developed through a series of benchmark tests recommended for most
analytical applications. Equipment and operating conditions used during
this evaluation are listed in Table
1. This 36-channel instrument was configured for the simultaneous
determination of 35 elements, as listed within the line library in Table
2. Two channels are used for Fe; one wavelength (259.94 nm) is
appropriate for dilute concentrations, while the other (271.44 nm) is
used for quantitating higher concentrations of Fe. The line library
contains information for the line array wavelengths and spectral orders
as set up by the instrument manufacturer. The resolution at a specific
wavelength is dependent upon the grating and spectral-order of the line.
Spectral resolution is defined as full width at half maximum
(FWHM), i.e., at half the height, the measured width of the peak signal.
Using the grating specified in Table
1, the resolution/spectral order relationship is described
below:
Table of Order vs. Spectral
Resolution
|
| Order |
Wavelength
Range
(nm)* |
Resolution
(nm) |
SAMI Range
(motor
steps) |
|
| 1st |
400-800 |
0.046 |
80 |
| 2nd |
235-400 |
0.023 |
| 3rd |
165-235 |
0.015** |
|
| * |
wavelength range is general -
slight overlaps into the next order are possible |
| ** |
third order provides the best
resolution | |
The SAMI is a spectral scanning device that is further
described in Section 3.1.
As shown above, a second-order peak
has a resolution of 0.023 nm. A ±80 motor step scan of a second-order
peak would easily cover a range of 0.046 nm.
The analysis of
additional elements beyond the eight validated allows for a more
complete spectral interference correction and provides for additional
screening capability. A list of the elements screened and validated is
provided in Table
2 and further displayed as calibration standards in Table
3.
1.3 Standard and Reagent Purity
a) Standards:
Standards used during this study
were prepared in a 32% HCl/4% HNO3 matrix except as noted in
the text. Single and multi-element standards were used to evaluate the
instrument. Chemical compatibility of the constituents was taken into
account in order to avoid precipitation when preparing the multi-element
standard solutions.
Two multi-element standards and a reagent
blank, as listed in Table
3, were used for instrument calibration of the validated elements.
The reagent blank consisted of a 32% HCl/4% HNO3 mixture. A
third standard solution (STD SOLN 3) is used to calibrate the screened
elements.
Note: The concentration and combination of elements
in each standard mixture was selected to minimize interferences for the
particular instrument and wavelengths used. Analysts using instruments
with different wavelengths/elements than stated may have to use
alternate mixtures.
All standard solutions used for instrument
calibration (Table
3) or spectral interference determinations were prepared by serial
dilution.
b) Chemicals Used for
Validation:
Sample Digestions and
Dilutions
Nitric Acid (HNO3, 69.0 - 71.0% w/w)
and Hydrochloric Acid (HCl, 36.5 - 38.0% w/w) ("Baker Analyzed"
Reagents, J.T. Baker Chemical Co., Phillipsburg, NJ).
Instrument Calibration
ICP Standard
Solutions (1,000 µg/mL) ("Instra-Analyzed" Atomic Spectral Standards,
J.T. Baker Chemical Co.)
Filter
Spiking
Most solutions used for filter spiking were
prepared from standards obtained from Inorganic Ventures, Inc. (Brick,
NJ). In addition, ICP standard solutions (10,000 µg/mL) from Specpure
(Aesar/Johnson Matthey Inc., Seabrook, NH) were used for any high
concentration spikes.
Inter-element
Corrections
Inorganic Venture and J.T. Baker standards
were primarily used to determine inter-element corrections.
1.4 Instrument Calibration
The
polychromator was calibrated according to the manufacturer's procedures.
The reagent blank and the multi-element calibration solutions (Table
3) were matched to the sample matrix of 32% HCl/4% HNO3.
During the calibration process, linear regression coefficients
were calculated by the Digital Equipment Corporation (DEC) 11/53
computer from standard response readings. All calibrations were
determined by first-order regression only.
1.5
Soldering and Brazing Discussion
This validation
primarily addresses air samples collected during soldering or brazing
operations. The eight elements most commonly found in these operations
were: Ag, Be, Cd, Cu, Pb, Sb, Sn, and Zn. Other elements can be added to
this list. The capability for expanding the analysis to other elements
is mainly dependent on laboratory instrumentation, element solubility,
and stability in the acid matrix used for digestion.
Applicable
OSHA Permissible Exposure Limits (PELs) (11.4)
are listed in Table
4. This Table lists both the Transitional and Final Rule PELs for
substances which may be present in solder and brazing operations. The
Transitional and Final Rule Limits are identical with the exception of a
Final Rule Ceiling Limit for Be, a TWA for tin oxide, and additional
STELs for zinc chloride and zinc oxide.
1.5.1 Welding,
Soldering, and Brazing Applications (11.5,
11.6)
According to Kirk-Othmer (11.5),
welding, brazing, and soldering are metal-to-metal bonding processes.
The temperature at which the joint is made is the primary feature
which differentiates between welding, soldering, or brazing. Solder
alloys melt below 800°F (427°C) and brazing alloys melt above 800°F.
a) Welding:
According to
Kirk-Othmer (11.5),
in welding operations, similar components are fusion-bonded at or just
below their melting points. The filler metal is either puddled into
relatively wide gaps, or the metal surfaces being joined are partially
melted and bonded by fusion or by a combination of puddling and
fusion.
b) Brazing and soldering:
In most brazing and in almost all soldering operations, the
components are molecularly bonded well below their melting points. An
exception is the brazing of aluminum and magnesium alloys. Generally,
both soldering and brazing involve the introduction of a non-ferrous
filler material. While similar in concept, brazing and soldering are
not identical. The temperatures at which the alloys melt provide the
primary difference between the two procedures.
The major
brazing filler metals are copper, brass, bronze, and silver alloys.
The filler metals are drawn into closely fitted joints by capillary
action and they bond and solidify without melting the components. Zinc
and cadmium can volatilize from zinc- and cadmium-containing brazing
alloys during brazing.
1.5.2 Specific
Components of Solders
The composition and use of some
common solders is shown in Table
5. A more detailed description of some of the common solder alloys
is given below:
a) Lead/tin:
Previously, most solder alloys were composed of combinations
of tin and lead.
b) "Lead-free":
Recently, "lead-free" solders have become more prevalent. A
sample of this type of solder was obtained and a qualitative analysis
by ICP and X-ray fluorescence (XRF) procedures at the SLTC confirmed
this solder contained an alloy of tin, silver, copper, and bismuth.
The "lead-free" solders have been reported as possessing
characteristics similar to "50 Tin/50 Lead" solders, and a greater
tensile and shear strength than those made with "50 Tin/50 Lead" or
"95 Tin/5 Antimony" solders. "Lead-free" solders have been substituted
for lead solders in plumbing applications, but have not been
noticeably used in the electronics industry. This lack of use in the
electronics industry is apparently due to the high temperature needed
to achieve the "lead free" solder's melting point.
c) Silver:
Many silver solders contain
cadmium in varying amounts.
d)
Antimony/tin:
These solders are also used as brazing
alloys.
e) Cadmium/silver:
These are used with much higher temperatures and are suitable
for use on copper and aluminum. This solder will also produce a very
high tensile strength.
f) Zinc:
Many of the alloyed solders may contain varying amounts of
zinc.
g) Indium:
This is used
for special applications, i.e., adhering glass to glass or glass to
metal. The low vapor pressure of these solders makes them useful for
seals in vacuum systems.
h) Other
metals:
Other trace contaminants present in base and
filler metals include arsenic, chromium, bismuth, cobalt, nickel,
selenium, thallium, and vanadium.
1.5.3
Solder and Brazing Health Hazards (11.5,
11.6)
Solder's
greatest danger to health lies in the presence of lead or cadmium in
the solder alloy. Historically, lead was present in large amounts in
most solder alloys, and some special alloys contained cadmium.
In the past, the four most hazardous metals commonly found
during soldering and brazing processes were lead, cadmium, beryllium,
and zinc. More recent solder formulations attempt to exclude most of
these elements. Some of the potential symptoms and hazards incurred
from exposure to these and other elements are listed below (11.6)
and current PELs for validated elements are listed in Table
4.
a) Lead:
Lead is used
in the soldering process in the form of lead/tin and lead/silver
filler metals. When heated, lead oxide fumes are formed. Excessive
exposure to lead oxide fumes can result in lead poisoning. Symptoms
include loss of appetite, indigestion, nausea, vomiting, constipation,
headache, abdominal cramps, nervousness, and insomnia. According to
Kirk-Othmer (11.5),
lead is absorbed through the mucous membranes of the lung, stomach, or
intestines and then enters the bloodstream.
b)
Cadmium:
Cadmium is found in some silver and zinc
solders, and in some base metals. When heated, cadmium oxide fumes can
be generated. Excessive exposure to these fumes can result in cadmium
poisoning, symptoms of which include dry cough, irritation of the
throat and nasal passages, ulceration of the nose, tightness of chest,
restlessness, and renal damage. Cadmium is a suspected carcinogen and
a higher incidence of prostate and lung cancers are noted among
workers in occupations that use cadmium in their
processes.
c) Beryllium:
Beryllium is used in magnesium filler metals for furnace
brazing and in some aluminum brazing filler metals. While soldering,
temperatures are normally too low to generate fumes from beryllium;
however, the heat involved in brazing can generate beryllium fumes,
which are extremely hazardous. Short-term exposure to these fumes may
result in a chemical pneumonia. Long-term effects include shortness of
breath, chronic cough, loss of weight, and fatigue. Beryllium is a
suspect human carcinogen.
d) Zinc:
Zinc is used in large amounts in zinc-cadmium and
zinc-aluminum solders and in some base metals. When heated, zinc oxide
fumes are generated. Excessive exposure to freshly formed zinc oxide
fumes can result in an illness called metal-fume fever or "zinc
chills." Symptoms include the presence of a sweetish or metallic taste
in the mouth, dryness and irritation of the throat, coughing, a
feeling of weakness, fatigue, and a general malaise condition similar
to the flu.
According to Kirk-Othmer (11.5),
zinc or tin chlorides are found in some fluxes.
e) Antimony/tin:
The potential health hazard
is moderate because harmful amounts of antimony or tin fumes are not
generally formed.
f) Indium:
Although human exposures concerning contact with indium or its
compounds have not been reported, animal studies indicate significant
lung impairment from respiratory exposures.
g)
Other Metals (11.6):
Other
trace metals present in base and filler metals which can give off
toxic fumes include arsenic, chromium, bismuth, cobalt, nickel,
selenium, thallium, and vanadium. It should be noted that a specific
PEL has not been assigned to bismuth at this time. NIOSH has stated
that arsenic is a suspected lung and lymphatic carcinogen, and
hexavalent chromium is a suspected lung carcinogen. The amount of
fumes generated from these trace metals is usually small, and
hazardous concentrations are not normally found in these operations.
Soldering and brazing with filler or base metals containing these
trace elements should be conducted in well-ventilated
areas to be certain that hazardous concentrations do not exist.
Compounds of these metals may also be present.
1.5.4 Selection of Solder/Brazing Elements for Method
Evaluation
The elements to be evaluated for analysis by
ICP were selected based upon several factors which included:
- The severity of potential health hazards from soldering and
brazing operations.
- Probability of occurrence of those hazards in the workplace
environment.
- The instrumental considerations including compatibility of the
selected analytes.
- The applicability of a dissolution procedure for the sample
matrix.
For example, due to its limited
use in industry, indium is not a validated element in this report.
Analysis can be performed for indium by using OSHA method no. ID-121.
An alternate ICP procedure (11.2,
11.7)
is available which can determine the elements Be, Cd, Cu, Pb, Sb, and
Zn. This alternate procedure is unable to accurately quantitate the
elements Ag and Sn due to solubility problems from the
H2S04/HCl digestion used.
2. Experimental
Procedure
An ICP Standard Operating
Procedure (SOP) is available for the ARL 3560 ICP, which details the
instrumental operating procedures used for this evaluation (11.8).
The procedure used for sample preparation has been described in OSHA
Method No. ID-206
(11.1).
Each sample taken from soldering and brazing operations is digested with
HCl and HNO3 (8:1 ratio), diluted to volume with deionized
water (DI H2O) to achieve a 32% HCl/4% HNO3 mixture,
and analyzed by ICP-AES. A systematic set of experiments for the purpose
of instrument evaluation was used. The experimental protocol
included:
- Investigation and correction for spectral line interferences.
- Determination of over-all analytical precision and accuracy.
Analyses of 18 samples for each element (6 samples at each test level)
except for Ag where 3 samples were determined at each test level.
- Determination of analytical detection limits.
- Determination of working ranges for the elements validated.
- Evaluation of spiked Quality Control (QC) samples.
- Discussion of the digestion procedure.
- Determination of a standard reference material.
- Determination using alternate wavelengths.
- Summary.
Each of these experiments is
discussed in Sections 3 through 9 below:
3.
Interferences
The determination of Inter-element Correction
(IEC) factors to compensate for spectral interferences is part of the
development procedure for any multi-element analytical method which uses
an atomic emission spectrometer. Spectral interferences have been
minimized by the careful selection of wavelengths for the line array of
the spectrometer. Spectral interferences have also been compensated for by
software which performs inter-element corrections. This
software assumes a linear relationship between analyte and interferant
within the working range limits.
3.1 Procedure
Inter-element corrections for elements likely to be found
in soldering and brazing operations were experimentally determined by
identifying and then evaluating the magnitude of each interference on
each analytical line. The interferences were first qualitatively
identified through peak scans and then a quantitative correction factor
was calculated. Inter-element corrections were experimentally determined
by identifying and then evaluating the magnitude of the interferences as
stated below.
The identification of interferences was first
determined by scanning peak profiles. Three types of peak scans were
conducted:
- the location of each analyte line peak position relative to the Mn
profile line.
- the aspiration of high concentration single-element standard
solutions and subsequent analysis using the spectrometer line array to
determine potential interferences.
- the re-scanning of each line during aspiration of a low
concentration analyte standard solution, and multiple higher
concentrations of the interfering element to determine the extent of
the interference.
The evaluated ARL ICP
has a mechanical spectral scanning device (this device is called a
"SAMI" by the manufacturer) that drives the primary slit using motor
steps in order to scan peak profiles over a specific wavelength range.
Scans were setup on the SAMI for ±80 motor steps to allow for
sufficient resolution of the analyte peak regardless of spectral order.
This "window" was sufficient to view any interferences close enough to
the analyte peak which may be erroneously identified as the analyte. The
scans were performed in 4-step increments and 2-s signal integration
time was used at each step.
Two sets of scans were analyzed in
order to determine spectral interferences.
a) First spectral interference scan
These scans were used as a screening technique to determine which
elements would pose a potential interference for any given spectral
line.
b) Second spectral interference
scan
Information from these scans demonstrated the extent
of each interference by showing the relative position of the analyte
peak to the interferant peak and the ratio of the response of the
interferant to the analyte standard for the particular spectral line.
After visually identifying potential spectral
interferences from these peak profiles, the quantitative effects from
these interferences were determined by measuring the intensities of each
interfering element. Apparent concentrations resulting from these
spectral line interferences on other channels were then determined. The
IEC factor was determined for each interference using the following
equation:
where:
| K |
= |
IEC factor |
| B |
= |
Apparent concentration of the affected
element |
| C |
= |
Concentration of the interferant
(single-element) standard |
Further details for determining spectral line
interferences for the ARL 3560 ICP has been described in reference 11.7.
3.2 Results
3.2.1 Peak Profiles (Motor Steps
Off-profile)
The peaks for the eight
element line array(s) were scanned using the "SAMI" in order to
determine how close they were to theoretical values. It has been
suggested by the instrument manufacturer, as a general rule, that each
spectral line should be within ±8 motor steps from the profile peak.
[Note: For this instrument, Mn and Cd are
used as profile elements. Prior to each analysis, a solution
containing the profile element(s) is scanned, the peak intensity
identified, and the slit settings adjusted to give maximum intensity
for the profile element(s). Further details regarding instrument
profiling can be found in references 11.1
- 11.3,
and 11.7.]
As summarized below, all the lines examined in this particular
ICP-AES instrument were within ±2 motor steps.
|
Motor Steps
"off
profile" |
Element |
|
| 2 |
Be |
| 0 |
Cd, Cu, Sb, Sn |
| -1 |
Ag, Pb |
| -2 |
Zn |
|
This indicates that,
as a general rule, when the instrument is profiled prior to each
analysis, the line peaks of the solder elements are well within
specifications.
3.2.2 Spectral Line
Interferences
The interference relationships on
specific wavelengths used for elements expected to be collected in
solder/brazing operations are shown in Figures 1 and 2. Figure
1 is for those elements affecting Pb and Sb. Figure
2 is for those elements affecting Ag, Be, Cd, Cu, Sn, and Zn.
A chart summarizing significant spectral line interferences
found during this evaluation is shown in Table
6. This Table lists the spectral line corrections in the order of
correction (i.e. the interference of Cd on the Co channel is corrected
before the interference of Co on the Cd or Pb channel).
As can
be seen from the first correction in Table
6, an IEC correction value of 0.00021 is necessary to compensate
for the affecting element Cd on the Co channel. If Cd is present,
0.00021 µg/mL is subtracted from any Co concentration for each 1 µg/mL
of Cd present in the sample. If 10 µg/mL of Cd is present, then a
0.002 µg/mL signal is subtracted from the Co channel.
The range
of IECs varied from a low of 0.000025 for Co interfering on the Cd
channel to a high value of 0.0039 for Be interfering on the Sb
line.
Because some solder samples may contain Bi, a study was
conducted to determine the feasibility of analyzing and correcting for
Bi. An interference from Bi on Sb and Pb was noted. The instrument as
set up did not contain a channel for Bi. Bismuth is a component of
some solders, especially the more recent "lead free" type. To
compensate for this, the Se channel at 196.09 nm was modified to allow
a scan of the Bi line at 196.006 nm using the SAMI scanning device.
This modification to include the semiquantitative determination of Bi
and any interferences from Bi on other lines requires additional
analysis time because the SAMI offsets from the Se to the Bi
wavelength for Bi measurements. 4. Precision and Accuracy
This experiment was designed to evaluate instrument
performance for determining analytes normally found in a sample matrix
from soldering and brazing fume operations.
4.1 Procedure
After the IEC factors were entered into the ARL computer
software, the precision and accuracy of the method were evaluated by
analyzing spiked samples. The spiking scheme for the multi-element
samples was conducted in the following manner:
The precision and
accuracy for each element was evaluated by analyzing 18 spiked filter
samples. Aqueous reference standards described in Section 1.3. were used
for each spike. Spike amounts were calculated for levels at about 0.5,
1, and 2 times the OSHA TWA PEL assuming a 480-L air volume. A worst
case scenario of air volumes < 400 L was assumed for samples
containing about 0.5 times the PEL for silver. Each multi-element spike
was delivered to a single mixed-cellulose ester (MCE) filter. The spikes
were delivered from stock solutions using calibrated micropipettes. A
calculation error occurred regarding the mass of silver during
preparation of the first multi-element spikes. Filters spiked with
silver at about 0.5 times the PEL were then prepared separately.
All spiked sample filters were digested and prepared for
analysis using the procedure specified in the method (11.1).
Each sample was diluted to a 25-mL solution volume. Samples separately
prepared for silver (approximately 0.5 times the PEL) were diluted to a
final volume of 10 mL. This solution volume is recommended in the method
(11.1)
to provide increased sensitivity for silver.
The instrument was
calibrated as stated in Section 1.4. These samples were determined using
a two-point calibration consisting of standards listed in Table
3 and a reagent blank.
4.2 Results
All sample results were examined in terms of precision (CV) and
amount of error. Analytical error (AE) for each element is calculated
as:
| ± AE % = 100 [|Mean Bias| + 2(CV)]
95%
confidence |
From the
summary in Table
7, it can be seen that the AE ranged from 2.3% for antimony to
approximately 17% for silver and lead. The precision range (CV) was from
0.01 for many of the analytes to 0.06 for silver and lead. The bias
varied from -0.011 for beryllium to +0.055 for zinc.
5. Detection limit, Background
Equivalent Concentration, and Short-Term Precision
5.1 Procedure
The procedure for the determination of detection limits,
Background Equivalent Concentration (BEC) and short-term reproducibility
for the ARL 3560 ICP has been previously described (11.7).
The detection limits in this evaluation were determined after aspirating
multielement calibration standards for a 5-s integration time. An 11
exposure sequence was used for the blanks and 10 exposures for the
standards. Multi-element calibration standards (STND SOLNS 1 and 2)
shown within Table
3 were used. The manufacturer's software algorithms calculate the
qualitative detection limit (DL) as two times the standard
deviation.
The qualitative detection limit for the ARL instrument
is calculated as:
| DL = (A × SDI × C)/(I -
Io) |
Where:
| A |
= |
2 (qualitative detection limit)
or |
| A |
= |
10 (quantitative detection
limit) |
| SDI |
= |
Standard Deviation of the Background
Intensity |
| I |
= |
Total Intensity |
| C |
= |
Analyte Concentration, the
concentration of the calibration standard |
| Io |
= |
Background Intensity (determined from
reagent blank) |
The BEC
is defined as the concentration of an analyte that is equal to the net
intensity of the background signal for that analyte:
Where:
m = (C)/(I - Io) = slope of the
calibration curve
Note: The manufacturer's software algorithms
automatically calculate the qualitative detection limit using two times
the standard deviation (2SD) of the blank, among other considerations.
Although the OSHA Inorganic Method Validation Protocol (11.9)
states a qualitative limit shall be determined with 3SDBL in
the calculations 2SDBL is accepted here to allow for future
performance comparisons using the same instrument and software.
5.2 Results
The results for short-term instrument precision are
reported in Table
8 as the Coefficient of Variation (CV) and Background Equivalent
Concentration (BEC). At concentration levels equal to or greater than
the BEC, short-term precision ranges are normally approximately 0.5% to
1.5% (as CV), provided linearity of the spectral response function is
maintained. Short-term precision should be 1% or better for simultaneous
systems (11.10).
As can be seen in the last column of Table
8, the CV is < 1% for all lines. The qualitative detection limits
varied from 0.001 µg/mL for beryllium to 0.05 µg/mL for lead. The BEC
values ranged from 0.02 µg/mL for beryllium to 1.9 µg/mL for lead.
Detection limits determined approximately one year after these
results were obtained show improved performance for most
elements:
|
| ELEMENT |
QUAL DL |
|
Ag
Be
Cd
Cu
Pb
Sb
Sn
Zn |
0.0043
0.0002
0.0054
0.0055
0.0425
0.0481
0.0396
0.0031 |
|
Detection limits are
very dependent on maintenance and operating conditions, and should be
periodically checked to assess instrument performance and the need for
maintenance. 6. Working
Range
The calibration used for routine
analysis of solder samples is a first order regression. For the
calibrations, the ARL 3560 ICP computer software calculates a linear
regression equation for each element from the intensities (counts) of two
measurements (a reagent blank and a reference solution usually in the
range of 1 to 10 µg/mL). An evaluation to determine the appropriate lower
and upper concentration ranges for each validated or screening element was
performed using these calibrations.
6.1. Procedure
The linearity was evaluated using a procedure previously
described (11.7).
The linearity of the calibration curves was checked by analyzing several
standard solutions within the range from 5 to 10 times the calculated
detection limit up to 1,000 µg/mL. The upper range was limited either by
reaching a detector saturation level or by exceeding the value of the
highest standard stock solution used (1,000 µg/mL).
6.2. Results
The highest concentration of most
of the standards used was 1,000 µg/mL. For a few elements, a 1,000 µg/mL
concentration did not saturate the detector nor was the linear range
exceeded. Standard solutions exceeding a concentration of 1,000 µg/mL
were available for these elements and linearity determinations beyond
1,000 µg/mL were performed. These exceptions were: aluminum, magnesium,
lead, bismuth, and antimony.
The upper working ranges (µg/mL)
and the concentrations at which the photomultiplier tubes (PMTs) become
saturated for elements at the wavelengths designated in the array are
summarized in Table
9. The PMTs should become saturated at a value between the Upper and
Saturation concentrations reported in the Range column.
In Table
8, column five contains the lower quantitative detection (LQD) limit
and column six the upper range of linearity for the brazing and solder
elements. For the ARL instrument, the LQD is five times the qualitative
detection limit. The quantitative detection limits ranged from 0.005
µg/mL for Be to 0.2 µg/mL for Pb and Sb.
The linear range was
evaluated using a 5-s integration time. The upper concentration limit
for the validated elements ranged from 20 µg/mL for Be to 1,000 µg/mL
for Pb and Sb. The optimum working range for most elements exceeded 100
µg/mL. 7. Evaluation of Spiked
Quality Control (QC) Samples
7.1. Procedure
Sets of QCs were determined for a final evaluation of the
precision and accuracy of the procedure. These QCs were independently
prepared on spiked filters. The concentration levels for some of the
analytes on these QCs were lower than those in the previous precision
and accuracy evaluation. With the exception of beryllium, the
concentrations for the prepared spikes were based upon calculations
using a 4-h sampling period and an air volume of 480 L.
The
concentrations ranged from 0.1 to 1 times the TWA PELs as
follows:
|
| ELEMENT |
× PEL |
|
Ag
Be
Cd
Cu
Pb
Sb
Sn
Zn |
0.5
(Ceiling or STEL)
0.1
0.5 to
1
1
0.5 to 1
0.5 to 1
1 |
|
To determine if
precision and accuracy could be improved using smaller solution volumes,
all samples were diluted to a final solution volume of 10
mL.
7.2. Results
The data, as
summarized from Table
10, provide the following method performance information:
With the exception of Be in one QC set, the AE was less than
25%. The Precision (CV) was better than 0.14 for all samples. With the
exception of Cu in the last QC set, the Mean varied within ±10%. For the
most part, these results are similar or better than those presented in
Section 4, and indicate a 10 mL solution volume can be used as an
alternative dilution. It is unknown why one set of beryllium samples was
± 35% AE. Samples to be analyzed for beryllium at Ceiling or STEL levels
should be diluted to 10 mL to improve sensitivity.
8. Digestion Procedure
Discussion
Experiments were previously
conducted (11.1,
11.11)
to evaluate the digestion procedure for solders. Following validation of
the method, spiked samples of the eight elements were prepared by an
independent group within the OSHA SLTC and had been routinely analyzed
using the JY-70 ICP. Some of the spiked samples had low antimony
recoveries.
As shown by the precision and accuracy, and
independent QC results within this report, the recoveries for antimony are
adequate if the digestion procedure in OSHA method no. ID-206
is followed. The loss of antimony was attributed to incomplete wetting of
the filters with HCl before addition of HNO3.
9. Determination of a Standard Reference Material
A Standard Reference Material (SRM) of solder from National
Institute of Standards and Technology (NIST), containing certified values
as reported below, was determined as "blind samples".
NIST SRM 127b Solder
(40 Sn-60 Pb)
|
| Element |
Percent by Weight |
|
| Tin |
39.3 |
| Antimony |
0.43 |
| Arsenic |
.01 |
| Bismuth |
.06 |
| Copper |
.011 |
| Nickel |
.012 |
| Silver |
.01 |
| Lead |
60.17* |
|
| * actual amount is not listed on the
certificate. The percent was calculated by subtracting the reported
elements from 100% and assuming the remainder as
lead. |
9.1. Procedure
Three "blind samples" containing the SRM were routinely
analyzed by a laboratory chemist. Sample preparation for bulk material
was carried out according to OSHA method no. ID-206.
Additional details are described below:
|
sample wt
range (mg) |
sample wt
median (mg) |
final sample
vol (mL) |
sample
(mg/mL) |
|
| 10-14 |
12 |
250 |
0.048 |
|
9.2.
Results
Due to the weights of SRM
used, the values found for Ag were below both the quantitative and
qualitative detection limits. The values for Cu, Sb, and Zn were below
the quantitative but above the qualitative detection limits. Therefore,
the values for Cu, Sb, and Zn could not be used and merely indicate that
these elements were present in the sample matrix.
Although Zn
was found in the SRM, it was not reported on the accompanying
certificate. It is possible that due to the large amounts of Pb and Sn
present in the bulk material these elements might possibly give an
apparent value for Zn, as the spectral corrections for this method are
for low concentrations typically found in workplace atmospheres. Caution
must be observed when using this method as a screening tool to identify
unknown elements in a bulk matrix. Minor elemental quantities found for
elements in bulks must he considered as apparent values only, and may
have to be confirmed by other methods such as Atomic Absorption
Spectroscopy.
From the results reported below it is evident that
if the minor components are to be reported, two sample aliquots will
have to be taken; one for the more concentrated aliquot used for
quantitating the values for Sb, Cu, and Ni, the other a larger weight
for minor components.
A suggested scheme for solder bulk
materials is to weigh a 10 to 20 mg aliquot; dilute to 250 mL to obtain
the major components within the linear working range; and prepare
another sample using approximately a 100-mg sample weight and 100-mL
sample solution volume. This will enable both major and minor components
to be identified, provided both sample results are carefully scrutinized
for exceeding upper linear range limits, detection limits, or
interferences potentially existing in a variable sample
matrix.
|
| |
------------------Wt %-------------------- |
| |
Pb |
Sn |
Sb |
Cu |
Ni |
Ag |
|
| Reference values |
60.17 |
39.3 |
0.43 |
0.011 |
0.012 |
0.01 |
| Avg Recovery |
60.4 |
38.1 |
n.d. |
n.d. |
n.d. |
n.d. |
| F/T |
100. |
96.9 |
- |
- |
- |
- |
|
| n.d. = none
detected |
10. Determinations
using Alternate Wavelengths
10.1.
Introduction
An ideal situation for
emission spectroscopy would be to have many alternate spectral lines
available for analysis of each element to assist in characterizing the
sample and minimize error. Unfortunately, there is a finite amount of
physical space available for the installation of spectral lines in the
array of a simultaneous direct reading emission spectrometer. This
limits the number of element channels that may be installed. An
alternative to increasing channels for the ARL 3560 ICP is to "create"
lines using the SAMI scanning mechanism. The SAMI, as previously
described in Section 3.1., is a stepper-motor-controller which allows
for mechanical profiling of the slit image and the relative wavelength
position. The SAMI is also routinely used for background corrections,
moving off and on a fixed wavelength at specified intervals. This
ability to move away from the fixed line position allows for examination
of other emission lines nearby. These emission lines, referred to as
"free SAMI" lines, are limited because they must be in close proximity
to element channels previously installed by the instrument manufacturer.
10.2. Procedure
Using the
scanning ability of the SAMI mechanism, free SAMI lines (optional
wavelengths) for the elements Bi, Cd, Be, and As were programmed into
the manufacturer's software as described in the table
below:
Selected Free SAMI Lines
|
| Ele(2) |
Wavelength(2)
(nm) |
Ele(1) |
Wavelength(1)
(nm) |
Offset
(Steps) |
Spectral
Order |
|
Bi
Cd1
Cd3
Be3
As1 |
196.006
228.801
214.433
217.506
228.812 |
Se
Co
Te
Sb
Co |
196.090
228.616
214.275
217.581
228.616 |
218.00
-320.00
-274.00
192.00
-338.00 |
3
2
2
3
2 |
|
Where:
Ele(1) = original
channel modified to allow a scan of Ele(2).
For example, the Co
228.616 nm line [Ele(1)] was modified by the SAMI (offset -320.00
divisions) to scan the 228.801 nm line for Cd [Ele(2)]. |
|
Detection limits and
BECs were then determined for these free SAMI lines using the procedure
discussed in Section 5.
10.3. Results
The following BEC and detection limit (DL) values were
calculated after determinations using the concentration of standards
specified (Std Concn). Reagent blanks were prepared in DI
H2O. The DL was reported as a qualitative detection limit
(A=2, as stated in Section 5.1).
BEC/DL - Free SAMI and Original Spectral
Lines
|
Channel
Name |
Blank
Int. |
Blank
SD |
Std
Int. |
Std
Concn |
BEC |
DL |
Wavelength
(nm) |
|
| As |
5.8799 |
0.2734 |
143.98 |
100.00 |
4.2578 |
0.3960 |
189.042 |
| As1 |
6.1419 |
0.1108 |
67.950 |
100.00 |
9.9371 |
0.3586 |
228.812 |
| As |
2.7624 |
0.1230 |
1,369.1 |
1,000.0 |
2.0217 |
0.1801 |
|
| As1 |
4.7699 |
0.0612 |
625.87 |
1,000.0 |
7.6798 |
0.1971 |
|
|
| Be |
4.5310 |
0.2074 |
1,450.4 |
10.000 |
0.0313 |
0.0029 |
313.042 |
| Be3 |
4.3889 |
0.0232 |
29.060 |
10.000 |
1.7790 |
0.0188 |
217.506 |
| Be |
4.5310 |
0.2074 |
|
100.00 |
saturated
detector |
| Be3 |
4.3889 |
0.0232 |
246.92 |
100.00 |
1.8096 |
0.0191 |
|
|
| Bi |
3.3873 |
0.0172 |
7.1453 |
100.00 |
90.134 |
0.9166 |
196.006 |
| Bi |
3.3527 |
0.0302 |
11.011 |
200.00 |
87.555 |
1.5754 |
|
|
| Cd1 |
4.2365 |
0.0168 |
203.77 |
10.000 |
0.2123 |
0.0017 |
228.801 |
| Cd3 |
3.5647 |
0.0164 |
314.33 |
10.000 |
0.1147 |
0.0011 |
214.433 |
| Cd |
1.7259 |
0.0164 |
98.449 |
10.000 |
0.1784 |
0.0034 |
226.502 |
| Cd1 |
4.2365 |
0.0168 |
1,000.0 |
|
saturated
detector |
| Cd3 |
3.5647 |
0.0164 |
|
1,000.0 |
saturated
detector |
| Cd |
1.7259 |
0.0164 |
|
1,000.0 |
saturated
detector |
|
The elements (Channel Name)
which are not followed by a number are the original installed
lines.
Blank Int. and Std Int. are blank and standard
intensities in K-pulses (this is a unique term used by the
manufacturer to designate intensity counts).
All
concentration (Concn) values (Std, Concn, BEC, and DL) are in
µg/mL. |
These additional
lines may be used as elemental confirmation to avoid the reporting of
"false positives" which can occur at concentrations near the detection
limit due to matrix effects, uncharacterized spectral, or other
interferences. The application of these alternate lines will extend the
analytical time taken for each sample, and subsequently affect the
amount of sample volume used during sample preparation. Sample solution
volumes of 10-mL may be insufficient if numerous additional lines are
scanned and more than one sample determination is necessary. Some notes
regarding the sensitivity of each line are listed below:
Bismuth:
The Bi channel is relatively
insensitive, yielding high BEC and DL values at concentrations of from
100 to 200 µg/mL. However, as previously discussed in Section 3, this
line may be used as confirmation for the presence of Bi in "lead free"
bulk solder samples.
Cadmium:
The
alternative lines for Cd have improved detection limits of a magnitude
of about three times that of the Cd 226.502 nm line. This increased
sensitivity is important as the PEL for Cd has recently been lowered
significantly (29 CFR 1910.1027 or 29 CFR 1926.63). It is important to
note there is a major As spectral interference on the alternate Cd
228.801 nm line.
Arsenic:
Although
at first glance it appears that the alternate As 228.812 line has a
comparable detection limit with the As 189.042 line, this is misleading.
Upon further examination, intensities from the same sample
concentrations are about two times greater for the As 189.042 line. The
reason for the similar detection limits results from the imprecision of
the As 189.042 blank (being about two times higher than that of the As
228.512 line). In addition, there is a major spectral interference on
the alternate As line resulting from cadmium.
Beryllium:
The original Be 313.042 line is
approximately ten times more sensitive than the alternate Be 217.506
line; however, the 217.506 line has a higher upper range and reduces the
requirement of further sample dilution for samples containing high
concentrations of Be.
Summary
In conclusion, this evaluation demonstrated
that the ARL ICP instrument adequately determined the eight elements
commonly found in soldering and brazing fumes within the ranges specified.
Compliance with OSHA Transitional or Final Rule PELs for solders can be
determined using this instrumental procedure (Note: At
the time of this writing the OSHA Final Rule Limits were stayed).
Precision and accuracy, detection limits, and linear working ranges were
all acceptable for air samples taken near the PELs for the eight elements
validated. As previously stated in Section 7.2., sample dilutions using 10
mL solution volumes should result in improved sensitivity for Be.
Sensitivity for other elements will also improve when using the 10-mL
final solution volume; however, a drawback is the amount of sample
available for repeat analysis. Due to the design of the automatic
sampler/nebulizer sample introduction system, about 5 to 6 mL of sample is
needed for each analysis.
A drawback to this method is the acid
digestion and dilution media; the acid matrix used (32% HCl/4%
HNO3) is very corrosive, and care must be exercised when
handling solutions. As discussed in a previous work (11.1,
11.11),
care must be taken during the digestion to prevent the loss of Sb or Sn.
Special attention must be taken regarding the order of addition of the
digestion acids. After adding the HCl, it is recommended to wait 5 min
before adding HNO3.
This analytical method should be
applicable with minor modifications to any ICP containing analytical lines
for the eight elements evaluated. Further work needs to be conducted to
evaluate the ability of this ICP for analyzing Cd at the new PEL of 0.005
mg/m³.
Addendum
During this and a previous evaluation, the existence of a
few "bugs" were noted in the software provided by ARL. Although this
software (and computer hardware) is no longer available, current users
should be made aware of the problems. One problem occurs during repeated
calibration of the instrument. During each calibration, an
inter-element correction is applied if two interfering
elements are present in the same calibration standard solution. For a few
of the elements having interferences, the respective
inter-element correction being applied became additive with
the next calibration (i.e. if 10 µg/mL Sb was present in a calibration
solution containing 10 µg/mL Zn, an inter-element correction
of 0.03 was applied such that the Sb concentration became 10.03 µg/mL to
compensate for the additive effect of Zn on the Sb line. The software
would not erase this 10.03 µg/mL calibration concentration and the next
calibration valid result in Sb being recognized as 10.06 µg/mL, even
though there was only 10 µg/mL Sb in the solution). The correction would
continue to be additive with each calibration unless the user applied a
software program called CSET to "reset" the concentrations to their
original values after each calibration. This was somewhat time-consuming
to perform and was not widely known by the manufacturer's personnel nor
was it documented in any manuals.
For other elements, the
concentration would be "reset" to the original concentration for the next
calibration. Most of the elements which had an interference present in the
same calibration solution were not affected.
11. References
11.1 Occupational
Safety and Health Administration Salt Lake Technical Center: ICP Analysis of Metal/Metalloid Particulates from Solder
Operations by D.C. Cook (USDOL/OSHA-SLTC Method No. ID-206).
In OSHA Analytical Methods Manual. 2nd ed.
Cincinnati, OH: American Conference of Governmental Industrial Hygienists,
1991.
11.2 Occupational
Safety and Health Administration Salt Lake Technical Center: Metal and Metalloid Particulates in Workplace Atmospheres
(ICP Analysis) by J. Septon (USDOL/OSHA Method No. ID-125G).
In OSHA Analytical Methods Manual. 2nd ed.
Cincinnati, OH: American Conference of Governmental Industrial Hygienists,
1991.
11.3 Occupational
Safety and Health Administration Salt Lake Technical Center: Metal and Metalloid Particulates in Workplace Atmospheres
(ICP Analysis) (Backup Data Report) by J. Septon (USDOL/OSHA Method
No. ID-125G).
In OSHA Analytical Methods Manual. 2nd ed.
Cincinnati, OH: American Conference of Governmental Industrial Hygienists,
1991.
11.4 "Air Contaminants; Final Rule":
Federal Register 54:12 (19 Jan. 1989). pp.
2923-2960; also 54:127 (5 July 1989). pp. 28054-28061.
11.5 Solders & Brazing Alloys. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd
Ed., Vol. 21, edited by H.F. Mark, D.F. Othmer, C.G. Overberger, and G.T.
Seaborg. New York, NY: John Wiley & Sons, 1983.
11.6 National Institute for Occupational
Safety and Health: Health and Safety Aspects of
Soldering and Brazing [DHEW(NIOSH) Pub. No. 78-197]. Cincinnati,
OH: Division of Technical Services, September, 1978.
11.7 Occupational Safety and Health
Administration Salt Lake Technical Center: Welding Fumes ICP Backup Data Report (ARL 3560) by J.
Septon. Salt Lake City, UT, 1991.
11.8 Occupational Safety and Health Administration Salt Lake
Technical Center: ICP Analysis Reference Guide,
OSHA Lab (Comprehensive Version) by J. Septon. Salt Lake City, UT,
1991 (unpublished).
11.9 Occupational Safety and Health Administration Salt Lake
Technical Center: Evaluation Guidelines of the
Inorganic Methods Branch In OSHA Analytical
Methods Manual. 2nd ed. Cincinnati, OH: American Conference of
Governmental Industrial Hygienists, 1991.
11.10 Arellano, S.D., M.W. Routh, and
P.D. Dalager: Criteria for evaluation of ICP-AES
performance. Amer. Lab.: 20-32 (August, 1985).
11.11 Occupational Safety
and Health Administration Salt Lake Technical Center: Recovery of Antimony and Other Elements Using the Digestion
Procedure for Solders by R. Douglas and D.C. Cook. Salt Lake City,
UT, 1991. (unpublished)
Table 1
Specifications
for ARL 3560 Simultaneous AES-ICP
|
R.F.
GENERATOR
Generator Model # [kW] Henry
Incident Power
[W]
Reflected RF Power [W]
P.A. Plate Supply Voltage
[V]
P.A. Plate Current [mA]
P.A. Grid Current [mA]
P.A.
Filament Voltage [V]
P.A. Tune Setting
P.A. Load
Setting
(where P.A. = Power Amplifier)
EXCITATION
Plasma Observation Height
[mm]
Coolant Gas Flow [L/min]
Coolant Gas Pressure
[psi]
Plasma Gas Flow [L/min]
Plasma Gas Pressure
[psi]
Carrier Gas Flow [L/min]
Carrier Gas Pressure
[psi]
Snout Gas Flow [L/min]
Nebulizer Uptake Rate
[mL/min]
Nebulizer [type]
Peristaltic Pump Used?
[yes/no]
Pre-Integration Flow Time [s]
Integration Time
[s]
SPECTROMETER
Instrument
Model
Grating [lines/mm]
Primary Slit Size [µm]
Profiling
Element
Profile Point [peak dial div.]
Vacuum [µm]
Path
Length [m] |
2.5
1175
0
4100
700
125
7.5
190
050
15
12
25.5
0.8
21.5
1
30.5
1.5
2
to 3
Meinhard-Type
C
no
30
5
3560
1080
20
Mn
496*
20
1 |
| * For the SAMI, 100 dial divisions = 400
motor steps |
COMPUTER
CONFIGURATION
Disk Drive Capacity
Computer
System
Software: |
30 MEG fixed and 1.2 MEG floppy
Micro 11/53
DEC Computer
DPS/TSX+ |
|
Table 2
Line Library for OSHA ARL 3560
Simultaneous AES-ICP
|
| Element |
Wavelength
(nm) |
Spectral
Order |
|
| |
Ag
Al*
As*
Au
B
Be
Bi*
Ca*
Cd
Co*
Cr*
Cu
Fe1*
Fe2*
Ga
Ge
In
Mg*
Mn*
Mo*
Ni*
Os
Pb
Pd
Pt
Rh
Sb
Se*
Si*
Sn
Te
Tl
U
V*
W
Zn
Zr |
328.068
308.215
189.042
242.795
249.680
313.042
196.006
393.366
226.502
228.616
267.720
324.754
259.940
271.440
417.205
209.430
230.606
279.080
257.610
202.030
231.604
225.585
220.353
360.955
265.945
343.489
217.581
196.090
288.158
189.980
214.275
190.864
409.014
310.230
239.709
213.856
343.823 |
2
2
3
3
3
2
3
2
3
2
3
2
3
3
1
3
3
2
3
3
3
3
3
2
2
2
3
3
2
3
2
3
1
2
3
3
1 |
|
| Measurement of
bolded elements are validated in this report. Elements with an
asterisk (*) are screened. Bismuth (Bi) is also in the line library
through modification of the Se
channel. |
Table 3
Calibration Standards
|
| NO |
ELE |
STD SOLN |
CONCN |
|
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22 |
Ag
Be
Cd
Pb
Cu
Sb
Sn
Zn
Al
As
Ca
Co
Cr
Fe
Mg
Mn
Mo
Ni
Se
Si
V
Bi |
1
1
1
1
2
2
2
2
3
3
3
3
3
3
3
3
3
3
3
3
3
3 |
1.
1.
10.
10.
1.
10.
10.
10.
10.
10.
10.
10.
10.
100.
10.
10.
10.
10.
10.
10.
10.
100. |
|
|
Where:
ELE
STD
SOLN
CONCN |
Element
Number of Calibration
standard
Concentration (µg/mL) of calibration
standard | |
STD SOLN 1 - was prepared in an amber-colored glass bottle
to protect the Ag from photo-decomposition.
STD SOLN 3 - This standard
is used only for calibration of the screened elements. The digestion and
analysis are not optimized for these elements.
Calibration is
accomplished using a two-point calibration curve with the concentration
for each element listed above. A reagent blank was used as the low
standard. Each element calibrated is contained in one of three separate
calibration standards (STD SOLN). For example, STD SOLN 1 contains Ag, Be,
Cd, and Pb.
All solutions were prepared in a 32% HCl/4%
HNO3 acid mixture including the reagent blank.
Table 4
Air Contaminants - OSHA Permissible
Exposure Limits1
|
| Element |
Substance Exposed to |
Transitional PEL
(mg/m³) |
Final
Rule PEL
(mg/m³) |
| |
|
TWA |
CEILING |
TWA |
STEL |
CEILING |
|
| Ag |
Metal dust and fume (as
Ag) |
0.01 |
|
0.01 |
|
|
| Be2 |
Be and compounds (as
Be) |
0.002 |
0.005³ |
0.002 |
0.005³ |
0.025 |
| Cd |
Fume
Dust |
0.1
0.2 |
0.3
0.6 |
0.1
0.2 |
0.3
0.6 |
|
| Cu |
Fume (as Cu)
Dusts amd
mists (as Cu) |
0.1
1 |
|
0.1
1 |
|
|
| Pb |
Inorganic |
(see
29 CFR 1910.1025, PEL is 0.05 mg/m³) |
| Sb |
Sb and compounds (as Sb) |
0.5 |
|
|
0.5 |
|
| Sn |
Inorganic compounds
except
oxides (as Sn)
Tin oxide (as Sn) |
2
|
|
2
2 |
|
|
| Zn |
Zinc chloride fume
Zinc
oxide fume |
1
5 |
|
1
5 |
2
10 |
|
| Note: underlined
values are those TWA PELs selected for validation. |
|
STEL duration is
for 15 min unless otherwise noted.
1 From reference 11.4
2
Beryllium also has Transitional Peak PEL concentration limit of
0.025 mg/m³.
3 Both the Transitional Ceiling Limit and the STEL
for beryllium are
for a maximum 30-min
duration. |
|
Note: This
validation was performed using the Final Rule Limits bolded above.
With a couple of exceptions the Final Rule Limits for the elements
or compounds listed are identical to the Transitional Limits.
Analysis of samples by the ARL 3560 using OSHA method no. ID-206
should perform well regardless of whether Transitional or Final Rule
Limits are used. After this validation was performed, the Final Rule
Limits were vacated.
For determination of exposures to rosin core
solder pyrolysis products, please see OSHA method no. 54 for
formaldehyde. |
|
Table 5
Common Solder Alloys*
|
| ---------Composition %--------- |
Melting
range, °C |
Use |
| Sn |
Pb |
Cd |
Bi |
Ag |
Sb |
|
|
|
| 63 |
37 |
- |
- |
- |
- |
183 |
eutectic solder for electronic
application |
| 60 |
40 |
- |
- |
- |
- |
183-190 |
high quality solder |
| 50 |
50 |
- |
- |
- |
- |
183-216 |
general-purpose solder, plumbing |
| 40 |
60 |
- |
- |
- |
- |
183-238 |
wiping solder, radiator solder |
| 30 |
70 |
- |
- |
- |
- |
183-255 |
machine and torch soldering |
| 20 |
80 |
- |
- |
- |
- |
183-277 |
automotive-body solder |
| 95 |
- |
- |
- |
- |
5 |
235-240 |
refrigeration soldering |
| x |
0 |
0 |
x |
x |
0 |
|
"Pb free" plumbing
solderinga |
| 62 |
36 |
- |
- |
2 |
- |
179 |
soldering silver surfaces |
| 1 |
97.5 |
- |
- |
1.5 |
- |
309 |
high temperature soldering |
| 15.5 |
32 |
- |
52.5 |
- |
- |
90 |
fusible links |
| 13 |
27 |
10 |
50 |
- |
- |
70 |
low melting solder |
|
* Modified from reference 11.5
-
not present or reported
a This solder also
contains copper, and is patented alloy containing unspecified
amounts of elements designated with an x. These were confirmed at
the SLTC by ICP and XRF analysis. A sample of "Safe Flo (TM) Silver"
solder, gauge 0.084, from Oatey, Cleveland, OH 44135 was used for
this confirmation.
Note: Because of recent requirements of
the Safe Drinking Water Act and certain state laws restricting
lead-solder has been developed that contains no lead, antimony, or
cadmium. These "lead free" solders have become popular in the
marketplace. |
Table 6
Interferences
|
| |
Channel |
Int. |
IEC factor |
|
|
Co
V
Cd
Cd
Sn
Sn
Sn
Cu
Ag
Ag
Pb
Pb
Pb
Pb
Sb
Sb
Sb
Sb
Zn
Be
Sb
Sb
Zn
Ag
Sn
Sb
Sb
Pb
Bi*
Bi*
Bi* |
Cd
Be
Ni
Co
Al
V
Mo
Mo
Mn
V
Ni
Co
Al
Mo
Ni
Mo
Fe1
Al
V
Ni
V
Zn
Pb
Cu
Cu
Be
Be
Bi*
Bi*
Be
Fe
Al |
0.00021
0.00093
0.00011
0.000025
0.00040
0.0016
0.00013
0.00061
0.00012
0.00016
0.00074
0.00044
0.0010
0.000235
0.0015
0.00035
0.00022
0.0012
0.0014
0.0038
0.00046
0.0015
0.00081
0.0013
0.000062
0.00042
0.0039
0.0003
0.001
0.009
0.37
0.049 |
|
Int.
Channel |
= Interference
= The Affected
Channel |
| * Bi line available
through modified Se
channel. | |
This table is organized in the following manner:
The
first two columns list two elements. The first element is the channel that
is affected by the second element (the affecting element). The
inter-element Correction Factor (IEC) for the affected
element is shown in the third column.
Table 7
Precision and Accuracy
|
| |
Element |
|
|
|
|
|
Ag |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
2.21 to
10.1
+0.036
0.066
±16.9% |
|
Be |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
0.5 to
2.0
-0.011
0.015
±4.0% |
|
Cd |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
24.0 to
100.
+0.054
0.026
±10.5% |
|
Cu |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
24.0 to
100.
+0.017
0.039
±9.5% |
|
Pb |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
12.0 to
48.0
+0.050
0.058
±16.7% |
|
Sb |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
120. to
480.
+0.008
0.007
±2.3% |
|
Sn |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
480. to
1,920.
+0.033
0.015
±6.3% |
|
Zn |
Range taken
(µg)
Bias
CV1 (Pooled)
Analytical Error
(Total) |
=
=
=
= |
600. to
4,800.
+0.055
0.013
±8.0% |
|
| Note: Analytical errors (AE)
of < ±25% are acceptable for all elements validated except
lead. [Lead is required to have < ± 20%, see 29 CFR
1910.1025(d)(9) for further
information] |
Table 8
Detection Limits, BEC, Ranges, and
Short-Term Precision
|
| ELEMENT |
WAVELENGTH |
*QUAL |
----*RANGE---- |
|
|
(nm) |
*BEC |
DL |
LOWER |
UPPER |
CV |
|
Ag
Bi
Be
Cd
Cu
Pb
Sb
Sn
Zn |
328.068
196.006
313.042
226.502
324.754
220.353
217.581
189.980
213.856 |
0.4857
90.
0.0177
0.1645
0.4982
1.8816
1.5882
0.6104
0.1897 |
0.0109
1.0
0.0010
0.0131
0.0142
0.0470
0.0388
0.0139
0.0241 |
0.0545
5.0
0.0050
0.0655
0.071
0.235
0.194
0.0695
0.1205 |
500
1,000
20
250
250
1,000
1,000
900
500 |
0.0035
0.03
0.0045
0.0031
0.0019
0.0066
0.0092
0.0015
0.0028 |
|
Results were obtained from
determinations performed on 21-Jan-92
| * |
= |
BEC, DL, and RANGE values
reported as µg/mL |
| DL |
= |
Qualitative Detection Limit (SD =
2) |
| BEC |
= |
Background Equivalent
Concentration |
| CV |
= |
Short-Term Precision (based on
three exposures) |
| LOWER |
= |
Quantitative Detection Limit (5 ×
DL) |
| UPPER |
= |
Upper Working Range |
| Integ. time |
= |
5
s | |
Table 9
Upper Range and Saturation
Concentrations
|
|
|
|
-----Range----- |
Element
Name |
Wavelength
(nm) |
Order |
Upper
> |
Saturation
< |
|
Ag*
Al
As
Be*
Bi
Ca
Cd*
Co
Cr
Cu*
Fe1
Fe2
Mg
Mn
Ni
Pb*
Sb*
Se
Si
Sn*
V
Zn* |
328.068
308.215
189.042
313.042
196.006
393.366
226.502
228.616
267.720
324.754
259.940
271.440
279.080
257.610
231.604
220.353
217.581
196.090
288.158
189.980
310.230
213.856 |
2
2
3
2
3
2
3
2
3
2
3
3
2
3
3
3
3
3
2
3
2
3 |
500
5,000
700
20
10,000
15
250
300
300
250
200
400
3,000
60
600
1,000
1,000
1,000
1,000
900
1,000
500 |
|
1,000
10,000
50
10,000
500
500
500
500
5,000
100
1,000
3,000
3,000
1,000
1,000
1,000 |
|
|
Range is listed as
µg/mL
* Validated elements |
Table 10
Quality Control Samples
|
|
N |
MEAN |
STD DEV |
CV |
AE
(±%) |
µg
TAKEN
---(range)--- |
|
QC Set 1
|
|
Ag
Be
Cd
Cu
Pb
Sb
Sn
Zn |
3
3
3
3
3
3
3
3 |
0.930
0.962
0.935
1.089
0.939
0.898
0.917
0.937 |
0.023
0.015
0.021
0.027
0.024
0.047
0.023
0.011 |
0.025
0.015
0.023
0.025
0.026
0.052
0.025
0.012 |
11.9
6.9
11.1
14.0
11.2
20.6
13.3
8.7 |
|
2.41
0.201
5.02
25.2
20.1
126.
504.
315. |
3.66
0.305
7.62
47.4
30.5
237.0
948.
592. |
|
QC Set 2
|
|
Ag
Be
Cd
Cu
Pb
Sb
Sn
Zn |
3
3
3
3
3
3
3
3 |
0.998
1.091
0.998
0.993
1.023
0.933
0.964
0.976 |
0.013
0.141
0.010
0.011
0.021
0.023
0.012
0.007 |
0.013
0.130
0.010
0.011
0.020
0.024
0.013
0.007 |
2.9
35.0
2.3
3.0
6.4
11.5
6.1
3.9 |
|
2.77
0.228
5.77
33.
23.1
165.
660.
412.5 |
3.46
0.267
7.20
51.
28.8
258.
1032.
645. |
|
QC Set 3
|
|
Ag
Be
Cd
Cu
Pb
Sb
Sn
Zn |
3
3
3
3
3
3
3
3 |
0.927
0.934
0.898
1.139
0.930
0.901
0.894
0.912 |
0.005
0.039
0.043
0.029
0.035
0.025
0.023
0.029 |
0.006
0.041
0.048
0.026
0.038
0.027
0.025
0.032 |
8.5
14.9
19.8
19.0
14.6
15.3
15.7
15.2 |
|
2.60
0.217
5.42
34.8
21.7
174.
696.
435. |
3.43
0.286
7.15
54.0
28.6
270.
1080.
675. |
|
| AE = Analytical
Error |
SPECTRAL LINE
INTERFERENCES
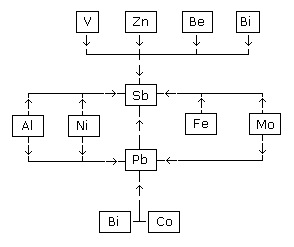
Figure 1:
Interactions of Pb and Sb |
SPECTRAL LINE INTERFERENCES
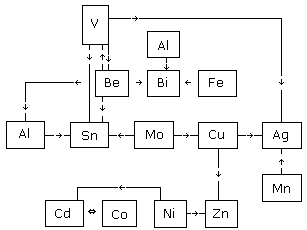
Figure 2:
Interactions of Ag, Be, Cd, Cu, Sn, Bi and
Zn |
i.e. as shown from Figure 2, Be has an interference from V; Be
interferes with V, Sn, Bi and Al.
|
|

