|
| Method no.: |
ID-125G |
| |
|
| Control no.: |
T-ID125G-FV-03-0209-M |
| |
|
| Matrix: |
Air, Wipe, or
Bulk |
| |
|
| OSHA Permissible
Exposure Limits: |
Permissible Exposure
Limits (PELs) are listed in Table
1 for elements commonly found in industrial environments.
This method has the capability of sampling and analyzing more
than these elements, the number being limited by instrumental
capability, as well as digestion solubility and
stability. |
| |
|
| Collection
Procedure: |
A calibrated personal
sampling pump is used to draw a known volume of air through a
mixed-cellulose ester membrane filter contained
in a styrene cassette. |
| |
|
| Minimum Recommended
Air Volumes: |
Time Weighted Average
Samples - 480 L
Short-Term Exposure Limit Samples - 30
L*
Ceiling Samples - 30 L |
| |
|
| Recommended Sampling
Rate: |
2 L/min |
| |
|
| Analytical
Procedure: |
Filters are digested
with nitric acid, sulfuric acid and hydrogen peroxide.
Dissolution of the elements is facilitated by addition of
hydrochloric acid. Analysis is performed using Inductively
Coupled Argon Plasma-Atomic Emission Spectroscopy
(ICAP-AES). |
| |
|
| Detection Limits: |
See Table
2 |
| |
|
| Validation Level: |
See Table
3 |
| |
|
| Precision and
Accuracy: |
See Table
3 |
| |
|
| Method
Classification: |
Validated analytical
method |
| |
|
| Chemist: |
Jerry Septon, Ray
Abel, Michael Simmons |
| |
|
| Date (Revised): |
November, 1988
(September, 2002) |
| |
|
| * Take 60-L samples when evaluating STEL
exposures to beryllium. |
| |
|
Commercial manufacturers and
products mentioned in this method are for descriptive use only
and do not constitute endorsements by USDOL-OSHA. Similar
products from other sources can be substituted.
|
| |
|
Division of Physical Measurements and Inorganic
Analyses
OSHA Technical Center
Sandy City,
Utah
|
1. Introduction
1.1 Scope
1.1.1 This method describes the collection and
subsequent analysis of airborne metal and metalloid
particulate by Inductively Coupled Argon Plasma-Atomic
Emission Spectroscopy (ICAP-AES).
1.1.2 This method provides rapid simultaneous
analysis and data reduction for a wide range of elements,
eliminating the necessity of separate analyses by
conventional atomic absorption techniques.
1.1.3
This method was validated for 13 elements (Be, Cd, Co, Cr,
Cu, Fe, Mn, Mo, Ni, Pb, Sb, V, and Zn). Other
elements can be added to or subtracted from the method.
The capability for expanding the analysis to other
elements is mainly dependent on laboratory instrumentation
and element solubility and stability in the acid matrix
used for digestion. 1.2 History
1.2.1.Previous to the introduction of
ICAP-AES, samples containing metallic particulates were
digested in a variety of ways and analyzed by Atomic
Absorption Spectroscopy (AAS) at the OSHA Analytical
Laboratory.
1.2.2 A first generation plasma source
and spectrometer (Jarrell-Ash Model 975 Atomcomp) was then
used by the OSHA Analytical Laboratory. The analytical
procedure for this instrument is described in OSHA Method
No. ID-125 (8.1).
1.2.3 Procurement of new inductively coupled
plasma (ICP) instruments, computers, and software allowed
samples to be determined using later technology. This
technology includes more sophisticated computer systems
for data reduction and instrument control.
1.2.4
When this method was originally written, three different
ICP instruments at the OSHA Salt Lake Technical Center
(OSHA-SLTC) are used to apply this method:
Jobin-Yvon (JY) Model 32 (Instruments SA,
Edison, NJ)
Jarrell-Ash Model 975 Atomcomp* (Thermo
Jarrell-Ash Corp., Franklin, MA)
Applied Research
Lab. (ARL) Model 3560 (ARL, Sunland, CA)
These instruments are further referred to as
ICP1, ICP2, or ICP3, respectively.
The Jarrell-Ash
system was upgraded with a new computer, generator, and
software in 1989.
This method is applicable to any
simultaneous spectrometer. This method was validated using
ICP1 and the data is presented in a backup report (8.2).
An additional evaluation was performed using ICP3 (8.3).
2. Detection Limits and Working Ranges (8.2)
2.1 OSHA Permissible Exposure Limits (PELs) (8.4)
for the elements screened and validated are listed in Table
1. Detection limits and working ranges are in Table
2. All reported detection limits were calculated for
50-mL solution volumes.
2.2 The optimum working
range for each element listed in Table
2 extends several orders of magnitude above each
detection limit. 3. Method Performance (8.2)
3.1 The precision and accuracy data for the 13
validated elements using ICP1 are listed in Table
3. These values are based on six samples at each
concentration level tested. Solutions of the 13 elements
were spiked on mixed-cellulose ester filters. These samples
were then digested and analyzed using procedures mentioned
in this method and in reference 8.2.
3.2 Nine of the thirteen elements reported in Table
3 were spiked at 0.5, 1, and 2 times the PEL, assuming a
120-L air volume. Spikes for manganese were calculated
assuming a 30-L air volume. Approximately 200-L air volumes
were assumed for Pb, Ni, and Sb.
3.3 The analytical
error (AE) at 95% confidence for each element listed in Table
3 was calculated as:
±AE% = 100 × [|Mean Bias| +
2(CV)]
Analytical errors for all elements tested
were within ±25%; the greatest value was ±18.1% for V. This
element was validated near it's detection limit.
4.
Interferences (8.6)
High temperatures present in the plasma (5,000
to 8,000°C) minimize most chemical and matrix interferences.
Interferences do exist, however, and can be categorized as
follows:
4.1 Physical interferences such as nebulization
and transport effects are influences that determine the rate
and particle size in which analytes are delivered to the
plasma. These effects are minimized by matching the acid
concentrations of samples and standards.
4.2
Chemical interferences are characterized by molecular
compound formation, ionization effects, and solute
volatilization effects. These effects are not severe in ICP
analysis and are minimized by matrix matching and careful
selection of operating conditions such as: incident plasma
source power, sample uptake rate and plasma observation
height.
4.3 Spectral interferences include:
- Unresolved overlap of molecular band spectra.
- Overlap of a spectral line from another element.
- Background from continuous or recombination phenomena.
- Background from stray light.
4.4 The first effect (a) can be minimized by a
careful selection of wavelengths for the reported elements.
The other types of spectral interferences (spectral overlap
and elevated background) are minimized by software which
performs interelement corrections. This software assumes a
linear relationship between the analyte and interference
within the working range limits. A spectral interference
correction equation typically used by ICP manufacturers is:
Corrected Concn = Calculated Concn - Ai ×
CPi
where:
| Ai |
is |
Correction factor |
| CPi |
is |
Concentration of the
interfering element |
Samples having analyte concentrations above
the working range limits should be diluted into range;
interelement corrections may not be accurate above the
working range. Experimentally determined interelement
corrections for the validated elements are listed in
reference 8.2.
4.5. If necessary, supplemental background
correction can be performed with additional software
supplied by the instrument manufacturer.
5.
Sampling
5.1 Equipment
5.1.1 Mixed cellulose ester (MCE) filters
(0.8-µm pore size), cellulose backup pads, and cassettes,
37-mm diameter, part no. MAWP 037 AO (Millipore Corp.,
Bedford, MA). Cassettes, filters (MCE) and backup pads of
25-mm diameter can also be used.
5.1.2 Gel bands
(Omega Specialty Instrument Co., Chelmsford, MA) for
sealing cassettes.
5.1.3 Sampling pumps capable of
sampling at 2 L/min.
5.1.4 Assorted flexible
tubing.
5.1.5 Stopwatch and bubble tube or meter
for pump calibration.
5.1.6 Scintillation vials,
20-mL, part no. 74515 or 58515, (Kimble, Div. of
Owens-Illinois Inc., Toledo, OH) with polypropylene or
Teflon cap liners. If possible, submit bulk or wipe
samples in these vials for ICP analysis.
5.1.7
Smear tabs, part no. 225-24 (SKC Inc., Eighty Four, PA),
or Whatman no. 41 or no. 42 filters (Whatman LabSales Inc.
, Hillsboro, OR) for wipe sampling.
5.1.8 Gloves,
disposable (for wipe sampling).
5.1.9 Ghost Wipes,
4" × 4" 1000/cs Wet with DI Water, part no. SC4250
(Environmental Express, Mt. Pleasant, SC) for wipe
sampling. 5.2 Sampling Procedure - Air
Samples
Welding fumes and samples requiring sample weights can be
characterized using this method. Collect samples on
pre-weighed 37-mm polyvinyl chloride (PVC) filters at 2
L/min flow rate. Conduct the welding fume sampling with the
filter cassette located inside the welding helmet (8.6).
If the free-space inside the hood precludes the use of 37-mm
diameter cassettes and filters, 25-mm sampling assemblies
with pre-weighed PVC filters can be used.
Desiccate
and post-weigh each sample and then calculate total welding
fume exposure:
net
weight (µg) - net weight of blank (µg)
air volume (liters) |
=
mg/m3 |
and determine compliance with the 5
mg/m3 TLV for welding fumes. Submit the samples
to the laboratory for welding fume/ICP analysis to further
characterize the samples.
5.2.1 Place a MCE filter and a cellulose
backup pad in each two- or three-piece cassette. Seal each
cassette with a gel band.
5.2.2 Calibrate each
personal sampling pump with a prepared cassette in-line to
approximately 2 L/min flow rate.
5.2.3 Attach
prepared cassettes to calibrated sampling pumps (the
backup pad should face the pump) and place in appropriate
positions on the employee or workplace area. Collect the
samples at about 2 L/min flow rates. Minimum sampling
times recommended are:
Recommended Sampling Times
|
| Time
Weighted Average Samples |
240 min |
| Short-Term Exposure
Limit Samples |
15
min* |
| Ceiling Samples |
15
min |
The analytical sensitivity of a specific
analyte may dictate using a larger sampling sampling time.
| * |
When determining
compliance with the STEL for beryllium, take 30-min
samples. |
| Note: |
If soluble compounds
(i.e. Cr2+, Cr3+, soluble
salts of Al, Fe, Mo, Ni, ZnCl2, etc.) are
suspected to be present in the sampled air, take
separate samples. Request analysis for the specific
compound(s). These samples are analyzed using OSHA
Method No. ID-121
and not by this method. |
5.2.4 If the filter becomes
overloaded while sampling, another filter cassette should
be prepared. Consecutive samples using shorter sampling
periods should be taken if overloading occurs.
5.2.5 Place plastic end caps on each cassette
after sampling.
5.2.6 Attach an OSHA-21 seal
around each cassette in such a way as to secure the end
caps. 5.3 Sampling
Procedure - Wipe Samples
5.3.1 Wear clean, impervious, disposable
gloves when taking wipe samples to prevent sample
contamination. Change gloves between samples to reduce the
possibility of cross contamination.
5.3.2 Moisten
Smear Tabs and Whatman filters with deionized water prior
to use.
5.3.3 If using a Ghost Wipe remove it from
its package and unfold it. Next fold the Ghost Wipe in
half and wipe a 10-cm × 10-cm area by starting at the
outside edge of the surface, applying firm pressure, wipe
the surface and progress towards the center by making
concentric squares of decreasing size. Fold wipe in half,
with contaminant side in, and wipe the surface again by
making concentric squares of decreasing size. Fold the
wipe in half, contaminant side in, and wipe surface a
third time. If using a Smear Tab or Whatman filter, wipe a
10-cm × 10-cm area by starting at the outside edge of the
surface, applying firm pressure, wipe the surface and
progress towards the center by making concentric squares
of decreasing size. If possible wipe the area at least 3
times.
5.3.4 Fold the wipe sample with exposed side
in. 5.3.5 Transfer the wipe sample into a 20-mL
scintillation vial and seal with vinyl or electrical tape.
Securely wrap an OSHA-21 seal length-wise from vial top to
bottom. 5.4 Sampling Procedure - Bulk Samples
In order of laboratory preference, bulk samples may
be one of the following:
- a high-volume filter sample,
- a representative settled dust (i.e. rafter) sample,
- a sample of the bulk material in the workplace.
Transfer the bulk material
into a 20-mL scintillation vial and seal with vinyl or
electrical tape. Securely wrap an OSHA-21 seal length-wise
from vial top to bottom.
5.5.Shipment
When
other compounds or elements are known or suspected to be
present in the sampled air, such information should be
transmitted with the sample(s) to the laboratory.
5.5.1 Submit at least one blank sample with
each set of air or wipe samples. Blank filter samples
should be handled in the same manner as other samples,
except no air is drawn through the blank.
5.5.2
Send the samples to the laboratory with the OSHA 91A
paperwork requesting ICP analysis.
5.5.3 Bulk
samples should be shipped separately from air samples.
They should be accompanied by Material Safety Data Sheets
if available. Check current shipping restrictions and ship
to the laboratory by the appropriate method.
6.
Analysis
6.1 Safety Precautions
6.1.1
Prepare 1:1 H2SO4 in DI
H2O cautiously.
- Use a 1- or 2-L thick-walled, break- and
heat-resistant bottle.
- Wear thick rubber gloves, plastic apron, labcoat,
and face shield.
- Add 500 mL DI H2O to the bottle.
- Place the bottle over the drain in a sink which has
a slot vent to provide ventilation. Begin running cold
tap water over the side of the bottle, being careful not
to get any tap water in the bottle. Let the level of
water rise in the sink to provide cooling of the bottle.
- Carefully and slowly begin adding 500 mL
concentrated H2SO4 to the DI
H2O. Add a small quantity, swirl to mix, and
allow contents to cool. Do NOT
allow boiling of solution within the container.
- After the acid has been added, loosely cap the
bottle and allow it to remain in the sink with the water
running for at least 15 min. Allow the solution to cool
to room temperature.
- A thick-walled beaker, Teflon-coated stirring bar,
electronic stirrer and a ventilation hood can also be
used to prepare 1:1 H2SO4 if
precautions are taken to prevent solution overheating
and splattering.
6.1.2 Digest all samples
within a suitable exhaust hood.
6.1.3 To prevent
splattering, add H2O2 (30%) to
beakers in 2- to 3-drop groups.
6.1.4 Perchloric acid added to organic
substances can produce fires and/or explosions. If
HClO4 solutions darken in color while heating,
immediately remove beakers from the hotplate and carefully
add a small amount of HNO3. Only use
HClO4 in exhaust hoods designed and reserved
for HClO4 use.
6.1.5 Do not directly
view the plasma.
6.1.6 Do not override the rf
generator or torch box safety interlocks.
6.2 Equipment
6.2.1 Inductively coupled argon plasma/atomic
emission direct-reading spectrometer, cooling unit for
torch assembly, computer, and radio-frequency (rf)
generator.
6.2.2 Nebulizer.
6.2.3
Automatic sampler.
6.2.4 Peristaltic pumps
(optional). Use one pump for automatic sampler rinse. Use
the other pump for sample introduction into the nebulizer.
6.2.5 Mass Flow Controller (optional). Use the
controller to regulate nebulizer argon flow and sample
uptake rate.
6.2.6 Borosilicate glass conical
beakers, 125- and 250-mL.
6.2.7 Borosilicate glass
volumetric flasks, 25-, 50-, 100-, 250-mL, and 1- or 2-L.
Use the larger flasks for standard preparation.
6.2.8 Thick walled, 1- or 2-L heat- and
break-resistant bottle.
6.2.9 Mixed cellulose
ester filters (0.45-µm pore size) and a filtering
apparatus. Use this system to remove any insoluble
particulates from sample solutions.
6.2.10 Hot
plate capable of reaching 300°C.
6.2.11 Volumetric
pipets, glass of various sizes.
6.2.12 Analytical
balance (0.01 mg). 6.3 Reagents (reagent grade or better)
6.3.1 Deionized water (DI
H2O).
6.3.2 Concentrated sulfuric
(H2SO4), hydrochloric (HCl), nitric
(HNO3), and perchloric (HClO4)
acids.
6.3.3 Prepare 1:1
H2SO4 (V/V) solutions as described
in Section 6.1.1
6.3.4 Sample dilution solution or reagent blank
(8% HCl/4% H2SO4):
In an
exhaust hood, slowly and carefully add 40 mL concentrated
H2SO4 to approximately 500 mL of DI
H2O contained in a thick-walled, heat- and
break-resistant bottle. Gently stir and allow the solution
to cool to room temperature. Slowly and carefully add 80
mL concentrated HCl, allow to cool, and dilute to 1 L with
DI H2O.
6.3.5 Stock solutions of 1,000
µg/mL for standard preparation of the various elements.
6.3.6 Hydrogen peroxide,
(H2O2), 30%.
| Note: |
Some manufacturers use
organotin compounds to stabilize
H2O2. Since Sn is one of the
elements screened, use H2O2
that does not contain this type of
stabilizer. |
6.3.7 Argon - quality as specified
by the ICP manufacturer. 6.4 Standard Preparation
Prepare
multielement calibration standards (STD) and continuing
calibration verification (CCV) solutions (see Addendum A for
examples of combinations) using 1,000 or 10,000 Fg/mL stock
solutions. An independent calibration verification (ICV)
standard is a mixture of elements whose concentrations are
within their respective analytical linear ranges and is used
to verify the calibration standards. Whenever possible,
prepare the ICV standard from different stock solutions than
those used for calibration standards. The ICV standard
should contain elements and concentrations reflecting what
is expected in the majority of the samples, or problem
elements. The final acid concentration of the STD, CCV and
ICV standards is 8% HCl/4% H2SO4.
These standards should be stable for at least 6 months.
6.5 Sample Preparation
The final acid
concentration for the different sample matrices should be 8%
HCl/4% H2SO4. All of the elements
validated are soluble when using the following acid
digestion procedures. Other elements not included in the
validated element list (Table
3) should be evaluated for solubility and stability
before using these procedures.
| Note: |
Requests for analysis of
compounds which have a PEL that specifically addresses
the soluble fraction (i.e., Fe, Ni, Mo, etc.) are
analyzed using OSHA Method No. ID-121
and not by this method. |
Filters, backup pads, wipes, and
bulks are prepared by the following procedures:
6.5.1
Mixed-cellulose ester (MCE) membrane filters
- If the beakers used for the digestion have not been
cleaned using a appropriate automated system, clean the
insides of the 125-mL conical beakers by refluxing 1:1
HNO3 using a hot plate in a ventilated hood.
Carefully pour the used 1:1 HNO3 into an
appropriate labeled container. Allow beakers to cool,
then rinse several times with DI H2O and
allow to dry. Using forceps, place sample filters in
separate labeled and washed beakers (If the backup pad
appears contaminated, see Section 6.5.2
below). If the cassette or vial contains loose dust,
carefully pour the dust into the beaker. Always rinse
the cassette with DI H2O and pour the water
into the beaker and wipe out the cassette with a
moistened, clean filter and place this filter in the
sample beaker.
- For samples with air volumes >200 L: Add 4 mL of
1:1 H2SO4, followed by 2 mL of
concentrated HNO3 to each beaker containing
the filter sample. For samples with air volumes < or
= 200 L: Add 2 mL of 1:1 H2SO4,
followed by 2 mL of concentrated HNO3.
- To facilitate the digestion, allow the filters to
sit at least an hour in the 1:1
H2SO4. Add several drops of
H2O2 (30%) to each beaker before
placing it on the hotplate.
- Heat the beakers on a hot plate for approximately 10
min. The solutions should turn brown.
- Cautiously add H2O2 in 2- to
3-drop groups until each solution becomes clear,
colorless, or slightly yellow (the color is dependent on
the concentration and type of analyte present).
- Heat several more minutes until dense, white fumes
of SO3 just become evident. Remove the
beakers from the hotplate and allow to cool.
- Slowly and carefully add the following amount of
concentrated HCl
(CAUTION: SPLATTERING MAY OCCUR IF
THE HCl IS ADDED TOO RAPIDLY OR THE
H2SO4 SOLUTION IS STILL
HOT):
| Acid Addition Data |
Acid Volume
|
|
Air Volume
|
| 4 mL |
|
> 200 L |
| 2 mL |
|
< or = 200 L |
|
- Rinse the sides of the beakers with DI
H2O and return the beakers to the hotplate.
Heat the beakers until near boiling to promote
solubilization of all elements present. Remove the
beakers from the hotplate and allow to cool.
- Quantitatively transfer the solutions into
volumetric flasks using DI H2O. For samples
having air volumes > 200 L, dilute to 50 mL; volumes
< or = 200 L, dilute to 25
mL, if the filter contains a lot of material, dilute to
50 mL.
6.5.2 Backup Pads
- If the backup pad has been contaminated during
collection, digest the pad along with the filter. Also,
separately digest and analyze the blank filter with a
clean backup pad.
- Place each contaminated backup pad and corresponding
filter into individual beakers. Allow to sit at least an
hour in the appropriate amount of 1:1
H2SO4 (Section 6.5.1,
Step 2). Add 10 mL of concentrated HNO3 and
proceed as in Section 6.5.1,
Step 3 above.
6.5.3 Wipe or Polyvinyl Chloride (PVC)
Filter Samples
Polyvinyl chloride filters can be used
for sample collection and analysis by ICP. The industrial
hygienist may sample for gravimetric determinations of
total dust or total welding fumes using PVC filters and
also submit these samples for ICP analysis if a sample
weight is required.
- If the beakers used for the digestion have not been
cleaned using a appropriate automated system, reflux 1:1
HNO3 in 250-mL (for wipes) or 125-mL (for PVC
filters) conical beakers, empty and allow to cool. Rinse
the beakers several times with DI H2O and
allow to dry.
- Place each filter or wipe in a separate washed
beaker.
- Add the appropriate amount of 1:1
H2SO4 as listed below:
Acid Addition Data
|
Acid
Volume*
|
|
Sample
Type
|
| 8 mL |
|
Wipe |
| 4 mL |
|
PVC filter
(> 200 L Air Volume) |
| 2 mL |
|
PVC filter
(>< or = 200 L Air Volume) |
| * Concentrated HCl or 1:1
H2SO4
|
- Add 10 mL concentrated HNO3 to each
beaker. Place the beakers on the hotplate.
- Add H2O2 in 2- to 3-drop
groups. PVC filters and wipes require more
H2O2 for digestion than MCE
filters.
- If HClO4 digestion precautions are
followed (Section 6.1.4),
1 to 2 mL of HClO4 can also be used to
complete digestion. The PVC filter will not
completely digest if HClO4 is not added;
therefore, thoroughly rinse the filter residue with DI
H2O during quantitative transfer of the
sample solution.
- Allow digested samples to cool and carefully add the
same volume of concentrated HCl as the 1:1
H2SO4 used in Section 6.5.3,
Step 3. above.
- Rinse the sides of the beaker with DI H2O
and then heat to near boiling.
- After beakers have cooled to room temperature,
dilute digested wipe solutions to 100 mL. Dilute PVC
filter solutions to final volumes as stated in Section
6.5.1,
Step 9.
6.5.4 Ghost Wipes
- If the beakers used for the digestion have not been
cleaned using a appropriate automated system, reflux 1:1
HNO3 in 125-mL conical beakers, empty and
allow to cool. Rinse the beakers several times with
deionized water and allow them to dry.
- Place each Ghost Wipe in a separate washed beaker.
- Add 4 mL of 1:1 H2SO4 to each
beaker. Let sample sit for 5 minutes.
- Add 2 mL of deionized water followed by 2 mL
concentrated HNO3 to each beaker. Let samples
sit for 15 minutes. After 15 minutes, up to 8 mL more of
concentrated HNO3 can be added to facilitate
digestion, especially if samples are heavily loaded.
Place the beakers on the hotplate.
- When solutions turns brown or black cautiously add
H2O2 in 2- to 3-drop groups until
each solution becomes clear, colorless, or slightly
yellow (the color is dependent on the concentration and
type of analyte present). Ghost Wipes may require up to
10 mL H2O2 before solution clears.
- Heat solutions several more minutes until dense,
white fumes of SO3 just become evident.
Remove beakers from hotplate and allow them to cool.
- After samples have cooled carefully add 4 mL of
concentrated HCl to each beaker.
- Rinse the sides of the beaker with deionized water
and then heat again on hot plate until samples are near
boiling.
- After beakers have cooled to room temperature,
dilute digested Ghost Wipes solutions to 50 mL.
6.5.5 Bulks
- Review any available material safety data sheets to
determine safe bulk handling. The safety data may also
offer a clue as to the aliquot amount needed for
adequate detection of the element(s) of interest.
- Measure by volume or weight an appropriate aliquot
of any liquid bulk sample. Weigh the appropriate amount
of any solid bulk sample. Weigh an aliquot of any paint
bulk by placing a small amount on a MCE filter, allow to
air dry then take the dry weight.
| Note: |
Aliquot amounts of
bulks are dependent on the analytical sensitivity,
detection limit, and solubility of the material
used. If uncertain, a 20- to 50-mg aliquot of a
solid material can be taken as a starting point.
Make sure the aliquot taken is representative of the
entire bulk sample. If needed, use a mortar and
pestle to grind any nonhomogeneous particulate bulk
samples in an exhaust hood. |
After measuring,
transfer the aliquot to a previously cleaned or
acid-washed 250-mL conical beaker.
- Add 20 mL of 1:1 H2SO4 and
digest on a hotplate. Hydrogen peroxide (dropwise) and a
few mLs of HNO3 can be carefully added to
break up the matrix.
| Caution: |
Do not add the
HNO3 to wet bulk materials containing
organic solvents. Significant reactions could
occur. |
- Remove the beaker from the hotplate and allow to
cool. Carefully add 20 mL of concentrated HCl and then
heat the solution to near boiling.
- Allow to cool and quantitatively transfer to a
250-mL volumetric flask. Dilute to volume with DI
H2O.
Air, wipe, and bulk samples: If
particulates are present in any of the sample solutions,
filter this solution through a MCE filter (0.45-µm pore
size) and then re-digest the particulate and filter. Save
the filtrates for analysis. 6.6 Instrument Startup and Calibration
Follow the manufacturer's instructions for
instrument start-up and calibration. An example of ICP
operating parameters is shown below. These settings will
vary from instrument to instrument:
| Gas Used |
Argon |
|
Gas
Flow
(Rotameter settings) |
Plasma
Nebulizer
Auxiliary Plasma |
12 - 16
L/min
*
0.14 - 0.18 L/min |
| RF Power |
Incident
Reflected |
1.1 kW
< 5 W |
| Observation
Height |
Plasma |
15 mm above work
coil |
| Integration
Time |
Peak Signal |
3 to 10 s |
| Wash Time |
Automatic
Sampler
Without Automatic Sampler |
60 s
10 s |
| Number Of
Exposures |
Standards &
Samples |
2 to 10 |
| Nebulizer* |
Solution Uptake
Rate
Pressure |
0.8 - 1.6 mL/min
~30
psig |
| Mass Flow
Controller |
Flow Rate Range |
varies* |
| * This flow
will vary depending on the type of nebulizer in
use. |
6.6.1 Profile the
instrument before beginning the calibration and analysis.
Follow the Standard Operating Procedure (SOP) (8.7)
or manufacturer's instructions for computer initialization
and profiling.
6.6.2 Obtain a two-point
calibration curve by nebulizing the working standards into
the plasma and measuring atomic emission intensities. For
most instruments, a first-order linear fit of the data is
computer calculated and slope and intercept coefficients
are obtained. Perform calibrations by following the
instrument manufacturer's guidelines.
6.6.3 See
Addendum A for the proper sequence of standards and
samples during the analysis. 6.7 Analytical Procedure
For more
details regarding analytical procedures, refer to the
instrument manufacturer's software manual(s) or the SOP (8.7).
6.7.1
If necessary, determine detection limits using the
manufacturer's software (if available). These limits
normally do not significantly change during short time
spans. A general rule is to recalculate detection limits
when an integral component (nebulizer, torch, mass flow
controller, etc.) of the ICP has been replaced or
adjusted. A typical calculation of detection limit (DL) is
shown:
| DL =
|
(K ×
SDI × C)
(I - Io) |
×
S |
where:
| S |
is |
Solution volume in
mL |
| K |
is |
Degree of confidence
(sigma value)* |
| SDI |
is |
Standard deviation of
reagent blank intensity (Io.) |
| C |
is |
Concentration of the
calibration standard in µg/mL |
| I |
is |
Total intensity of
standard containing concentration C |
| Io |
is |
Background intensity
(reagent blank) | * In most cases, K=2 or 3 for qualitative
and K=10 for quantitative determinations.
Reporting limits may be equal to or larger than the
calculated detection limits. Reporting limits should be
verified annually for each matrix.
6.7.2 Analysis
using an automatic sampler is described below:
- Fill automatic sample vials to the minimum sample
volume for one analysis and a potential rerun.
- Load the automatic sampler with labeled standard and
sample vials. A multielement working standard should be
analyzed after every 5 to 6 samples. A control standard
should be occasionally analyzed to ensure proper
instrument operation. If an element or elements
contained in the control standard are not within
specification (a general rule is to use a value less
than ±10 to 15% of the known concentration), the analyst
should recalibrate before proceeding with the analysis.
- Aspirate each sample or standard for approximately 1
min prior to initiating the exposure cycle. This ensures
equilibration in the plasma and minimizes carry-over
effects.
- Dilute and reanalyze any samples containing elements
(both screened and validated) exceeding the working
range (Table
2). In particular, notice the reduced upper
limit (8 µg/mL) for lead. Interelement corrections may
not be accurate above the working range. Prepare the
dilutions by pipetting an appropriate aliquot from the
original solution and dilute with 8% HCl/4%
H2SO4.
- Based on the calibration curve initially obtained,
convert the sample intensities to concentrations. Then,
using the air volume, solution volume, dilution factor
and sample weight, calculate the concentration for each
element analyzed as mg/m3 (air samples),
total micrograms (wipes), or percentage of total weight
(bulks) using the equations shown below.
6.8
Calculations
6.8.1 Total amount of
analyte in the sample:
| µg A =
(µg/mL A) × (mL S) × (DF) |
(1) |
where:
| µg A |
is |
Total µg of
analyte in the sample |
| µg/mL A |
is |
Measured
concentration of analyte in sample solution (derived
from calibration curve) |
| mL S |
is |
total volume
of the solution analyzed |
| DF |
is |
amount of
dilution applied to an aliquot of the original
solution (ratio of final volume divided by the
aliquot volume) | 6.8.2 The blank value, if any, is subtracted
from each sample:
where:
| µgc A |
is |
µg of analyte, blank
corrected |
| µgb A |
is |
µg of analyte in
blank |
6.8.3 For air samples, the concentration of
analyte in the sample is expressed in mg analyte per cubic
meter for each element or compound analyzed:
| mg/m3 = |
(µgc A) × (GF)
air volume (L) | |
(3) |
where:
GF is Gravimetric Factor
For those elements having a
PEL listed as an oxide, the gravimetric factors for the
validated elements are:
1.4298 for
Fe2O3
1.2447 for
ZnO 1.7852 for
V2O5
6.8.4 Convert bulk
sample analytes to % composition using:
| %(w/w) = |
(µgc A) (100%)
(sample weight) (1,000
µg/mg) | |
(4) |
where:
| µgc A |
is |
analyte amount
(µg) |
| Sample wt |
is |
aliquot (in mg) of bulk
taken in Section 6.5.4 | 7. Reporting Results
7.1 Air sample results are reported as
mg/m3. Results for analytes having a PEL as an
oxide are reported as mg/m3 of the oxide.
7.2 Wipe sample concentrations are calculated and
reported as total micrograms for each element.
7.3
Bulk sample results are calculated and reported as elemental
percent by weight (or volume if liquid aliquots were used).
Due to differences in sample matrices between bulks and
standards, bulk results are approximate for each element
determined. (Elemental values are to be reported for all
bulk analyses, do not use a gravimetric factor.)
7.4
Determinations of the screened elements or compounds are not
routinely reported. Spectral interference corrections for
these analytes are not included and validations have not
been performed. If a sample has a screened analyte over the
PEL, the analyst should contact her/his supervisor.
Additional sampling, or if possible, additional analysis of
the original sample should be performed to quantitate the
potential overexposure. 8. References
8.1 Occupational Safety and Health Administration
Analytical Laboratory: OSHA
Analytical Methods Manual (USDOL/OSHA-SLCAL Method
No. ID-125). Cincinnati, OH: American Conference of
Governmental Industrial Hygienists (Pub. No. ISBN:
0-936712-66-X), 1985.
8.2 Occupational Safety and Health Administration
Technical Center: ICP Backup Data
Report (ID-125G) by J.C. Septon. Salt Lake City, UT.
Revised 1991.
8.3 Occupational Safety and Health Administration
Technical Center: ICP Backup Data
Report (ARL 3560) by J.C. Septon. Salt Lake City, UT.
In progress.
8.4 "Toxic and
Hazardous Substances," Code of Federal
Regulations Title 29, Pt. 1910.1000, Subpart Z. 1987.
pp 676-682.
8.5 National Institute for Occupational Safety and
Health: NIOSH Manual of Analytical
Methods, 2nd ed., Vol. 7 (DHEW/NIOSH Pub. No.
82-100). Cincinnati, OH, 1981. Method No. 351.
8.6 United States Department
of Labor, OSHA: "Memorandum, Sampling for Welding
Fumes" by Patricia Clark, Director Designate, Directorate of
Compliance Programs. United States Department of Labor,
OSHA, Washington, DC, February 14, 1989. [Memo].
8.7 Occupational Safety and
Health Administration Analytical Laboratory: ICP Standard Operating Procedure by J.C.
Septon. Salt Lake City, UT. 1988 (unpublished).
Table 1
Air
Contaminants - OSHA Permissible Exposure Limits* |
|
| Element |
Substance
Exposed to |
PEL
(mg/m3)
|
|
|
|
TWA |
CEILING |
|
|
|
|
| Ag** |
Metal and
soluble compounds
(as Ag) |
0.01 |
|
|
|
| As** |
Inorganic
compounds (as As) |
See
29 CFR 1910.1018 for applications |
| Be# |
Beryllium and
compounds
(as Be) |
0.002 |
0.005## |
|
|
|
| Ca** |
Calcium
oxide |
5 |
|
|
|
| Cd |
Fume
Dust
(See 29 CFR 1910.1027) |
0.1
0.2 |
0.3
0.6 |
|
|
|
| Co |
Metal dust and
fume (as Co) |
0.1 |
|
|
|
| Cr |
Cr metal (as
Cr) |
1 |
|
|
|
| Cu |
Fumes (as
Cu)
Dusts and mists (as Cu) |
0.1
1 |
|
|
|
| Fe |
Dicyclopentadienyl iron Total dust
Iron
oxide fume (as Fe2O3) |
15
10 |
|
|
|
| Fe,V |
Ferrovanadium
dust |
1 |
|
|
|
|
| Mg** |
Magnesium oxide
fume Total particulate |
15 |
|
|
|
| Mn |
Mn compounds
(as Mn)
Mn fume (as Mn) |
|
5
5 |
|
|
|
| Mo |
Insoluble
compounds (as Mo) Total dust |
15 |
|
|
|
| Ni |
Metal and
insoluble
compounds (as Ni) |
1
1 |
|
|
|
| Pb |
Inorganic (see 29 CFR 1910.1025) |
| Sb |
Sb and
compounds (as Sb) |
0.5 |
|
|
|
| Se** |
Se and
compounds (as Se) |
0.2 |
|
|
|
| Sn** |
Inorganic
compounds except oxides (as Sn) |
2 |
|
|
| Te** |
Te and
compounds (as Te) |
0.1 |
|
|
|
| Zn |
Zinc oxide
fume
Zinc oxide Total dust
Zinc stearate Total
dust |
5
15
15 |
|
|
|
|
|
| * |
From reference
8.4 |
| ** |
Elements screened -
PELs are listed for information only, because
the screened elements are not digested or
analyzed using optimum conditions. |
| # |
Beryllium also has
a Peak PEL of 0.025 µg/m³. |
| ## |
Both the Ceiling
Limit and the STEL for beryllium are for a
maximum 30-min
duration. | |
|
| Note: |
Compounds having
total and respirable dust PELs of 15 and 5 mg/m³
respectively, are normally analyzed
gravimetrically. Elements contained in these
dust samples can be identified by this or other
methods, if
necessary. | |
Table 2
Detection Limits* and
Upper Limits |
Analyte
|
Qual. Det.
Limit
(µg)
|
Quan. Det.
Limit
(µg)
|
Upper
Limit
(µg/mL)
|
| Ag** |
1.1 |
3.8 |
** |
| Al** |
6.8 |
23. |
200 |
| As** |
1.7 |
5.7 |
20 |
| Be |
0.013 |
0.043 |
5 |
| Ca** |
0.79 |
2.6 |
20 |
| Cd |
0.14 |
0.47 |
50 |
| Co |
1.2 |
4.0 |
100 |
| Cr |
0.40 |
1.3 |
50 |
| Cu |
0.64 |
2.1 |
50 |
| Fe |
8.9 |
30. |
200 |
| Mg** |
2.1 |
7.1 |
20 |
| Mn |
0.061 |
0.20 |
15 |
| Mo |
0.52 |
1.7 |
100 |
| Pb |
2.1 |
7.0 |
8 |
| Ni |
0.59 |
2.0 |
100 |
| Sb |
4.2 |
14. |
100 |
| Se** |
3.2 |
11. |
20 |
| Si** |
2.4 |
8.1 |
20 |
| Sn** |
3.5 |
12. |
** |
| V |
0.57 |
1.9 |
100 |
| Zn |
0.14 |
0.47 |
50 |
| * |
Calculations are based on
a 50-mL solution volume and equations listed in
Section 6.7.1
Each detection limit is dependent on the spectral
wavelength and order used for analysis. |
| ** |
Screened elements -
Limits are approximate - the digestion and analysis
are not optimized for these
elements. |
Both the detection limits and upper limits
were determined using ICP1 (JY-32 ICP). Performance may vary
from instrument to instrument. Upper limits are the upper
linear range for each element. These were determined using a
linear model (8.2).
Table 3
Precision and Accuracy
Data* for Spiked Samples |
|
Element
|
CV
|
Bias
|
Range
(µg)
|
Analytical
Error (± %)
|
| Be |
0.010 |
0.010 |
0.12-0.48 |
2.9 |
| Cd1 |
0.014 |
-0.005 |
6-24 |
3.2 |
| Co |
0.020 |
-0.040 |
6-24 |
8.1 |
| Cr2 |
0.015 |
-0.074 |
60-240 |
10.3 |
| Cu3 |
0.017 |
0.054 |
60-240 |
8.7 |
| Fe |
0.018 |
-0.012 |
420-1680 |
4.8 |
| Mn |
0.032 |
0.077 |
75-300 |
14.1 |
| Mo4 |
0.047 |
-0.029 |
225-900 |
12.3 |
| Ni |
0.025 |
0.017 |
105-420 |
6.6 |
| Pb |
0.040 |
0.04 |
5-20 |
12.1 |
| Sb |
0.014 |
0.012 |
60-240 |
3.9 |
| V |
0.045 |
0.090 |
3.5-14 |
18.1 |
| Zn |
0.007 |
0.014 |
240-960 |
2.9 |
| * |
Reported values were
obtained from ICP1 (ISA JY-32 ICP). Results may vary
from instrument to
instrument. | The
following exceptions were used when calculating spike
amounts:
| 1 |
PEL for fume (0.1
mg/m3) used |
| 2 |
PEL for insoluble forms
(1 mg/m3) used |
| 3 |
PEL for dust form (1
mg/m3) was used |
| 4 |
5 mg/m3 target
concentration was used. This was performed because a
very large amount of spike would be necessary if the
15 mg/m3 PEL for Mo was used. |
|
| CV |
Coefficient of
Variation |
| Instrument Calibration & Quality Control
Sequence |
QC
Function |
Acronym |
Frequency |
Acceptance
Criteria |
Analytes & Nominal Concentrations
(ppm) |
Initial
Calibration1 |
CB |
At the beginning of each analytical
sequence
and
as
required by failing performance checks |
n/a |
|
| STD-A |
RSD <
2%
(replicate
readings) |
Target analytes reported to
clients:
1 - Be
5 - Cr, Cu, Pb, V
10 -
Cd. Co, Mn, Mo, Ni, Sb, Zn
100 - Fe |
| STD-B |
Anticipated interfering
analytes:
1 - Ag, Pt
10 - Al, As, Ce, Mg,
Nb, Se, Sn, Ti |
Initial
Performance
Checks |
ICV |
Immediately
following the initial
calibration |
90-110%
Recovery |
0.5 - Be
2.5 - Cr,
Cu, Pb, V
5 - Cd, Co, Mn, Mo, Ni, Sb, Zn
50 -
Fe |
| CCV |
Immediately following each calibration event in the
analytical sequence
(IB must follow ICS) |
0.5 - Be
2.5 - Cr,
Cu, Pb, V
5 - Cd, Co, Mn, Mo, Ni, Sb, Zn
50 -
Fe |
| RLV |
75-125%
Recovery |
0.002 - Be
0.01 -
Cd
0.03 - V
0.05 - Co, Cu, Mn
0.1 - Pb
0.2
- Cr
0.5 - Fe, Mo, Ni, Sb, Zn |
| ICS |
85-115%
Recovery
(Target
Analytes
Only) |
0.2 - Be, Cd
1 - Pb,
V, Ag
5 - Co, Cr, Co, Mn, Mo, Ni, Sb, Zn, As, Se,
Sn
10 - Ce, Nb, Pt
200 - Fe, Al, Mg, Ti |
| IB |
|X| <
Reporting Limit
(Target
Analytes Only) |
|
Continuing
Performance
Checks |
CCV |
Every 10
field
samples |
90-110%
Recovery |
0.5 -
Be
2.5 - Cr, Cu, Pb, V
5 - Cd, Co, Mn, Mo, Ni,
Sb, Zn
50 - Fe |
| RLV |
75-125%
Recovery |
0.002 -
Be
0.01 - Cd
0.03 - V
0.05 - Co, Cu,
Mn
0.1 - Pb
0.2 - Cr
0.5 - Fe, Mo, Ni, Sb,
Zn |
| IB |
|X| <
Reporting Limit
(Target
Analytes Only) |
|
Sequence
Termination
Performance
Checks |
CCV |
At the end
of
the
analytical
sequence |
90-110%
Recovery |
0.5 -
Be
2.5 - Cr, Cu, Pb, V
5 - Cd, Co, Mn, Mo, Ni,
Sb, Zn
50 - Fe |
| RLV |
75-125%
Recovery |
0.002 -
Be
0.01 - Cd
0.03 - V
0.05 - Co, Cu,
Mn
0.1 - Pb
0.2 - Cr
0.5 - Fe, Mo, Ni, Sb,
Zn |
| IB |
|X| <
Reporting Limit
(Target
Analytes Only) |
|
| Term: |
STD |
Calibration
Standard(s) |
|
CB |
Calibration
Blank |
|
ICV |
Independent
Calibration Verification (alternate source reference
material) |
|
CCV |
Continuing Calibration
Verification |
|
IB |
Instrument Blank (part
of continuing calibration verification) |
|
RVL |
Reporting Limit
Verification |
|
ICS |
Interference Check
Sample |
|
Target
Analyte: any analyte that is reported to clients in
the final report of
results | 1The linear calibration range for
target analytes may be established/verified for each
analytical sequence by analyzing a high level calibration
verification standard at the end of the analytical sequence.
In order to report sample results that are measured at
levels between this high standard and the initial
calibration standard, the high standard must exhibit a
recovery between 90 and 110%.
Addendum B
Ghost Wipe Backup Data
|
- Backup Data
General background
information about the determination of detection limits
and reproducibility of the overall procedure is found in
the "Evaluation Guidelines for Surface Sampling
Methods"1.
The Guidelines define analytical parameters, specific
laboratory tests, statistical calculations and acceptance
criteria.
Sample preparation of Ghost Wipes was
performed as described in Section 6.5.4 of OSHA Method
ID-125G. Instrument analytical conditions used are
described in Section 1.9 of this document. Lot
number
of Ghost Wipes used was 9901, manufactured on May 20,
1999.
1.1 Detection Limit of the overall procedure
(DLOP) and reliable quantitation limit (RQL).
The
DLOP is measured as mass per sample. Seven Ghost wipes
were spiked with descending increments of analyte. These
spiked Ghost Wipes, and a sample blank, were analyzed
and the data obtained used to calculate the required
parameters (standards error of estimate and the slope)
for the calculation of the DLOP.
The RQL is
considered the lower limit for precise quantitative
measurements. It is determined from the regression line
parameters obtained for the calculation of the DLOP,
providing 75% to 125% of the analyte is
recovered.
The analyte target, calculated
DLOPs, and RQLs for the 13 validated analytes are shown
below:
Table 1.1.1
Target Concentrations,
DLOPs, and RQLs |
|
analyte
and
wavelength |
target
concn
(µg) |
DLOP
(µg) in 50 mL |
RQL
(µg) in 50 mL |
recovery
at RQL*
(%) |
|
| BE
313.107 |
4 |
0.0052 |
0.017 |
112.8 |
| Cd
214.440 |
10 |
0.053 |
0.18 |
94.6 |
| Co
228.616 |
200 |
0.32 |
1.1 |
101.2 |
| Cr
267.716 |
1000 |
1.0 |
3.4 |
101.2 |
| Cu
324.752 |
200 |
0.45 |
1.5 |
106.0 |
| Fe
238.204 |
2000 |
1.5 |
5.1 |
105.5 |
| Mn
257.610 |
400 |
0.29 |
0.97 |
101.1 |
| Mo
202.031 |
1000 |
0.8 |
2.7 |
103.8 |
| Ni
232.003 |
1000 |
0.92 |
3.1 |
97.5 |
| Pb
220.353 |
100 |
0.55 |
1.8 |
81.9 |
| Sb
206.836 |
1000 |
1.5 |
5.1 |
105.8 |
| V
292.402 |
56 |
0.08 |
0.29 |
103.1 |
| Zn
213.857 |
800 |
2.2 |
7.3 |
95.4 |
| *Percent
recovery at or near the RQL.
Table 1.1.2
Detection Limit of the
Overall Procedure for Beryllium |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
-7.4 |
| 0.012 |
64.7 |
| 0.025 |
121.9 |
| Figure 1.1.2 Plot of data to
determine DLOP/RQL for Beryllium. (Y=44.36.7X
+2.2) |
| 0.05 |
224.5 |
| 0.1 |
436.1 |
| 0.15 |
666.4 |
| 0.2 |
895.1 |
| 0.25 |
1110.4 |
|
Table 1.1.3
Detection Limit of the
Overall Procedure for Cadmium |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
0.4 |
| 0.031 |
4.5 |
| 0.062 |
4.6 |
| 0.12 |
9 |
| 0.25 |
15.8 |
| 0.38 |
24.6 |
| 0.5 |
32.5 |
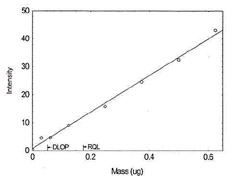
Figure 1.1.3. Plot of data to determine
the DLOP/RQL for Cadmium. (Y=65.2X + 0.7) |
| 0.63 |
43 |
|
Table 1.1.4
Detection Limit of the
Overall Procedure for Cobalt |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
-2.1 |
| 0.62 |
26.7 |
| 1.25 |
52.9 |
| 2.5 |
104.8 |
| 5 |
206.7 |
| 7.5 |
318.5 |
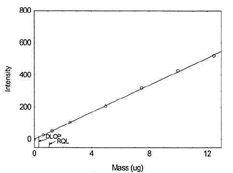
Figure 1.1.4. Plot of data to determine
DLOP/RQL for Cobalt. (Y=42.2X + 0.4) |
| 10 |
428.2 |
| 12.5 |
519.8 |
|
Table 1.1.5
Detection Limit of the
Overall Procedure for Chromium |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
46.3 |
| 3.1 |
673.8 |
| 6.2 |
1332.1 |
| 12.5 |
2749 |
| 25 |
5258 |
| 37.5 |
8015 |
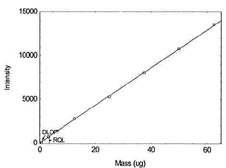
Figure 1.1.5. Plot of data to determine
DLOP/RQL for Chromium. (Y=215.6X - 6.5) |
| 50 |
10769.5 |
| 62.5 |
13546.3 |
|
Table 1.1.6
Detection Limit of the
Overall Procedure for Copper |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
1050.3 |
| 0.62 |
1404 |
| 1.3 |
1904.2 |
| 2.5 |
2687.7 |
| 5 |
4444.8 |
| 7.5 |
6128.2 |
| 10 |
7844.6 |
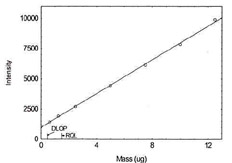
Figure 1.1.6. Plot of data to determine
DLOP/RQL for Copper. (Y=136.4X + 648.9) |
| 12.5 |
9893.2 |
|
Table 1.1.7
Detection Limit of the
Overall Procedure for Iron |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
699.2 |
| 6.2 |
1546.4 |
| 12.5 |
2371.5 |
| 25 |
4035.6 |
| 50 |
7338.3 |
| 75 |
10830.9 |
| 100 |
14309.3 |
Figure 1.1.7. Plot of data to determine
DLOP/RQL for Iron. (Y= 136.4X + 648.9) |
| 125 |
17759.7 |
|
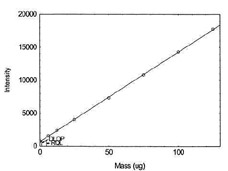
Table 1.1.8
Detection Limit of the
Overall Procedure for Manganese |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
166.6 |
| 1.2 |
2552.4 |
| 2.5 |
5015.1 |
| 5 |
9899.9 |
| 10 |
19028.4 |
| 15 |
28853.3 |
| 20 |
38573.7 |
Figure 1.1.8. Plot of data to determine
DLOP/RQL for Manganese. (Y= 1924.2X + 124.5) |
| 25 |
48431.3 |
|
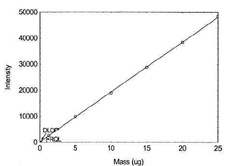
Table 1.1.9
Detection Limit of the
Overall Procedure for Molybdenum |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
-2.7 |
| 3.1 |
32.3 |
| 6.2 |
63.2 |
| 12.5 |
130.3 |
| 25 |
251.6 |
| 37.5 |
388 |
| 50 |
515.2 |
Figure 1.1.9. Plot of data to determine
DLOP/RQL for Molybdenum. (Y= 10.3X - 1.1) |
| 62.5 |
641.4 |
|
Table 1.1.10
Detection Limit of the
Overall Procedure for Nickel |
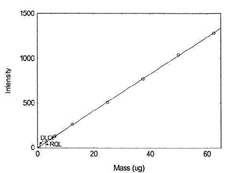 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
0.9 |
| 3.1 |
65.5 |
| 6.2 |
135.7 |
| 12.5 |
265.1 |
| 25 |
508.5 |
| 37.5 |
770.4 |
| 50 |
1040.4 |
Figure 1.1.10. Plot of data to determine
DLOP/RQL for Nickel. (Y= 20.6X + 3.0) |
| 62.5 |
1284 |
|
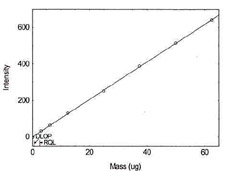
Table 1.1.11
Detection Limit of the
Overall Procedure for Lead |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
0.3 |
| 0.31 |
-0.4 |
| 0.62 |
2.7 |
| 1.25 |
4.3 |
| 2.5 |
11.6 |
| 3.8 |
19.5 |
| 5 |
24.8 |
Figure 1.1.11. Plot of data to determine
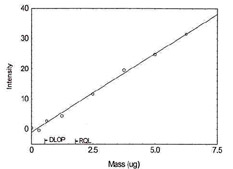
DLOP/RQL for Lead. (Y= 5.2X - 1.0) |
| 3.2 |
31.5 |
|
Table 1.1.12
Detection Limit of the
Overall Procedure for Antimony |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
-1.3 |
| 3.1 |
6 |
| 6.2 |
15.2 |
| 12.5 |
28.8 |
| 25 |
60.8 |
| 37.5 |
88.1 |
| 50 |
116.6 |
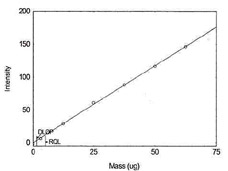
Figure 1.1.12. Plot of data to determine
DLOP/RQL for Antimony. (Y= 2.4X - 0.4) |
| 62.5 |
146.3 |
|
Table 1.1.13
Detection Limit of the
Overall Procedure for Vanadium |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
5.7 |
| 0.17 |
39.8 |
| 0.35 |
64.5 |
| 0.7 |
114.6 |
| 1.4 |
230.2 |
| 2.1 |
352.4 |
| 2.8 |
472.4 |
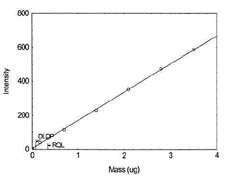
Figure 1.1.13 Plot of data to determine
DLOP/RQL for Vanadium. (Y= 165.9X + 4.6) |
| 3.5 |
586.7 |
|
Table 1.1.14
Detection Limit of the
Overall Procedure for Zinc |
 |
|
| mass per sample (µg) |
intensity |
|
| 0 |
805.8 |
| 2.5 |
879.8 |
| 5 |
1088.1 |
| 10 |
1445.5 |
| 20 |
2201.2 |
| 30 |
3046.4 |
Figure 1.1.14. Plot of data to determine
DLOP/RQL for Zinc (Y= 76.0X + 725.7) |
| 40 |
3825.4 |
| 50 |
4482.3 |
|
1.2
Storage Test
Storage samples were prepared by
liquid-spiking Ghost Wipes at the target concentration.
Twelve storage samples were prepared. Three samples were
analyzed on the day prepared. Nine of the samples were
stored at ambient temperature (about 22ºC). At 5-day
intervals three samples were analyzed. Results were blank
corrected.
Table 1.2.1
Storage Test for
Beryllium |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
90.2 |
91.3 |
92.8 |
| 5 |
90.7 |
93.7 |
91.2 |
| 10 |
90.1 |
88.9 |
92.0 |
Figure 1.2.1. Storage test for
Beryllium. |
| 15 |
91.1 |
91.3 |
91.9 |
|
Table 1.2.2
Storage Test for Cadmium |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
91.0 |
92.6 |
93.3 |
| 5 |
95.8 |
97.9 |
96.8 |
| 10 |
96.8 |
94.7 |
97.7 |
Figure 1.2.2. Storage test for
Cadmium. |
| 15 |
96.0 |
96.0 |
96.4 |
|
Table 1.2.3
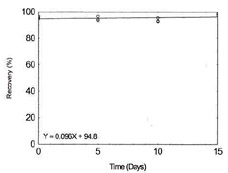
Storage Test for Cobalt |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
94.8 |
95.9 |
96.7 |
| 5 |
93.6 |
96.6 |
94.3 |
| 10 |
93.2 |
92.7 |
95.6 |
Figure 1.2.3. Storage test for
Cobalt. |
| 15 |
97.4 |
98.4 |
97.4 |
|
Table 1.2.4
Storage test for
Chromium |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
96.7 |
100.1 |
98.1 |
| 5 |
95.6 |
98.7 |
97.6 |
| 10 |
96.4 |
94.1 |
97.0 |
Figure 1.2.4. Storage test for
Chromium. |
| 15 |
96.7 |
98.5 |
97.0 |
|
Table 1.2.5
Storage Test for Copper |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
95.7 |
96.6 |
98.2 |
| 5 |
95.9 |
97.8 |
96.3 |
| 10 |
96.3 |
93.8 |
96.6 |
Figure 1.2.5. Storage test for
Copper. |
| 15 |
96.8 |
97.8 |
96.8 |
|
Table 1.2.6
Storage Test for Iron |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
97.5 |
98.6 |
100.3 |
| 5 |
97.1 |
99.0 |
97.7 |
| 10 |
97.7 |
95.9 |
98.8 |
Figure 1.2.6. Storage test for Iron. |
| 15 |
98.7 |
99.9 |
99.0 |
|
Table 1.2.7
Storage Test for
Manganese |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
95.2 |
98.5 |
96.8 |
| 5 |
94.6 |
97.5 |
96.5 |
| 10 |
96.0 |
93.8 |
96.6 |
Figure 1.2.7. Storage test for
Manganese. |
| 15 |
96.9 |
98.7 |
97.2 |
|
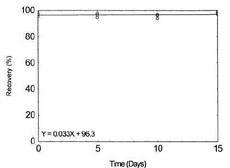
Table 1.2.8
Storage Test for
Molybdenum |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
96.0 |
97.0 |
98.0 |
| 5 |
96.3 |
97.0 |
95.0 |
| 10 |
94.7 |
93.6 |
96.5 |
Figure 1.2.8. Storage test for
Molybdenum. |
| 15 |
95.3 |
95.8 |
95.8 |
|
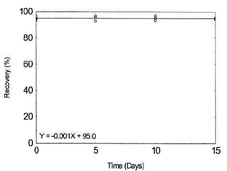
Table 1.2.9
Storage Test for Nickel |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
94.5 |
97.7 |
95.1 |
| 5 |
94.2 |
98.1 |
95.0 |
| 10 |
94.5 |
93.3 |
96.2 |
Figure 1.2.9. Storage test for
Nickel. |
| 15 |
94.4 |
94.6 |
95.3 |
|
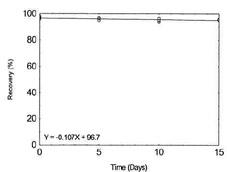
Table 1.2.10
Storage Test for Lead |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
93.6 |
94.8 |
96.3 |
| 5 |
93.0 |
96.6 |
95.7 |
| 10 |
95.2 |
93.4 |
96.7 |
Figure 1.2.10. Storage test for
Lead. |
| 15 |
94.3 |
95.3 |
95.0 |
|
Table 1.2.11
Storage Test for
Antimony |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
85.8 |
85.5 |
90.1 |
| 5 |
86.9 |
88.2 |
85.6 |
| 10 |
78.2 |
81.7 |
86.6 |
Figure 1.2.11. Storage test for
Antimony. |
| 15 |
85.5 |
89.6 |
89.0 |
|
Table 1.2.12
Storage Test for
Vanadium |
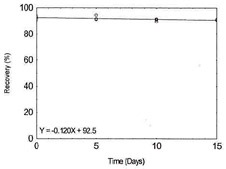 |
|
| time (days) |
recovery (%) |
|
| 0 |
91.3 |
93.6 |
92.4 |
| 5 |
91.2 |
94.4 |
91.7 |
| 10 |
91.0 |
89.5 |
91.6 |
Figure 1.2.12. Storage test for
Vanadium. |
| 15 |
91.1 |
90.7 |
91.2 |
|
Table 1.2.13
Storage Test for Zinc |
 |
|
| time (days) |
recovery (%) |
|
| 0 |
90.0 |
90.9 |
91.4 |
| 5 |
89.9 |
92.8 |
90.7 |
| 10 |
89.6 |
88.7 |
91.4 |
Figure 1.2.13. Storage test for
Zinc. |
| 15 |
90.3 |
91.4 |
90.9 |
|
1.3
Sampler Removal Efficiency
Six 100-cm2 glass plates
were liquid-spiked at the target concentrations and
allowed to dry. Samples were collected from each surface
by placing a Ghost Wipe folded in half at an outside edge
of the glass plate and progressing towards the center
making concentric squares of decreasing size while
applying firm pressure. The Ghost Wipe was folded in half
with the contaminant side in and the glass plate was wiped
a second time. The Ghost Wipe was again folded in half and
the surface wiped a third time. Results were blank
corrected.
Table 1.3.1
Sampler Removal Efficiency
Data for Beryllium on Ghost Wipes |
|
Table 1.3.2
Sampler Removal Efficiency
Data for Cadmium on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 4 |
3.770 |
94.2 |
|
10 |
9.300 |
93.0 |
| 4 |
3.755 |
93.9 |
|
10 |
9.168 |
91.6 |
| 4 |
3.938 |
98.4 |
|
10 |
9.625 |
96.2 |
| 4 |
3.880 |
97.0 |
|
10 |
9.535 |
95.4 |
| 4 |
3.848 |
96.0 |
|
10 |
9.595 |
96.0 |
| 4 |
3.785 |
84.4 |
|
10 |
8.330 |
83.3 |
|
|
|
Table 1.3.3
Sampler Removal Efficiency
Data for Cobalt on Ghost Wipes |
|
Table 1.3.4
Sampler Removal Efficiency
Data for Chromium on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 200 |
182.1 |
91.0 |
|
1000 |
964.5 |
96.4 |
| 200 |
182.0 |
91.0 |
|
1000 |
889.5 |
88.9 |
| 200 |
190.6 |
95.3 |
|
1000 |
1001.5 |
100.1 |
| 200 |
187.6 |
93.8 |
|
1000 |
917.5 |
91.7 |
| 200 |
187.8 |
93.9 |
|
1000 |
992.0 |
99.2 |
| 200 |
162.8 |
81.4 |
|
1000 |
927.0 |
92.7 |
|
|
|
Table 1.3.5
Sampler Removal Efficiency
Data for Copper on Ghost Wipes |
|
Table 1.3.6
Sampler Removal Efficiency
Data for Iron on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 200 |
194.1 |
97.0 |
|
2000 |
1914 |
95.7 |
| 200 |
193.6 |
96.8 |
|
2000 |
1913 |
95.6 |
| 200 |
200.7 |
100.4 |
|
2000 |
1987 |
99.4 |
| 200 |
195.6 |
97.9 |
|
2000 |
1940 |
97.0 |
| 200 |
197.3 |
98.6 |
|
2000 |
1957 |
97.8 |
| 200 |
172.5 |
86.2 |
|
2000 |
1704 |
85.2 |
|
|
|
Table 1.3.7
Sampler Removal Efficiency
Data for Manganese on Ghost Wipes |
|
Table 1.3.8
Sampler Removal Efficiency
Data for Molybdenum on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 400 |
385.1 |
96.3 |
|
1000 |
892.0 |
89.2 |
| 400 |
356.8 |
89.2 |
|
1000 |
698.5 |
69.8 |
| 400 |
400.0 |
100.0 |
|
1000 |
791.0 |
79.1 |
| 400 |
367.1 |
91.8 |
|
1000 |
780.5 |
78.0 |
| 400 |
395.7 |
98.9 |
|
1000 |
724.5 |
72.4 |
| 400 |
371.1 |
92.8 |
|
1000 |
749.0 |
74.9 |
|
|
|
Table 1.3.9
Sampler Removal Efficiency
Data for Nickel on Ghost Wipes |
|
Table 1.3.10
Sampler Removal Efficiency
Data for Lead on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 1000 |
913.5 |
91.4 |
|
100 |
90.95 |
91.0 |
| 1000 |
843.0 |
84.3 |
|
100 |
89.00 |
89.0 |
| 1000 |
955.0 |
95.5 |
|
100 |
93.60 |
93.6 |
| 1000 |
881.0 |
88.1 |
|
100 |
91.55 |
91.6 |
| 1000 |
941.5 |
94.2 |
|
100 |
92.75 |
92.8 |
| 1000 |
875.0 |
87.5 |
|
100 |
80.40 |
80.4 |
|
|
|
Table 1.3.11
Sampler Removal Efficiency
Data for Antimony on Ghost Wipes |
|
Table 1.3.12
Sampler Removal Efficiency
Data for Vanadium on Ghost Wipes |
|
|
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 1000 |
809.5 |
81.0 |
|
56 |
52.08 |
93.0 |
| 1000 |
654.5 |
65.4 |
|
56 |
49.41 |
88.2 |
| 1000 |
733.0 |
73.3 |
|
56 |
54.23 |
96.8 |
| 1000 |
717.5 |
71.8 |
|
56 |
50.23 |
89.7 |
| 1000 |
698.5 |
69.8 |
|
56 |
52.43 |
93.6 |
| 1000 |
690.5 |
69.0 |
|
56 |
50.98 |
91.0 |
|
|
|
Table 1.3.13
Sampler Removal Efficiency
Data for Zinc on Ghost Wipes |
|
| theoretical (µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
| 800 |
710.7 |
88.8 |
| 800 |
712.2 |
89.0 |
| 800 |
754.2 |
94.3 |
| 800 |
739.2 |
92.4 |
| 800 |
735.2 |
91.9 |
| 800 |
641.7 |
80.2 |
|
1.4 Analytical
Method Recovery and Stability of Digested Samples
1.4.1 Analytical Method
Recovery
Analytical method recovery (AMR) was
determined by liquid-spiking Ghost Wipes with the
analytes at the RQL, 0.1, 1.0, and 10 times the target
concentrations. These samples were stored overnight at
ambient temperature and then analyzed. Results were
blank corrected.
Table 1.4.1.1
Analytical Method
Recovery of Beryllium from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
0.017 |
114.7 |
82.4 |
94.1 |
82.4 |
93.4 |
| 0.1 |
0.4 |
95.6 |
99.5 |
96.1 |
97.4 |
97.2 |
| 1.0 |
4 |
94.5 |
95.1 |
90.6 |
94.4 |
93.6 |
| 10 |
40 |
92.2 |
92.9 |
93.6 |
94.4 |
93.3 |
| AMR |
|
|
|
|
|
94.4 |
|
Table 1.4.1.2
Analytical Method
Recovery of Cadmium from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
0.18 |
83.3 |
86.1 |
86.1 |
80.6 |
84.0 |
| 0.1 |
1 |
99.5 |
102.5 |
98.0 |
99.0 |
99.8 |
| 1.0 |
10 |
99.4 |
99.80 |
96.5 |
99.4 |
98.8 |
| 10 |
100 |
98.3 |
100.4 |
102.4 |
102.5 |
100.9 |
| AMR |
|
|
|
|
|
95.9 |
|
Table 1.4.1.3
Analytical Method
Recovery of Cobalt from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
1.1 |
105.9 |
92.7 |
94.1 |
97.7 |
97.6 |
| 0.1 |
20 |
99.2 |
101.9 |
100.2 |
101.1 |
100.6 |
| 1.0 |
200 |
98.2 |
98.1 |
93.7 |
97.7 |
96.9 |
| 10 |
2000 |
96.6 |
97.6 |
98.2 |
99.3 |
97.9 |
| AMR |
|
|
|
|
|
98.2 |
|
Table 1.4.1.4
Analytical Method
Recovery of Chromium from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
3.4 |
116.9 |
102.5 |
107.5 |
107.9 |
108.7 |
| 0.1 |
100 |
98.8 |
102.9 |
99.8 |
101.6 |
100.8 |
| 1.0 |
1000 |
99.3 |
98.9 |
95.3 |
99.1 |
98.2 |
| 10 |
10000 |
96.8 |
99.3 |
101.0 |
101.6 |
99.7 |
| AMR |
|
|
|
|
|
101.8 |
|
Table 1.4.1.5
Analytical Method
Recovery of Copper from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
1.5 |
110.0 |
111.7 |
100.0 |
89.7 |
102.8 |
| 0.1 |
20 |
99.8 |
102.7 |
101.4 |
101.0 |
101.2 |
| 1.0 |
200 |
98.0 |
97.4 |
97.4 |
97.4 |
96.6 |
| 10 |
2000 |
94.8 |
97.5 |
99.4 |
99.4 |
97.7 |
| AMR |
|
|
|
|
|
99.6 |
|
Table 1.4.1.6
Analytical Method
Recovery of Iron from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
5.1 |
131.9 |
112.9 |
107.4 |
93.0 |
111.3 |
| 0.1 |
200 |
102.3 |
106.2 |
102.9 |
104.2 |
103.9 |
| 1.0 |
2000 |
101.2 |
100.8 |
97.0 |
101.1 |
100.0 |
| 10 |
20000 |
98.5 |
101.7 |
103.8 |
103.8 |
102.0 |
| AMR |
|
|
|
|
|
104.3 |
|
Table 1.4.1.7
Analytical Method
Recovery of Manganese from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
0.97 |
121.0 |
107.4 |
137.7 |
165.6 |
132.9 |
| 0.1 |
40 |
99.2 |
103.0 |
100.3 |
101.9 |
101.1 |
| 1.0 |
400 |
99.1 |
98.6 |
95.1 |
98.7 |
97.9 |
| 10 |
4000 |
96.4 |
99.0 |
100.7 |
101.2 |
99.3 |
| AMR |
|
|
|
|
|
107.8 |
|
Table 1.4.1.8
Analytical Method
Recovery of Molybdenum from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
2.7 |
122.6 |
100.0 |
108.2 |
96.3 |
106.8 |
| 0.1 |
100 |
101.8 |
103.5 |
102.2 |
103.0 |
102.6 |
| 1.0 |
1000 |
98.9 |
98.7 |
94.6 |
98.5 |
97.7 |
| 10 |
1000 |
97.4 |
98.4 |
98.8 |
99.8 |
98.6 |
| AMR |
|
|
|
|
|
101.4 |
|
Table 1.4.1.9
Analytical Method
Recovery of Nickel from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
3.1 |
122.1 |
105.5 |
121.1 |
106.1 |
113.7 |
| 0.1 |
10 |
98.0 |
101.0 |
99.4 |
99.6 |
99.5 |
| 1.0 |
100 |
98.7 |
98.2 |
94.7 |
97.4 |
97.2 |
| 10 |
1000 |
96.9 |
97.7 |
97.8 |
99.1 |
97.9 |
| AMR |
|
|
|
|
|
102.1 |
|
Table 1.4.1.10
Analytical Method
Recovery of Lead from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
1.8 |
53.9 |
65.0 |
80.6 |
67.2 |
66.7 |
| 0.1 |
10 |
90.7 |
93.6 |
101.0 |
104.0 |
97.3 |
| 1.0 |
100 |
98.8 |
98.8 |
94.5 |
98.5 |
97.6 |
| 10 |
1000 |
96.6 |
99.8 |
101.3 |
102.4 |
100.0 |
| AMR |
|
|
|
|
|
90.4 |
|
Table 1.4.1.11
Analytical Method
Recovery of Antimony from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
5.1 |
76.9 |
59.8 |
37.1 |
47.8 |
55.4 |
| 0.1 |
100 |
90.9 |
90.6 |
90.6 |
92.5 |
91.2 |
| 1.0 |
1000 |
92.3 |
92.1 |
89.2 |
91.7 |
91.3 |
| 10 |
10000 |
88.7 |
85.9 |
93.2 |
94.5 |
90.6 |
| AMR |
|
|
|
|
|
82.1 |
|
Table 1.4.1.12
Analytical Method
Recovery of Vanadium from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
0.29 |
94.8 |
86.2 |
96.6 |
101.7 |
94.8 |
| 0.1 |
5.6 |
93.0 |
96.8 |
94.2 |
97.2 |
95.3 |
| 1.0 |
56 |
94.6 |
93.9 |
91.1 |
93.3 |
93.2 |
| 10 |
560 |
92.6 |
93.6 |
93.9 |
94.4 |
93.6 |
| AMR |
|
|
|
|
|
94.2 |
|
Table 1.4.1.13
Analytical Method
Recovery of Zinc from Ghost Wipes |
|
| level |
sample number
|
| ×
target concn |
µg per
sampler |
1 |
2 |
3 |
4 |
mean |
|
| RQL |
7.3 |
125.8 |
132.7 |
104.6 |
91.1 |
113.6 |
| 0.1 |
80 |
92.3 |
96.8 |
91.7 |
95.4 |
94.0 |
| 1.0 |
800 |
93.6 |
93.2 |
89.6 |
92.9 |
92.3 |
| 10 |
8000 |
93.5 |
92.6 |
93.2 |
94.1 |
93.4 |
| AMR |
|
|
|
|
|
98.3 |
|
1.4.2 Stability of Digested
Samples
Stability of the digested samples were
investigated by reanalyzing the four 1.0 times the target
concentration samples seven days after initial analysis.
After the original analysis was performed the samples were
stored at ambient temperature in the 50 mL volumetric
flasks in which they were brought to volume in. For the
second analysis new aliquots were taken from each
volumetric. Results were blank corrected.
Table 1.4.2.1
Stability of Digested
Samples for Beryllium |
|
Table 1.4.2.2
Stability of Digested
Samples for Cadmium |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 94.5 |
91.9 |
2.9 |
|
99.4 |
97.2 |
2.2 |
| 95.1 |
92.3 |
2.8 |
|
99.8 |
98.4 |
1.4 |
| 90.6 |
87.3 |
3.3 |
|
96.5 |
94.0 |
2.5 |
| 94.4 |
9191 |
3.3 |
|
99.4 |
97.9 |
1.5 |
|
|
|
Table 1.4.2.3
Stability of Digested
Samples for Cobalt |
|
Table 1.4.2.4
Stability of Digested
Samples for Chromium |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 98.2 |
98.3 |
0.1 |
|
99.3 |
97.8 |
1.5 |
| 98.1 |
98.7 |
0.6 |
|
98.9 |
97.2 |
1.7 |
| 93.7 |
94.0 |
0.3 |
|
95.3 |
93.4 |
1.9 |
| 97.7 |
97.7 |
0.0 |
|
99.1 |
97.2 |
1.9 |
|
|
|
Table 1.4.2.5
Stability of Digested
Samples for Copper |
|
Table 1.4.2.6
Stability of Digested
Samples for Iron |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 98.0 |
96.8 |
1.2 |
|
101.2 |
99.2 |
1.0 |
| 97.4 |
95.9 |
1.5 |
|
100.8 |
98.6 |
2.2 |
| 93.4 |
91.9 |
1.5 |
|
97.0 |
94.1 |
2.9 |
| 97.4 |
95.8 |
1.6 |
|
101.1 |
98.7 |
2.4 |
|
|
|
Table 1.4.2.7
Stability of Digested
Samples for Manganese |
|
Table 1.4.2.8
Stability of Digested
Samples for Molybdenum |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 99.0 |
97.4 |
1.6 |
|
98.9 |
99.2 |
0.3 |
| 98.6 |
96.6 |
2.0 |
|
98.7 |
99.8 |
1.1 |
| 95.1 |
93.0 |
2.1 |
|
94.6 |
99.8 |
5.2 |
| 98.7 |
96.6 |
2.1 |
|
98.5 |
98.6 |
0.1 |
|
|
|
Table 1.4.2.9
Stability of Digested
Samples for Nickel |
|
Table 1.4.2.10
Stability of Digested
Samples for Lead |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 98.7 |
96.6 |
2.1 |
|
98.8 |
97.0 |
1.8 |
| 98.2 |
96.8 |
1.9 |
|
98.8 |
97.6 |
1.2 |
| 94.7 |
92.4 |
2.3 |
|
94.5 |
94.2 |
0.3 |
| 97.4 |
95.4 |
2.0 |
|
98.5 |
98.2 |
0.3 |
|
|
|
Table 1.4.2.11
Stability of Digested
Samples for Antimony |
|
Table 1.4.2.12
Stability of Digested
Samples for Vanadium |
|
|
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
initial
(%) |
after 7
days (%) |
difference (%) |
|
|
|
| 92.3 |
93.2 |
0.9 |
|
94.6 |
92.1 |
2.5 |
| 92.1 |
93.7 |
1.6 |
|
93.9 |
92.0 |
1.9 |
| 89.2 |
89.9 |
0.7 |
|
91.0 |
87.3 |
3.7 |
| 91.7 |
93.6 |
1.9 |
|
93.3 |
90.3 |
3.0 |
|
|
|
Table 1.4.2.13
Stability of Digested
Samples for Zinc |
|
| initial
(%) |
after 7
days (%) |
difference (%) |
|
| 93.6 |
93.5 |
0.1 |
| 93.2 |
93.9 |
0.7 |
| 89.6 |
89.6 |
0.0 |
| 92.9 |
92.7 |
0.2 |
|
1.5
Reproducibility
1.5.1 Sampling Reproducibility Six 100-cm2
glass plates were liquid-spiked at the target
concentrations and allowed to dry. Two chemist, other
than the one developing the method,
conducted surface
sampling. Results were blank corrected.
Table 1.5.1
Sampling Reproducibility
Data for Beryllium on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 4 |
3.821 |
95.5 |
3.621 |
90.5 |
| 4 |
3.788 |
94.7 |
3.646 |
91.2 |
| 4 |
3.687 |
92.2 |
3.678 |
91.9 |
| 4 |
3.973 |
99.3 |
3.640 |
91.0 |
| 4 |
4.023 |
100.6 |
3.711 |
92.8 |
| 4 |
3.899 |
97.5 |
3.599 |
90.0 |
|
Table 1.5.2
Sampling Reproducibility
Data for Cadmium on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 10 |
9.615 |
96.2 |
9.480 |
94.8 |
| 10 |
9.545 |
95.4 |
9.520 |
95.2 |
| 10 |
9.310 |
93.1 |
9.510 |
95.1 |
| 10 |
9.865 |
98.6 |
9.550 |
95.5 |
| 10 |
10.06 |
100.6 |
9.665 |
96.6 |
| 10 |
9.805 |
98.0 |
9.390 |
93.9 |
|
Table 1.5.3
Sampling Reproducibility
Data for Cobalt on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 200 |
182.1 |
91.1 |
183.5 |
91.8 |
| 200 |
179.5 |
89.7 |
185.5 |
92.9 |
| 200 |
173.3 |
86.6 |
185.4 |
92.7 |
| 200 |
187.6 |
93.8 |
185.0 |
92.5 |
| 200 |
190.5 |
95.2 |
188.2 |
94.1 |
| 200 |
184.0 |
92.0 |
182.5 |
91.2 |
|
Table 1.5.4
Sampling Reproducibility
Data for Chromium on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 1000 |
976.0 |
97.6 |
918.0 |
91.8 |
| 1000 |
981.0 |
87.1 |
956.0 |
95.6 |
| 1000 |
905.5 |
90.6 |
944.5 |
94.4 |
| 1000 |
991 |
99.1 |
935.0 |
93.5 |
| 1000 |
1025 |
102.5 |
980.5 |
98.0 |
| 1000 |
1012 |
101.2 |
954.5 |
95.4 |
|
Table 1.5.5
Sampling Reproducibility
Data for Copper on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 200 |
196.0 |
98.0 |
189.5 |
94.8 |
| 200 |
193.9 |
96.9 |
190.4 |
95.2 |
| 200 |
185.1 |
92.5 |
189.6 |
94.8 |
| 200 |
198.9 |
99.5 |
188.2 |
94.0 |
| 200 |
202.3 |
101.2 |
190.7 |
95.3 |
| 200 |
195.4 |
97.7 |
185.6 |
92.7 |
|
Table 1.5.6
Sampling Reproducibility
Data for Iron on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 2000 |
1961 |
98.1 |
1933 |
96.6 |
| 2000 |
1939 |
97.0 |
1949 |
97.5 |
| 2000 |
1838 |
91.9 |
1948 |
97.4 |
| 2000 |
2002 |
100.0 |
1936 |
96.8 |
| 2000 |
2038 |
101.9 |
1962 |
98.1 |
| 2000 |
1975 |
98.7 |
1909 |
95.5 |
|
Table 1.5.7
Sampling Reproducibility
Data for Manganese on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 400 |
390.5 |
97.6 |
369.0 |
92.3 |
| 400 |
392.4 |
98.1 |
383.7 |
95.9 |
| 400 |
363.5 |
90.9 |
379.0 |
94.8 |
| 400 |
396.2 |
99.1 |
374.8 |
93.7 |
| 400 |
409.1 |
102.2 |
391.8 |
98.0 |
| 400 |
403.3 |
100.8 |
382.6 |
95.6 |
|
Table 1.5.8
Sampling Reproducibility
Data for Molybdenum on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 1000 |
737.5 |
73.8 |
906.0 |
90.6 |
| 1000 |
862.0 |
86.2 |
932.0 |
93.2 |
| 1000 |
803.5 |
80.4 |
952.0 |
95.2 |
| 1000 |
934.0 |
93.4 |
908.0 |
90.7 |
| 1000 |
839.0 |
83.9 |
970.5 |
97.0 |
| 1000 |
929.5 |
93.0 |
938.5 |
93.8 |
|
Table 1.5.9
Sampling Reproducibility
Data for Nickel on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 1000 |
925.0 |
92.5 |
895.5 |
89.6 |
| 1000 |
928.0 |
92.8 |
932.5 |
93.2 |
| 1000 |
875.5 |
87.6 |
920.5 |
92.0 |
| 1000 |
945.0 |
94.5 |
915.0 |
91.5 |
| 1000 |
967.0 |
96.7 |
959.0 |
95.9 |
| 1000 |
959.0 |
95.9 |
936.0 |
93.6 |
|
Table 1.5.10
Sampling
Reproducibility Data for Lead on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 100 |
94.55 |
94.6 |
92.65 |
92.6 |
| 100 |
92.30 |
92.3 |
93.3 |
93.3 |
| 100 |
89.45 |
89.5 |
92.9 |
92.9 |
| 100 |
96.15 |
96.2 |
92.4 |
92.4 |
| 100 |
97.10 |
97.1 |
94.6 |
94.6 |
| 100 |
94.95 |
95.0 |
91.6 |
91.6 |
|
Table 1.5.11
Sampling
Reproducibility Data for Antimony on Ghost
Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 1000 |
757.0 |
75.7 |
751.5 |
75.2 |
| 1000 |
836.0 |
83.6 |
780.5 |
78.0 |
| 1000 |
813.0 |
81.3 |
797.5 |
79.8 |
| 1000 |
911.5 |
91.2 |
814.5 |
81.4 |
| 1000 |
823.0 |
82.3 |
882.5 |
88.2 |
| 1000 |
925.0 |
92.5 |
827.5 |
82.8 |
|
Table 1.5.12
Sampling
Reproducibility Data for Vanadium on Ghost
Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 56 |
52.58 |
93.9 |
48.54 |
86.7 |
| 56 |
51.68 |
92.3 |
50.88 |
90.9 |
| 56 |
49.37 |
88.2 |
50.78 |
90.7 |
| 56 |
53.28 |
95.2 |
50.48 |
90.2 |
| 56 |
54.38 |
97.1 |
52.48 |
93.7 |
| 56 |
53.83 |
96.1 |
51.23 |
91.5 |
|
Table 1.5.13
Sampling
Reproducibility Data for Zinc on Ghost Wipes |
|
| |
Chemist 1 |
Chemist 2 |
|
| theoretical (µg/surface) |
recovered (µg/sample) |
recovery (%) |
recovered (µg/sample) |
recovery (%) |
|
| 800 |
725.5 |
90.7 |
729.2 |
91.2 |
| 800 |
720.7 |
90.1 |
727.7 |
91.0 |
| 800 |
697.7 |
87.2 |
742.2 |
92.8 |
| 800 |
750.7 |
93.8 |
731.2 |
91.4 |
| 800 |
754.7 |
94.3 |
751.7 |
94.0 |
| 800 |
732.7 |
91.6 |
728.2 |
91.0 |
|
1.5.2 Analytical
Reproducibility
Six samples were prepared by
spiking media in the same manner that was used in the
preparation of samples for the storage study. The
samples were submitted to the OSHA SLTC for analysis and
the samples were analyzed by a chemist other than the
one developing the method. The samples were analyzed
after being stored for 15 days at ambient temperature.
Results were blank corrected.
Table 1.5.2.1
Analytical Reproducibility
Data for Beryllium on Ghost Wipes |
|
Table 1.5.2.2
Analytical Reproducibility
Data for Cadmium on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 4 |
3.767 |
94.2 |
|
10 |
10.10 |
101.0 |
| 4 |
3.903 |
97.6 |
|
10 |
10.30 |
103.0 |
| 4 |
3.958 |
99.0 |
|
10 |
10.46 |
104.6 |
| 4 |
4.008 |
100.2 |
|
10 |
10.74 |
107.4 |
| 4 |
4.024 |
100.6 |
|
10 |
10.50 |
105.0 |
| 4 |
3.892 |
97.3 |
|
10 |
10.34 |
103.4 |
|
|
|
Table 1.5.2.3
Analytical Reproducibility
Data for Cobalt on Ghost Wipes |
|
Table 1.5.2.4
Analytical Reproducibility
Data for Chromium on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 200 |
193.8 |
96.9 |
|
1000 |
97.3 |
97.3 |
| 200 |
199.4 |
99.7 |
|
1000 |
1004 |
100.4 |
| 200 |
201.3 |
100.6 |
|
1000 |
1025 |
102.5 |
| 200 |
205.2 |
102.6 |
|
1000 |
1033 |
103.3 |
| 200 |
203.9 |
102.0 |
|
1000 |
1016 |
101.6 |
| 200 |
199.2 |
99.6 |
|
1000 |
1006 |
100.6 |
|
|
|
Table 1.5.2.5
Analytical Reproducibility
Data for Copper on Ghost Wipes |
|
Table 1.5.2.6
Analytical Reproducibility
Data for Iron on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 200 |
193.0 |
96.5 |
|
2000 |
2016 |
100.8 |
| 200 |
198.0 |
99.0 |
|
2000 |
2070 |
103.5 |
| 200 |
202.1 |
101.0 |
|
2000 |
2119 |
106.0 |
| 200 |
203.0 |
101.5 |
|
2000 |
2134 |
106.7 |
| 200 |
202.7 |
101.4 |
|
2000 |
2104 |
105.2 |
| 200 |
196.4 |
98.2 |
|
2000 |
2069 |
103.4 |
|
|
|
Table 1.5.2.7
Analytical Reproducibility
Data for Manganese on Ghost Wipes |
|
Table 1.5.2.8
Analytical Reproducibility
Data for Molybdenum on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 400 |
392.4 |
98.1 |
|
1000 |
982.4 |
98.2 |
| 400 |
404.4 |
101.1 |
|
1000 |
879.9 |
88.0 |
| 400 |
412.6 |
103.2 |
|
1000 |
1157 |
115.7 |
| 400 |
415.6 |
103.9 |
|
1000 |
1040 |
104.0 |
| 400 |
409.9 |
102.5 |
|
1000 |
1043 |
104.3 |
| 400 |
404.8 |
101.2 |
|
1000 |
1016 |
101.6 |
|
|
|
Table 1.5.2.9
Analytical Reproducibility
Data for Nickel on Ghost Wipes |
|
Table 1.5.2.10
Analytical Reproducibility
Data for Lead on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 1000 |
955.1 |
95.5 |
|
100 |
96.10 |
96.1 |
| 1000 |
983.6 |
98.4 |
|
100 |
97.75 |
97.8 |
| 1000 |
990.1 |
99.01 |
|
100 |
98.00 |
98.0 |
| 1000 |
1005 |
100.5 |
|
100 |
101.2 |
101.2 |
| 1000 |
997.6 |
99.8 |
|
100 |
99.6 |
99.6 |
| 1000 |
981.6 |
98.16 |
|
100 |
97.85 |
97.8 |
|
|
|
Table 1.5.2.11
Analytical Reproducibility
Data for Antimony on Ghost Wipes |
|
Table 1.5.2.12
Analytical Reproducibility
Data for Vanadium on Ghost Wipes |
|
|
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
|
|
| 1000 |
907.4 |
90.7 |
|
56 |
51.53 |
92.0 |
| 1000 |
729.9 |
73.0 |
|
56 |
53.55 |
95.6 |
| 1000 |
1107 |
111 |
|
56 |
53.85 |
96.2 |
| 1000 |
942.4 |
94.2 |
|
56 |
54.60 |
97.5 |
| 1000 |
943.4 |
94.3 |
|
56 |
54.80 |
97.9 |
| 1000 |
917.9 |
91.8 |
|
56 |
53.50 |
95.5 |
|
|
|
Table 1.5.2.13
Analytical Reproducibility
Data for Zinc on Ghost Wipes |
|
| theoretical
(µg/surface) |
recovered
(µg/sample) |
recovery
(%) |
|
| 800 |
733.4 |
91.7 |
| 800 |
751.9 |
94.0 |
| 800 |
761.4 |
95.2 |
| 800 |
771.9 |
96.5 |
| 800 |
770.9 |
96.4 |
| 800 |
750.9 |
93.9 |
|
1.6 Interferences
1.6.1 Media, Reagents, and Surface.
Six blank Ghost Wipes were analyzed, after each
being used to wipe a separate cleaned glass plate, to
determine interferences due to contamination from the
glass surface, media, and reagents.
Table 1.6.1.1
Contamination to
Analysis from the Media and Reagents (µg
found) |
|
| analyte |
wipe
1 |
wipe
2 |
wipe
3 |
wipe
4 |
wipe
5 |
wipe
6 |
average |
|
| Be |
0.009 |
0.004 |
0.000 |
0.008 |
0.008 |
0.008 |
0.006 |
| Cd |
0.000 |
0.015 |
0.070 |
0.000 |
0.000 |
0.000 |
0.014 |
| Co |
0.090 |
0.060 |
0.100 |
0.000 |
0.020 |
0.035 |
0.051 |
| Cr |
0.030 |
0.015 |
0.000 |
0.09 |
0.020 |
0.100 |
0.042 |
| Cu |
1.690 |
1.190 |
1.040 |
1.010 |
1.280 |
1.355 |
1.261 |
| Fe |
4.900 |
3.265 |
3.140 |
3.475 |
3.840 |
4.795 |
3.902 |
| Mn |
0.103 |
0.022 |
0.021 |
0.020 |
0.037 |
0.026 |
0.038 |
| Mo |
0.000 |
0.000 |
0.000 |
0.000 |
0.060 |
0.075 |
0.022 |
| Ni |
0.000 |
0.000 |
0.000 |
0.070 |
0.000 |
0.000 |
0.012 |
| Pb |
0.430 |
0.000 |
0.000 |
0.515 |
0.000 |
0.000 |
0.158 |
| Sb |
0.000 |
0.435 |
0.000 |
0.000 |
0.000 |
0.000 |
0.072 |
| V |
0.040 |
0.000 |
0.015 |
0.020 |
0.030 |
0.000 |
0.018 |
| Zn |
13.88 |
8.755 |
8.300 |
6.065 |
9.080 |
9.560 |
9.273 |
|
Six blank Ghost Wipes were analyzed, after
each being used to wipe a separate cleaned glass plate,
to determine interferences due to contamination from the
glass surface, media, and reagents.
Table 1.6.1.2
Contamination to
Analysis from the Glass Surface, Media and
Reagents (µg found) |
|
| analyte |
wipe
1 |
wipe
2 |
wipe
3 |
wipe
4 |
wipe
5 |
wipe
6 |
average |
|
| Be |
0.000 |
0.000 |
0.000 |
0.000 |
0.002 |
0.000 |
0.000 |
| Cd |
0.030 |
0.020 |
0.020 |
0.005 |
0.005 |
0.020 |
0.017 |
| Co |
0.090 |
0.055 |
0.090 |
0.075 |
0.000 |
0.145 |
0.076 |
| Cr |
0.155 |
0.355 |
0.220 |
0.070 |
0.370 |
0.370 |
0.257 |
| Cu |
1.325 |
1.170 |
1.015 |
1.040 |
1.340 |
1.405 |
1.216 |
| Fe |
4.865 |
5.130 |
4.425 |
4.170 |
6.200 |
6.130 |
5.153 |
| Mn |
0.132 |
0.198 |
0.138 |
0.088 |
0.120 |
0.205 |
0.147 |
| Mo |
0.125 |
0.355 |
0.410 |
0.000 |
0.000 |
0.165 |
0.176 |
| Ni |
0.440 |
0.450 |
0.470 |
0.355 |
0.305 |
0.675 |
0.449 |
| Pb |
0.000 |
0.000 |
0.335 |
0.245 |
0.640 |
0.120 |
0.223 |
| Sb |
1.390 |
0.950 |
0.000 |
0.360 |
0.525 |
1.240 |
0.744 |
| V |
0.105 |
0.055 |
0.040 |
0.000 |
0.020 |
0.015 |
0.039 |
| Zn |
21.59 |
12.30 |
11.07 |
14.79 |
12.07 |
9.970 |
13.63 |
|
1.6.2 Spectral Interferences
The
following Inter-Element Corrections (IEC) were used to
correct for spectral
interferences:
Table
1.6.2.1
Radial ICP IEC Factors a,b,c |
| analyte & wavelength |
interfering analytes |
| Co |
Cr |
Cu |
Fe |
Mn |
Mo |
Ni |
Ti |
V |
| Cd
313.107 |
- |
- |
- |
0.0576 |
- |
- |
- |
- |
- |
| Co
214.44 |
- |
- |
- |
- |
- |
- |
0.1681 |
1.6467 |
- |
| Cr
228.616 |
- |
- |
- |
- |
0.2 |
- |
- |
- |
- |
| Cu
267.716 |
- |
- |
- |
- |
- |
0.4168 |
- |
- |
- |
| Fe
238.204 |
- |
0.0478 |
- |
- |
- |
- |
- |
- |
- |
| Ni
232.003 |
- |
47.35 |
- |
- |
- |
6.187 |
- |
- |
- |
| Pb
220.353 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Sb
206.836 |
0.1 |
11.628 |
- |
- |
- |
- |
- |
- |
- |
| V
292.402 |
0.7907 |
- |
- |
- |
- |
- |
- |
0.8331 |
- |
| Zn
213.857 |
- |
- |
1.329 |
0.0518 |
- |
- |
4.691 |
0.2 |
- |
- The IEC factor2
is the apparent analyte concentration in ppb divided by
the interferent concentration in ppm and is calculated as
follows:
| IEC
Factor = |
Apparent Analyte Cocnc (ppb)
|
| Interferent Concn
(ppm) |
Where the apparent analyte concentration is
the connection of the interferent measured at the analyte
wavelength in the absence of the analyte.
The
corrected analyte concentration is calculated as
follows:
Corrected Analyte Concn (ppb)=
Measured
Analyte Concn (ppb) - (Interferent Concn (ppm) * IEC
Factor)
- A dash indicates that no interference
was observed on the analyte from the interfering analyte.
Interfering analytes were analyzed at the following
concentration:
200 ppm: Fe
100
ppm: Cr, Cu, Co, Mo, Ni
50 ppm: Mn, V, Ti
- IEC factors are instrument dependent.
Interferences listed in this table do not represent all
possible interferences.
1.7 Solubility and
Stability of Lead Sulfate
The solubility of lead
sulfate was investigated by weighing out various amounts
and placing it in a conical beaker, along with a Ghost
Wipe, and digested. The samples were reanalyzed four days
later to determine if any lead had precipitated out of
solution. Results were not blank
corrected.
Table 1.7.1
Lead Sulfate Solubility and
Stability |
|
|
|
|
day 1 |
day 4 |
| PbSO4used (mg) |
theoretical for lead* (µg) |
lead
found (µg) |
recovery
(%) |
lead
found (µg) |
recovery
(%) |
|
| 0.025 |
16.73 |
15.92 |
95.2 |
16.82 |
100.5 |
| 0.078 |
52.23 |
49.82 |
95.4 |
50.30 |
96.3 |
| 0.162 |
108.5 |
105.9 |
97.6 |
107.8 |
99.3 |
| 0.230 |
154.0 |
153.2 |
99.5 |
155.2 |
100.7 |
| 0.252 |
168.7 |
162.7 |
96.4 |
167.0 |
98.9 |
| 0.477 |
319.4 |
313.7 |
98.2 |
319.8 |
100.1 |
| 0.595 |
398.4 |
395.9 |
99.4 |
402.7 |
101.1 |
| 0.698 |
467.4 |
472.6 |
101.1 |
472.6 |
101.1 |
| 0.821 |
549.7 |
544.1 |
99.0 |
555.7 |
101.1 |
| 1.125 |
753.3 |
739.1 |
98.1 |
751.2 |
99.7 |
| 1.254 |
839.6 |
826.1 |
98.4 |
838.7 |
99.9 |
| 1.715 |
1148 |
1121 |
97.6 |
1144 |
99.6 |
| 2.025 |
1356 |
1326 |
97.8 |
1346 |
99.3 |
| 2.738 |
1833 |
1821 |
99.3 |
1845 |
100.6 |
| 4.584 |
3069 |
2993 |
97.5 |
3048 |
99.3 |
|
*Purity
of lead sulfate used was 98%
1.8 Analysis of three
NIST Standard Reference Materials
Approximately
100 mg of material was placed in a conical beaker along a
Ghost Wipe. Samples were digested and then filtered
through an MCE filter to remove all particulate. The MCE
filter for each sample, along with the remaining
particulate, were digested a second time. Results were not
blank corrected.
Table 1.8.1
Standard Reference Material
2580 Lead Paint |
| element |
sample 1
weight = 101.6 mg |
sample 2
weight = 100.9 mg |
sample 3
weight = 100.6 mg |
| first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
| Pb |
2466 |
1653 |
4409 |
93.4 |
2345 |
992.0 |
4379 |
76.2 |
1730 |
1674 |
4366 |
78.0 |
| Fe* |
533.5 |
27.08 |
508.0 |
110 |
520.0 |
17.50 |
504.0 |
107 |
513.0 |
24.42 |
503.0 |
107 |
| Zn* |
2842 |
127.1 |
3048 |
97.4 |
2790 |
83.95 |
3027 |
94.9 |
2750 |
114.2 |
3018 |
94.9 |
*Results not certified by NIST
Table 1.8.2
Standard Reference Material
2583 Trace Elements in Indoor Dust |
| element |
sample 1
weight = 103.8 mg |
sample 2
weight = 100.3 mg |
sample 3
weight = 100.4 mg |
| first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
| Cd |
0.565 |
0.000 |
0.757 |
74.6 |
0.695 |
0.000 |
0.732 |
94.9 |
0.465 |
0.000 |
0.733 |
63.4 |
| Cr |
5.215 |
0.900 |
8.304 |
73.6 |
5.585 |
0.905 |
8.024 |
80.8 |
5.395 |
0.755 |
8.032 |
76.6 |
| Pb |
6.820 |
0.365 |
8.916 |
80.6 |
6.425 |
0.050 |
8.616 |
75.2 |
6.710 |
0.160 |
8.624 |
79.7 |
Table 1.8.3
Standard Reference Material
1648 Urban Particulate Matter |
| element |
sample 1
weight = 101.4 mg |
sample 2
weight = 101.7 mg |
sample 3
weight = 101.1 mg |
| first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
first digest
(µg) |
second
digest (µg) |
theor
(µg) |
recovery
(%) |
| Pb |
608.0 |
30.88 |
664.2 |
96.2 |
618.0 |
33.12 |
666.1 |
97.8 |
595.0 |
30.35 |
662.2 |
94.4 |
| Cd |
7.185 |
0.255 |
7.605 |
97.8 |
7.215 |
0.280 |
7.628 |
98.3 |
7.185 |
0.270 |
7.583 |
98.3 |
| Cr |
10.38 |
3.990 |
40.86 |
35.2 |
9.595 |
2.920 |
40.99 |
30.5 |
10.76 |
3.185 |
40.74 |
34.2 |
| Cu |
53.95 |
2.925 |
61.75 |
92.1 |
54.70 |
3.020 |
61.94 |
93.2 |
53.50 |
2.645 |
61.57 |
91.2 |
| Mn |
71.80 |
4.101 |
79.70 |
95.2 |
73.63 |
4.495 |
79.93 |
97.7 |
71.44 |
3.766 |
79.46 |
94.6 |
| Ni |
6.600 |
0.490 |
8.315 |
85.3 |
6.660 |
0.500 |
8.339 |
95.9 |
6.760 |
0.530 |
8.290 |
87.9 |
| V |
9.805 |
0.990 |
12.88 |
83.8 |
9.725 |
1.070 |
12.92 |
83.6 |
9.680 |
0.900 |
12.84 |
82.4 |
1.9 Instrument
Analytical Conditions
| Instrument: |
Perkin-Elmer Optima
4300 DV ICP |
| Replicates: |
2 |
| Read Time: |
Auto, 5-20 sec |
| Sample Flow Rate: |
2.20 mL/min |
| Gas: |
Argon |
| Plasma Gas Flow: |
15 L/min |
| Auxiliary Gas
Flow: |
0.20 L/min |
| Nebulizer Gas
Flow: |
0.60 L/min |
| RF Power: |
1300 watts |
| Plasma View: |
Radial |
| View Distant: |
15.0 mm |
| Peak Algorithm: |
Peak Area |
| Overlap
Correction: |
IEC |
| Background
Correction: |
2-Point |
| Calibration: |
2-Point |
1 Lawrence, R. Evaluation Guidelines for Surface Sampling
Methods ; OSHA Salt Lake Technical Center, U.S.
Department of Labor: Salt Lake City, UT, 2001.
2 The Perkin-Elmer Corporation. WinLab32 Instrument Control Software Guide
; Norwalk, Ct, 1999.
| |

