Introduction
The procedure for the air sample collection and analysis of nitric
oxide (NO) is described in OSHA Method No.
This method has been evaluated near the OSHA Transitional Permissible Exposure Limit (PEL) for 240-min samples. At the time of this study, the Time Weighted Average (TWA) PEL for NO is 25 ppm. The Final Rule PEL is also 25 ppm as a TWA.
Test atmospheres were generated and samples were collected and analyzed according to the procedures listed below.
Generation System
All generations of NO test atmospheres, and hence all experiments, with two exceptions, were performed using the apparatus shown in Figure 1. The analysis (Section 1) and detection limit experiments did not use a test atmosphere generation for sample preparation. Instead, samples were spiked with solutions of sodium nitrite. For further details regarding the detection limit experiment, see reference 11.2.
A cylinder of NO in nitrogen (1.05% NO, Air Products and Chemicals, Long Beach, CA) was used as the contaminant source. The NO was mixed, using a glass mixing chamber, with filtered, tempered air. A flow, temperature, and humidity control system (Miller-Nelson Research Inc., Model HCS-301) was used to condition the diluent air for mixing. A Teflon sampling manifold was attached to the mixing chamber. Flow rates for the diluent air were determined using a dry test meter. Contaminant gas flows were measured using mass flow controllers and soap bubble flowmeters.
Sample Collection
Air samples were collected from the Teflon manifold using calibrated
SKC Model
- SKC NO2-NO collection device (SKC Cat. No. 226-40,
water-washed):
The sampling device consists of three separate glass tubes. A description of the tubes is given in reference 11.1. The SKC tubes used for all validation experiments were from lot no. 374 except for the storage stability experiment where lot no. 444 tubes were used. - Supelco combination tube:
This combination tube contains all three sections in a single tube. Two 400-mg sections ofTEA-IMS are separated by an oxidizer section. The Supelco tube uses a smaller mesh size of molecular sieve and only approximately 800 mg of oxidizer. Tubes from lot no. 564-07 were only used for a preliminary sampling and analysis experiment. Due to the low recoveries found during this preliminary study, further experiments using the Supelco combination tube were not performed.
Sample analysis
Note: The analytical portion of the method for NO is the same as the NO2 method; both analyses are performed by determining the amount of NO2- produced from the NO2-TEA reaction.
Samples prepared for all experiments were analyzed by IC using the conditions specified in the method (11.1.). For the conversion of NO2 to nitrite, a conversion factor (C.F.) of 0.72 was first reported (11.3.). Later experiments indicated an average C.F. of 0.63 (11.2., 11.4.-11.5.). The 0.63 C.F. was used for all experiments in this evaluation which were conducted with concentrations less than 10 ppm NO. A C.F. of 0.5 was used for concentrations above 10 ppm NO.
Sample Results
Results were calculated using peak areas and linear regression concentration-response curves. A statistical protocol (11.6.) was used to evaluate results. Any calculation of error follows the general formula:
Errori = ± [|mean biasi| + 2CVi] × 100% (95% confidence)
where i is the respective sample pool being examined
Data were subjected to the Bartlett's test (11.7.) and a test for outliers (11.8.) to determine homogeneity of variance and identify any outliers. Both tests were conducted using the 99% confidence level.
Validation
The following experiments were conducted for the validation of Method
No.
- Analysis - Desorption efficiency (DE) of spiked samples.
- Sampling and Analysis - generation and analysis of NO samples.
- Collection efficiency.
- Breakthrough tests.
- Storage stability.
- Sampling at different humidities.
- Determination of the conversion factor for NO concentrations of 10 to 200 ppm.
- Sampling and analysis of a mixture of NO and NO2.
This analytical method was also compared to the polarographic method
previously used by the OSHA laboratory. This method comparison and the
detection limit determinations were performed during the NO2
method validation (See reference 11.2.
for more information). The quantitative detection limit was determined to
be 0.08 µg/mL
A preliminary sampling and analysis experiment using Supelco tubes was also performed and is discussed in Section 9.
1. Analysis (Desorption Efficiency, DE)
Procedure: Eighteen spiked samples (6 samples at each test
level) were prepared and analyzed. Samples were prepared by spiking known
amounts of sodium nitrite solutions into
Results: The results are listed in Table 1. Recoveries at these levels represent analytical DE. Results also provide recoveries, analytical error (AE), and extent of variability for the analytical portion of the method.
All analysis data passed the Bartlett's and outlier tests. Sample results were pooled. The analytical data for the method (Table 1) gave acceptable precision and accuracy (11.7.) and does not indicate a need for a desorption correction factor. The coefficient of variation for analysis (CV1) was 0.045 and the average analytical recovery was 107.3%.
2. Sampling and Analysis
Procedure: A total of 20 samples were collected from dynamically generated test atmospheres and analyzed. The concentrations generated were about 0.5, 1, and 2 times the PEL. The generation system shown in Figure 1 was used. Samples were taken for 240 min at a RH and temperature of 50% and 25°C, respectively.
Results: The results, as shown in Table 2, provide the overall error (OE) and precision of the sampling and analytical method. Overall error should be less than ±25% when calculated using the equation listed in the Introduction.
The Sampling and Analysis data show acceptable precision and accuracy (11.7.). All data passed both the outlier and Bartlett's test and the results were pooled. The coefficients of variation for spiked CV1 (pooled) samples, generated CV2 (pooled) samples and overall CVT (pooled) are:
The sampling and analytical bias was +3.3%. Overall error was within
guidelines
3. Collection Efficiency
Procedure: Dynamically generated samples were used to measure
the sorbent collection efficiency at the upper concentration limit (50 ppm
NO) of the validation. Six SKC sampling devices were connected to backup
1)
This train was used to collect NO at 2 times the OSHA PEL for 240 min.
A pump flow rate of approximately 0.025 L/min was used. The amount of NO
collected in each
Results: Results are reported in Table 3. The collection efficiency was calculated as:
| % Collection Efficiency = | µg NO found in tube 3
µg NO found in tube 3 + tube 4 |
× 100% |
Collection efficiency was 100% at 2 times the PEL, which indicates the sorbent media has adequate capacity for collecting NO within the validation range.
4. Breakthrough
Procedure: Test atmospheres were generated at a concentration greater than the validation level to determine if any breakthrough of NO occurs from the primary solid sorbent sampling tube (following the oxidizer) into a second tube. Breakthrough is considered significant if the concentration collected with the second tube is >5% of the results from the first tube. Twelve sampling devices were connected to backup tubes (as mentioned in Section 3.) and then to sampling pumps. All samples were collected at a concentration of 200 ppm and 0.025 L/min flow rate. Three sampling devices were removed from the generation system at 60, 120, 180, and 240 min. The generation system was set at 30% RH and 25°C. The low humidity level was used as a "worst case" test since the presence of water is necessary for the conversion reaction of NO2 to NO2- to proceed (11.1., 11.4.).
Results: Results are shown in Table 4. The extent of breakthrough was assessed by:
| % Breakthrough = | µg NO found in tube 4
µg NO found in tube 3 + tube 4 |
× 100% |
Breakthrough studies indicate the SKC sorbent tube and oxidizer capacity for NO is adequate for air concentrations up to 200 ppm when using air volumes and flow rates described. Further research to determine the actual breakthrough concentration was not conducted. It should be unlikely that industrial environments will exceed an exposure of eight times the PEL.
5. Storage Stability
Procedure: A study was conducted to determine if any storage
problems existed for
5.1. Twelve samples were collected at the OSHA PEL as described in the Introduction.
5.2. These samples were stored at 20 to 25°C on a laboratory bench for the duration of the storage period.
5.3. Three samples were analyzed at 0, 5, 15, and 30 days.
Results: The results of the storage stability study are shown in Table 5. The mean of samples analyzed after 30 days was within ±5% of the mean of samples analyzed after 1 day. Samples may be stored in environmental conditions found in a laboratory setting for 30 days without a significant change in results.
6. Humidity Study
Procedure: A study was conducted to evaluate any effects on recovery when sampling at different humidities. Contaminant atmospheres conditioned at 30, 50, and 80% RH were generated at 25°C. Six or seven SKC sampling devices were used at each RH level.
Results: Results are shown in Table 6. Data from sampling at different humidities displayed an apparent effect on sampling efficiency. As shown in Table 6, an analysis of variance (F test) was performed on the data to determine if a significant difference in the results existed from changes in humidity. Sample recoveries and OE for the three different humidity levels were also considered. The calculated F value is greater than the critical value and a significant effect from humidity appears to exist. A slight decrease in average recovery is apparent at low humidity (30% RH); however, results are still within OE limits (< ±25%) and corrective action when sampling at low humidities appears unnecessary.
7. Conversion Factor (C.F.)
As described in OSHA Method No.
Procedure: The following two procedures were used to experimentally determine the C.F. for concentrations greater than 10 ppm.
7.1. Determination of C.F. using oxidation of NO
- 7.1.1. The same generation system shown in Figure
1 was used. Nitrogen dioxide was produced by flowing a diluted
NO mixture through SKC oxidizer sections.
7.1.2. The generation system was set at 50% RH and 25°C.
7.1.3. The NO2 produced was then collected using impingers containing 1.5% TEA solutions. Variable time periods (30 to 360 min) and different concentration ranges were used. The TEA solutions were used in an attempt to avoid any extraneous background contribution or intrinsic contamination that is sometimes noted when using the impregnated solid sorbent. Samples were taken at a flow rate of about 0.025 L/min primarily to assure complete oxidation of the NO and secondarily to provide sufficient residence time of NO2 in the TEA solutions.
7.2. Determination of C.F. using NO2 permeation tubes
- 7.2.1. A second study was performed using permeation tubes
(Thermedics Inc., Woburn, MA) as the NO2 source. The
system was setup as mentioned in reference 11.2.
7.2.2. The generation system was set at 50% RH and 25°C.
7.2.3. Samples were taken using impingers containing 1.5% TEA. Flow rates of 0.15 mL/min were used to collect samples for 30 to 60 min (Note: A higher sample flow rate was possible because NO2 was used instead of NO).
Results: The results for C.F. calculations from about 1 to 193 ppm are listed in Table 7. This data shows the C.F. for the 10 to 100 ppm concentration range averaged approximately 0.50; at about 200 ppm the factor apparently decreased to 0.37. Further work may be necessary to determine why the factor decreased at the 200 ppm level. As mentioned in Section 4, no breakthrough was found on backup tubes when sampling at 200 ppm.
Proposed curve fits for the C.F. are shown in Figure 2a and Figure 2b. Figure 2b is an expanded scale version of Figure 2a. As a comparison with other authors experiments, some of the data (<15 ppm NO2) used in the curve fit were taken from the following studies found in literature:
| NO2 ppm | C.F. | Literature Source (reference no.) | ||
|
|
|
| ||
| 0.01 | 1* | 11.13., 11.14. | ||
| 3.4 | 0.73 | 11.4., 11.5. | ||
| 9.05 | 0.61 | 11.4., 11.5. | ||
| 10.7 | 0.56 | 11.4., 11.5. | ||
| * The first data set (0.01, 1) is used to force a value of unity for a concentration well below the limit of detection. The C.F. value of unity was determined only for a passive monitor (11.13., 11.14.) where the NO2 concentration at the monitor face is apparently very low (11.13.). | ||||
The conversion factor appears to follow either general curve fit:
| Y = (a) x (NO)b | (1) | ||
| or | |||
| Y = (a) + (b) × ln(NO) | (2) | ||
where:
Y = calculated C.F. NO = uncorrected ppm NO a = slope; for equation (1), a = 0.7140, for (2), a = 0.7372 b = intercept; for equation (1), b = -0.09714, for (2), b = -0.06368
The standard deviation about the regression line (Sy/X) for (1) was 0.0536 and 0.0393 for equation (2).
According to the reaction proposed by Gold (11.4.), NO2- and triethanolammonium nitrate are formed in the reaction of NO2 with TEA. The amount of nitrate (NO3-) produced has not been documented at different NO2 concentrations. As can be seen by Figure 2a and Figure 2b, as the concentration of NO2 (or NO) decreases, the subsequent formation of NO2- (in relation to NO2) increases. As the NO2 concentration decreases, theoretically the NO3- concentration should also decrease. Although bubblers with TEA solutions were used at one point in the experiment in an attempt to rule out NO3- contamination, the NO3- concentrations could not be confirmed due to the apparent contamination of NO3- found in the generation system and sorbent material. The measured concentration of NO3- did not appear to change in relation to NO2 concentration. Comparison of the ratios of peak areas for the two analytes (NO2-/NO3-) across the concentration range tested gave variable, almost random results. When considering NO2 concentrations below 25 ppm, this ratio would be expected to increase as the concentration of NO2 decreases.
The correction for the conversion of NO2 to NO2- has been approximated using an average C.F. of 0.63 for less than 10 ppm NO (or NO2) and 0.50 for concentrations above 10 ppm. A computer simulation using the approximate 0.63 and 0.5 C.F. values for a concentration range of 1 to 100 ppm gave results within +11% of those calculated using equation (1). The approximate C.F.s were within +5% of the calculated factors for most of the concentration range. The greatest disagreement between calculated and approximate C.F.s occurs at about 10 ppm.
The two approximate C.F. values were used for all data contained in this backup report and were recommended in the method (11.1.). These two C.F. values appeared to be more convenient to use and the potential difference between calculated and approximate C.F. values in the concentration range tested is minor.
Further work to accumulate a larger data base of C.F. values and consequently more accurate slope and intercept values should be performed before extensive use of these equations (especially below 1 ppm NO2). This work may also reveal whether one equation is more suitable to use. Also, a more controlled study of the NO3- concentration and contamination may shed light on the reaction mechanism at low concentrations.
8. Sampling and Analysis of a Mixture of NO and NO2
Procedure: A determination of the ability of the three-tube sampling device to sample NO/NO2 mixtures was assessed. A mixture of NO and NO2 was generated using equipment described in the Introduction (for NO) and as mentioned in reference 11.2. (for NO2). Samples were taken using the sampling device for 1 h at a flow rate of 0.15 L/min (50% RH and 25°C).
Results: Results are shown in Table 8. The mixture study indicates the sampling tube is capable of collecting a mixture of NO and NO2 at their respective PEL concentrations for 1 h.
9. Sampling and Analysis - Supelco Tubes
Procedure: A preliminary evaluation of the combination device manufactured by Supelco was conducted using the same conditions and equipment mentioned in the Introduction. Samples were collected using the procedure mentioned in Section 2. Two sets of six samples were taken at the PEL and 50% RH. A sampling flow rate of about 0.025 L/min and a sampling time of 4 h was used.
Results: Results are listed in Table
9. The Supelco tube results indicate extremely variable and mainly low
recoveries when sampling at the PEL. The oxidizer in the Supelco tube
contained only about 800 mg and may have contributed to the low recovery
by not having sufficient oxidizing power to convert all of the NO to
NO2. Preliminary tests conducted by NIOSH (11.12.,
11.15.)
indicated 800 mg of oxidizer gave significantly lower recoveries for NO
concentrations greater than 12 ppm. The SKC tubes tested for this
evaluation (Method No.
10. Discussion
The data generated during the validation indicate this method is an acceptable alternative to the polarographic method. The ion chromatographic method offers an accurate and precise determination of compliance with the OSHA 25 ppm TWA PEL for NO. A concentration-dependent conversion factor is required in calculations. Although data was not presented in this backup report regarding sorbent contamination, previous studies have indicated serious contamination problems (11.2., 11.16.). The molecular sieve solid sorbent must be washed with deionized water before impregnation and tube packing. This water washing will remove any soluble contaminants such as chloride or nitrite salts present in the molecular sieve. An attempt to identify the NO2-TEA reaction products has been performed (11.17.); however, future work needs to be conducted to further identify and characterize the mechanism and conversion factors of this reaction.
11. References
- 11.1. Occupational Safety and Health
Administration Technical Center: Nitric Oxide in Workplace
Atmospheres by J.C. Ku (USDOL/ OSHA-SLTC Method No.
11.2. Occupational Safety and Health Administration Technical Center: Nitrogen Dioxide Backup Data Report (ID-182), by J.C. Ku. Salt Lake City, UT. Revised 1991.
11.3. Saltzman, B.E.: Colorimetric Microdetermination of Nitrogen Dioxide in the Atmosphere. Anal. Chem. 26:1949 (1954).
11.4. Gold, A.: Stoichiometry of Nitrogen Dioxide Determination in Triethanolamine Trapping Solution. Anal. Chem. 49:1448-1450 (1977).
11.5. Blacker, J.H.: Triethanolamine for Collecting Nitrogen Dioxide in the TLV Range. Am. Ind. Hyg. Assoc. J. 34:390 (1973).
11.6. Occupational Safety and Health Administration Analytical Laboratory: Precision and Accuracy Data Protocol for Laboratory Validations. In OSHA Analytical Methods Manual. Cincinnati, OH: American Conference of Governmental Industrial Hygienists (Pub. No. ISBN: 0-936712-66-X), 1985.
11.7. National Institute for Occupational Safety and Health: Documentation of the NIOSH Validation Tests by D. Taylor (DHEW/NIOSH Pub. No. 77-185). Cincinnati, OH, 1977.
11.8. Mandel, J.: Accuracy and Precision, Evaluation and Interpretation of Analytical Results, The Treatment of Outliers. In Treatise on Analytical Chemistry. 2nd ed. edited by Kolthoff, I.M. and P.J. Elving. New York: John Wiley and Sons, Inc., 1978. p 282.
11.9. Morgan, G.B., C. Golden, and E.C. Tabor: "New and Improved Procedures for Gas Sampling and Analysis in the National Air Sampling Network" Paper presented at the 59th Annual Meeting of the Air Pollution Control Association, San Francisco, CA, 1966.
11.10. Occupational Safety and Health Administration Analytical Laboratory: OSHA Analytical Methods Manual (USDOL/OSHA-SLCAL Method No. ID-109). Cincinnati, OH: American Conference of Governmental Industrial Hygienists (Pub. No. ISBN: 0-936712-66-X), 1985.
11.11. National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 2nd ed., Vol. 4 (DHEW/NIOSH Pub. No. 78-175, Method No. S321). Cincinnati, OH, 1978.
11.12. Willey, M.A., C.S. McCammon, Jr., and L.J. Doemeny: A Solid Sorbent Personal Sampling Method for the Simultaneous Collection of Nitrogen Dioxide and Nitric Oxide in Air. Am. Ind. Hyg. Assoc. J. 38:358-363 (1977).
11.13. Palmes, E.D., A.F. Gunnison, J. DiMattio and C. Tomczyk: Personal Sampler for Nox. Am. Ind. Hyg. Assoc. J. 37:570-577 (1976).
11.14. National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 3rd ed. (Method 6700) edited by P.M. Eller (DHHS/NIOSH Pub. 84-100), Washington, D.C.: Government Printing Office, 1984.
11.15. Jones, V., and T.A. Ridjik: Nitric oxide oxidation method for field calibration of nitrogen dioxide meters. Am. Ind. Hyg. Assoc. J. 41:433-436 (1980).
11.16. Occupational Safety and Health Administration Analytic Laboratory: Special Project - Evaluation of TEA Tubes for Contamination. by D.C. Cook. Salt Lake City, UT. 1985 (unpublished).
11.17. Aoyama, T., Yashiro, T.:
Investigation of the reaction by trapping nitrogen dioxide in air
using the triethanolamine method. J. Chromatogr.
Analysis - Nitric Oxide
|
| |||||||
| µg* Taken | µg* Found | F/T | N | Mean | Std Dev | CV | AE |
| (0.5 × PEL) | |||||||
| 103.04 103.04 103.04 103.04 103.04 103.04 |
105.19 110.45 105.26 117.47 113.68 111.08 |
1.0209 1.0719 1.0215 1.1400 1.1033 1.0780 |
|||||
| 6 | 1.073 | 0.046 | 0.043 | 15.9 | |||
| (1 × PEL) | |||||||
| 206.09 206.09 206.09 206.09 206.09 206.09 |
226.24 239.92 226.80 241.83 215.14 210.37 |
1.0978 1.1642 1.1005 1.1734 1.0439 1.0208 |
|||||
| 6 | 1.100 | 0.062 | 0.056 | 21.2 | |||
| (2 × PEL) | |||||||
| 412.17 412.17 412.17 412.17 412.17 412.17 |
415.69 447.42 422.43 429.73 448.54 424.95 |
1.0085 1.0855 1.0249 1.0426 1.0882 1.0310 |
|||||
| 6 | 1.047 | 0.033 | 0.031 | 11.0 | |||
| * Results are listed as micrograms nitric oxide. These values already have the Conversion Factor applied. | |||||||
|
| |||||||
F/T = Found/Taken = Desorption Efficiency AE = Analytical Error (±%) Bias = +0.073 CV1 (Pooled) = 0.045 Analytical Error (Total) = ±16.3%
Sampling and Analysis - Nitric Oxide
|
| |||||||
| ppm* Taken | ppm* Found | F/T | N | Mean | Std Dev | CV | OE |
| (0.5 × PEL) | |||||||
| 13.04 13.04 13.04 13.04 13.04 13.04 13.04 |
10.70 12.57 12.55 12.58 13.77 14.87 14.17 |
0.8206 0.9640 0.9624 1.0560 1.1403 1.0867 |
|||||
| 7 | 0.999 | 0.105 | 0.105 | 21.1 | |||
| (1 × PEL) | |||||||
| 25.93 25.93 25.93 25.93 25.93 25.93 |
27.04 26.51 26.23 28.99 28.92 29.55 |
1.0428 1.0224 1.0116 1.1180 1.1153 1.1396 |
|||||
| 6 | 1.075 | 0.056 | 0.052 | 17.8 | |||
| (2 × PEL) | |||||||
| 50.52 50.52 50.52 50.52 50.52 50.52 50.52 |
54.02 48.50 48.77 48.29 57.02 55.49 52.87 |
1.0693 0.9600 0.9654 0.9559 1.1287 1.0984 1.0465 |
|||||
| 7 | 1.032 | 0.072 | 0.069 | 17.1 | |||
| * Results are listed as ppm nitric oxide | |||||||
|
| |||||||
F/T = Found/Taken OE = Overall Error (±%) Bias = +0.033 CV2 (Pooled) = 0.080 CVT (Pooled) = 0.082 Overall Error (Total) = ±19.7%
Collection Efficiency - Nitric Oxide
(25°C and 50% RH)
|
| |||||||||
| ----------µg NO Found in---------- | |||||||||
| Sample No. | First Tube | Second Tube | % Collection Efficiency | ||||||
|
|
|
|
| ||||||
| 1 2 3 4 5 6 7 |
277.95 215.05 254.07 258.54 292.02 279.74 265.27 |
ND ND ND ND ND ND ND |
100 100 100 100 100 100 100 | ||||||
| |||||||||
|
| |||||||||
Breakthrough Study - Nitric Oxide
(25°C, 30% RH)
|
| ||||||||||||||||
| ----------µg NO Found in---------- | ||||||||||||||||
| Time, Min | n | First Tube | Second Tube | % Breakthrough | ||||||||||||
|
|
|
|
|
| ||||||||||||
| 60 120 180 240 |
3 3 3 3 |
291.18 657.64 960.63 1,074.23 |
ND ND ND ND |
0 0 0 0 | ||||||||||||
| ||||||||||||||||
|
| ||||||||||||||||
Storage Stability* - Nitric Oxide
|
| |||||
| Storage Day | Found µg | Air Vol (L) | Found ppm | Taken ppm | % Recovery |
|
| |||||
| Day 1 | 361.30 358.45 374.25 |
6.45 6.37 6.66 |
29.77 29.91 29.87 |
28.45 28.45 28.45 |
104.6 105.1 105.0 |
| n Mean Std Dev CV |
3 104.9 0.26 0.0025 | ||||
| Day 3 | 345.52 348.59 345.59 |
6.58 6.45 6.66 |
27.91 28.72 27.59 |
28.66 28.66 28.66 |
97.4 100.2 96.3 |
| n Mean Std Dev CV |
3 98.0 2.0 0.021 | ||||
| Day 15 | 370.67 339.51 331.44 |
6.60 6.31 6.66 |
29.85 28.60 26.45 |
28.66 28.66 28.66 |
104.2 99.8 92.3 |
| n Mean Std Dev CV |
3 98.8 6.01 0.061 | ||||
| Day 30 | 362.52 366.26 353.78 |
6.59 6.40 6.72 |
29.24 30.42 27.98 |
28.51 28.51 28.51 |
102.6 106.7 98.1 |
| n Mean Std Dev CV |
3 102.4 4.30 0.042 | ||||
| * SKC sampling devices, Lot No. 444 were used | |||||
|
| |||||
Humidity Test (25°C) - Nitric Oxide
|
| |||
| % RH | 30 | 50 | 80 |
|
|
|
|
|
| NO Found, ppm | 22.94 23.51 22.60 22.67 26.11 24.87 25.18 |
27.04 26.51 26.23 28.99 28.92 29.55 |
26.73 26.54 25.49 25.70 31.13 27.81 |
| n Mean, ppm Std Dev, ppm CV Known Conc., ppm Recovery, % |
7 23.98 1.40 0.058 26.17 91.6 |
6 27.87 1.44 0.052 25.93 107.5 |
6 27.23 2.08 0.076 25.78 105.6 |
|
| |||
| F test
results: Fcalc = 10.5 Fcrit = 6.23 p <0.01 df = 2, 16 | |||
|
| |||
Nitrogen Dioxide Conversion Factor
|
| |||||
| NO2 ppm* | n | Std Dev | CV | Average C.F.** | Source |
|
|
|
|
|
|
|
| 0.82 12.89 13.72 15.74 19.85 25.20 39.65 49.79 77.85 97.90 158.57 192.57 |
4 7 5 5 4 7 5 6 5 6 5 7 |
0.082 0.038 0.023 0.037 0.032 0.037 0.031 0.022 0.024 0.020 0.018 0.025 |
0.150 0.074 0.041 0.072 0.063 0.070 0.058 0.043 0.050 0.044 0.042 0.068 |
0.817 0.519 0.569 0.513 0.509 0.533 0.529 0.517 0.480 0.450 0.437 0.368 |
1 1 1 2 2 1 2 1 2 1 2 1 |
|
| |||||
| * NO2 ppm <=>
NO ppm
n = number of samples - collection media for all samples was 1.5% TEA solution ** Average C.F. (conversion factor) was calculated from sample results assuming 100% recovery Source 1 = NO cylinder + oxidizers Source 2 = NO2 permeation tubes | |||||
|
| |||||
Nitrogen Dioxide - Nitric Oxide Mixture Study
(25°C & 50% RH)
|
| ||||
| Nitrogen Dioxide | Nitric Oxide | |||
|
|
| |||
| Air Vol, L | Found ppm | Taken ppm | Found ppm | Taken ppm |
|
|
|
|
|
|
| 7.61 8.14 9.16 7.61 8.14 9.16 |
5.38 5.34 5.52 5.25 6.48 4.82 |
5.24 5.24 5.24 5.24 5.24 5.24 |
25.91 26.24 28.23 25.26 34.74 23.26 |
28.76 28.76 28.76 28.76 28.76 28.76 |
| n Mean Std Dev CV Recovery |
6 5.47 0.55 0.101 104.4% |
6 27.27 3.99 0.146 94.8% |
||
|
| ||||
Preliminary Sampling & Analysis - Nitric Oxide
Supelco Tubes
|
| |||||||
| ppm* Taken | ppm* Found | F/T | N | Mean | Std Dev | CV | OE |
| (1 × PEL Set 1) | |||||||
| 25.96 25.96 25.96 25.96 25.96 25.96 25.96 |
6.07 20.14 22.02 20.42 9.99 26.62 10.52 |
0.234 0.776 0.848 0.787 0.385 1.025 0.405 |
|||||
| 7 | 0.637 | 0.294 | 0.461 | 128. | |||
| (1 × PEL Set 2) | |||||||
| 26.08 26.08 26.08 26.08 26.08 26.08 26.08 |
13.22 22.34 9.63 22.47 4.88 8.46 9.19 |
0.507 0.857 0.369 0.862 0.187 0.324 0.352 |
|||||
| 7 | 0.494 | 0.266 | 0.539 | 158. | |||
| * Results are listed as ppm nitric oxide | |||||||
|
| |||||||
F/T = Found/Taken OE = Overall Error (±%) Supelco tubes, lot no. 564-07, were used.
A block diagram of the major components of the dynamic generation system is shown below. The system consists of four essential elements, a flow, temperature and humidity control system, a nitric oxide vapor generating system, a mixing chamber and an active sampling manifold.
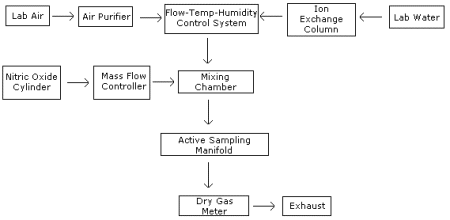
Figure 1
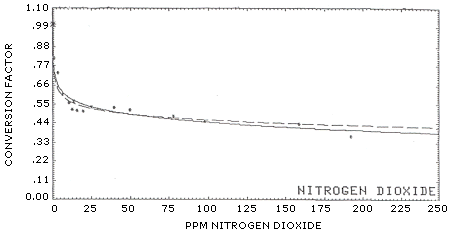
| Solid Line Broken Line |
y = (a) + (b) × ln(X) y = (a) × (X)b |
| See Section 7 of the text for further descriptions | |
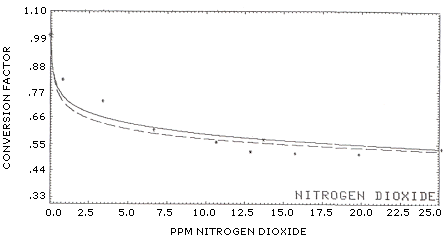
| Solid Line Broken Line |
y = (a) + (b) × ln(X) y = (a) × (X)b |
| See Section 7 of the text for further descriptions | |