1. Introduction
This method describes the collection and analysis of
airborne hydrogen chloride using Ion Chromatography. Other acids which
can be collected and analyzed simultaneously are HBr,
H3PO4, HNO3, and
H2SO4.
1.1 History (9.1,
9.2)
Prior to the use of this method, HCl was collected using 0.1
N NaOH in an impinger and analyzed by specific
ion electrode, or by adding AgNO3 and measuring the turbidity
of the AgCl formed.
1.2 Uses (9.3)
Hydrogen chloride is
used in the manufacture of pharmaceutical hydrochlorides, making vinyl
chloride from acetylene, alkyl chlorides from olefins, and arsenious
chloride from arsenious oxide. Hydrogen chloride is also used in the
chlorination of rubber and in organic reactions involving isomerization,
polymerization, and alkylation. HCl is also used to make chlorine, when
it is economical.
1.3 Physical and Chemical Properties
(9.4, 9.5)
Specific
Gravity:
Melting Point:
Boiling Point:
Molecular
Weight: |
1.268
-114.8°C
-84.9°C
36.46 | 2. Working Range and Detection Limit
2.1 The working range for a 7.5-liter air sample is 0.01
to 1.0 ppm for Cl¯ for a 10-mL sample volume. This corresponds to 0.10
to 10.0 µg of chloride. The upper range can be extended by sample
dilution.
2.2 The qualitative detection limits for Cl¯ were
calculated using the Student's T-Test. The detection limit for Cl¯ is
0.10 µg at a confidence level of 95%. This detection limit was
calculated based on a sample volume of 10 mL and an injection volume of
100 µL. The detection limit may be improved by using a larger injection
volume (for auto sampler only), or by using a smaller volume than 10 mL
to desorb the sample.
3. Stability, Collection
Efficiency, and Coefficient of Variation.
3.1 The storage stability of HCl on silica gel tubes was
found to be acceptable. Aqueous HCl was spiked onto 9 silica gel tubes
at a level corresponding to 1.0 × PEL. Air with 80% humidity and 25°C
was pumped through the tubes at 0.5 Lpm for 15 minutes. Three tubes were
analyzed on that day (Day 0). Three tubes each were analyzed 7 and 14
days later. There were no significant losses of HCl.
| SAMPLE |
DAY |
PPM |
AVE |
STD.
DEV. |
| |
|
|
|
|
1STA
2STA
3STA
4STA
5STA
6STA
7STA
8STA
9STA |
0
0
0
7
7
7
14
14
14 |
6.12
7.31
6.38
6.06
6.40
5.94
5.46
6.10
6.64 |
6.60
6.13
6.07 |
0.511
0.192
0.482 |
All samples were re-analyzed on day 14, and it was
found that there were no losses. This suggests that once the tubes were
desorbed the HCl is not lost for at least 2 weeks.
3.2
Aqueous HCl was spiked onto 9 silica gel tubes at a level corresponding
to 1.0 × PEL. Air with 80% humidity and 25°C was pumped through the
tubes at 0.5 Lpm for 15 minutes. Part B of the sample were analyzed and
no breakthrough was found. This is in accordance with a NIOSH study
(9.6) which found that the collection efficiency of silica gel tubes in
generated HCl test atmospheres was 100%.
3.3 The
coefficient of variation (CVA) for the analytical method in
the range of 35 to 114 µg Cl¯ was 0.225 (9.7).
4. Interferences
Large quantities of fluoride will cause some masking of
the chloride peak.
5. Advantage and Disadvantages
5.1 The method can be automated and is quick and
accurate.
5.2 Thin procedure uses silica gel tubes instead of
impingers which are used in other sampling methods for HCl. This
eliminates the inherent problems of using impingers.
5.3
Unlike previous methods, particulates are not an interference in this
method, since they can be captured on the glass fiber filter in the tube
and analyzed separately, if necessary.
6. Sampling Procedure
6.1 Apparatus - Silica gel sorbent tubes, SUPELCO, Inc.
ORBO-53 (or equivalent silica gel tubes which have been demonstrated to
show low levels of the anions of interest), personal sampling pump with
calibrated flow in line with a silica gel tube to an accuracy of ±10% at
the 95% confidence limit at the recommended flow rate.
6.2
The silica gel tube is attached to a calibrated personal sampling pump
and the sampling tube is placed in the sampling area or worker's
breathing zone. At a flow rate of 0.5 liters per minute, 7.5 liters of
air are drawn through the sampling tube.
6.3 After
sampling, the silica gel tube is removed from the tubing, sealed and
identified with OSHA Form 21, and shipped to the Laboratory for
analysis.
6.4 With each batch of up to 20 samples, a blank
tube which has had no air drawn through it is submitted for analysis.
The blank tube should be from the same lot of tubes used for
sampling.
6.5 It is very important to list as interferences
any particulate acids or salts known to be present In the workplace
atmosphere.
7. Analytical Procedure
7.1 Apparatus - Ion exchange chromatograph, equipped
with electrical conductivity detector and recorder, or integrator, 10 mL
pipette, 1 mL plastic syringe with male Luer fitting, Anion Separator
Column 3 × 250 mm with Concentrator Column, Anion Suppressor Column 10 ×
100 mm, and appropriate volumetric glassware for dilutions and standard
preparation.
7.2 Reagents - All reagents used should be ACS
analyzed reagent grade or better.
7.2.1 Deionized water with a specific conductance of
10 µmho/cm or less for preparation of eluents and other solutions
which will be used in the analysis.
7.2.2 Chloride Stock
Standard (1000 µg/mL Cl¯) -- Dissolve 1.648 g NaCl and dilute to 1
liter with deionized water. Chloride working standards are made by
diluting the stock solution with standard eluent.
7.2.3
Standard Eluent (0.003 M
CO3=/0.0024 M
HCO3¯) Dissolve 5 g Na2HCO3 and 5 g
NaHCO3 in 20-liter carboy with deionized
water.
7.2.4 Regenerant Solution (1 N H2S04). Carefully add 111
mL of concentrated H2SO4 to 2 liters of
deionized water and dilute to 4 liters.
7.3 Safety
Precautions
7.3.1 When using the Ion Chromatograph, the column
door should be kept closed during the analysis in case the columns
burst. To avoid this danger the pressure should be checked at the
beginning of the analysis and periodically during the analysis. The
pressure should not exceed 500 psi.
7.3.2 Care should be
used when handling reagents, especially the regenerant solution (1
N H2SO4), to avoid
chemical burns.
7.3.3 Care should be exercised when using
laboratory glassware. Chipped pipettes, volumetric flasks, beakers, or
any glassware with sharp edges exposed should not be used in order to
avoid the possibility of cuts, abrasions, and lost
samples.
7.3.4 Pipetting should never be done by mouth -- a
bulb should always be used.
7.4 Standard Preparation
7.4.1 Working standards are prepared in the analytical
range of 0.2 µg/mL to 50 µg/mL from dilutions of the 1000 µg/mL stock
solution. These standard solutions should be prepared fresh
weekly.
7.4.2 If an auto sampler capable of variable
volume injections is used, a 3 µg/mL Cl¯ standard is used. This
intermediate working standard should be prepared fresh monthly.
7.5 Sample Preparation
7.5.1 The sample tube used with this analysis can be
separated into 3 parts. The first part is the glass fiber filter plug
which collects any particulate. The second part is a silica gel backup
section (section A) which collects the acid mists. The third part is a
silica gel section (section B) which collects any acid mists not
collected by section A. The second and third parts are separated by a
foam plug, which is discarded.
7.5.2 Score the sample
tube with a file in front of the primary sorbent section (section A)
and break the tube at the score line. Transfer the glass fiber filter
plug and section A to a clean 20 mL vial. If the analysis is to be
done only for HCl, the glass fiber filter plug can be discarded. If
sulfuric and/or phosphoric acids are requested, the glass fiber filter
plug must be analyzed separately.
7.5.3 Place silica gel
section B in a separate clean 20 mL vial.
7.5.4 If the air
volume is greater than or equal to 1liter, pipette about 5 mL of
eluent (0.003 M
CO3=/0.0024 M
HCO3¯) into each sample vial and cap tightly. If the air
volume is less than 1 liter, a smaller volume of eluent is
used.
7.5.5 Place the vial in a large beaker with DI
water and boil for 10 minutes. Let cool and dilute to 10.0 mL with
eluent in a volumetric flask (if the air volume is less than 1 liter,
dilute to 5 mL in a volumetric flask). When particulate acids are
listed as interferences, the glass fiber plug should be discarded.
Sample solutions which are not clear should be filtered before
analysis.
7.5.6 If using an auto sampler, transfer some
of the sample into an appropriate sampling vial. The vial should be at
least half full. Label each vial with the appropriate laboratory
identification number.
7.5.7 For hand injection, use 1 mL
of the eluent to flush the 0.1 mL injection loop thoroughly. When
using automatic injection try to use about a 100 µL injection volume.
The autosampler is less accurate below 100 µL.
7.6
Analysis
7.6.1 For general instrument set up refer to
Section 7 of the Ion Chromatography Standard Operating Procedure
(9.8)
7.6.2 The normal instrument parameters
are:
Sensitivity: 30 µmho full scale
Eluent: 0.003 M Na2CO3 and 0.0024 M NaHCO3
Flow Rate: 138 mL/hr,
approximately 30% on vernier
Concentrator Column: 3-mm I.D. ×
50-mm
Anion Separator Column: 3-mm I.D. × 250-mm
Suppressor
Column:10-mm I.D. × 100-mm
Run Time: Approximately 20 minutes,
depending upon analytical conditions.
7.6.3 With the instrument
set up and stabilized, place the auto sampling vial into the sampling
tray using tray positions one through five for standards.
7.6.4
Enter the proper parameters into the auto sampler. See Section 4 of
the Ion Chromatography Standard Operating Procedure (9.8)
7.6.5
Start the auto sampler and observe the first few chromatograms to
ensure proper operation. Periodically check the zero offset between
samples to correct any baseline drift and to ensure proper sensitivity
and retention time of the analytes.
7.6.6 Use the timer to stop
the run if the auto sampler is to be left unattended.
7.6.7 For
hand injection, a 1-mL aliquot is taken up in a syringe from the 20-mL
vial and injected into the injection port with the toggle switch in
the load position. After the sample is loaded, switch the toggle to
the inject position and start the integrator or push the PIP button if
a strip chart recorder is being used.
7.6.8 For both hand
and auto sample injections, record the sample number onto the
chromatogram. A record of the sample identity and instrument
conditions should be kept.
7.6.9 As the analysis proceeds,
check the retention times of standards vs. samples to ensure
uniformity.
7.6.10 If interfering substances are present,
establish positive identity of the peaks by spiking known amounts of
standard solution, or try to obtain better separation by changing the
eluent concentration or by reducing the flow rate.
7.7 Calculations
7.7.1 Peak areas or heights of the standards are used
to construct a standard curve using the OSHA Auto Colorimetric
Program. The samples results are obtained from a plot of peak height
or peak area vs. concentration. The blank corrected sample values are
then calculated using the Auto Colorimetric
Program.
7.7.2 When using the OSHA Auto Colorimetric
Program, sample numbers and volumes are entered into the calculator in
the following manner:
Sample Number, Peak Area or Height,
L Air Volume, mL Solution Volume, mL Aliquot Volume.
7.7.3 Air
Concentration values are calculated by the following
equation:
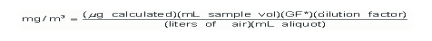
GF* = Gravimetric
Factor = 1.03 for HCl
7.7.4 HCl is reported in ppm rather than
in mg/m³. To convert
the mg/m³ values to ppm, the mg/m³ value
must be multiplied by the conversion factor 0.671.
8. Reporting Results
for Compounds Determined by Ion Chromatography
8.1 Results are reported on the OSHA Form 91 in ppm for
HCl, using two significant figures.
8.2 The estimated
detection limit calculated by the Auto Colorimetric Program is reported
on the OSHA Form 91 when no analyte is detected.
8.3 The
presence of significant unidentifiable peaks is noted on the OSHA Form
91.
8.4 All data processor printouts and chart recorded
chromatograms are filed in a central file according to laboratory sample
identification.
8.5 Calculations are checked by a fellow
chemist before the completed OSHA Form 91's are given to the
supervisor.
9. References
9.1 Hydrogen Chloride in Workplace Atmospheres, OSHA
Method # ID-173SG.
9.2 Hydrogen Chloride, OSHA Method #
VI-5, last revised on January, 1978.
9.3 Merck Index, 10th Edition, pg. 696,
1983.
9.4 CRC Handbook of Chemistry
& Physics, 62nd Edition, 1981-1982.
9.5 The Condensed Chemical Dictionary, 10th Edition, pg.
544, 1981.
9.6 Monitoring for Airborne
Inorganic Acids, M.E. Cassinelli and D.G; Taylor, National
Institute for Occupational Safety and Health, 4676 Columbia Parkway,
Cincinnati, OH, 45226.
9.7 OSHA Laboratory Quality Control
Data, Cl¯ by IC to September 6, 1979.
9.8 OSHA Ion
Chromatography Standard Operating Procedure, Prepared by the Ion
Chromatography Committee, Occupational Safety & Health
Administration Analytical Laboratory, Inorganic
Division.
9.9 NIOSH Manual of Analytical Methods, Second
Edition, Volume 7, Method Number P&CAM 339 (revised), Issued on
2/15/84.
|

