1. Introduction:
1.1 Scope
This method describes the collection and
analysis of airborne selenium. It is applicable for both ceiling
and time-weighted average (TWA) exposure evaluations. The analysis
is based on the utilization of a graphite furnace.
1.2 Previous
analysis of selenium was by an atomic absorption spectro-photometer
(AAS). This method did not take into account the possibility of
interfering complexing species such as Ni, Cu, Hg, etc. in order to
dissociate these complexing species, it was necessary to develop a
method in which a higher temperature, such as with the graphite furnace,
was used. This proved to be a very effective and accurate
method.
1.3 Uses
Selenium exhibits both photovoltaic
action, where light is converted directly into electricity, and
photo-conductive action, where the electrical resistance decreases
with increased illumination. These properties make selenium
useful in the production of photo cells and exposure meters for
photographic use, as well as solar cells. Selenium is also able to
convert ac electricity to dc and is extensively, used in rectifiers.
Below its melting point, selenium is a p-type semiconductor, and is
finding many uses in electronic and solid state applications. It
is used in zerography for reproducing and copying documents, letters,
etc. It is used in the manufacture of pigments, in insecticides, in
rubber compounding, to remove the green (iron) tint of glass, to
produce pink, ruby, and black glass glaze, to improve the machinability
of copper alloys and stainless steel, to improve the grain,
structure, and ductility of cast steel, to increase the depth of chill
in cast iron, as a flameproofing agent for textiles and wire-cable
coverings, and in chemical and ceramic manufacture. Exposures to
selenium may result during the smelting and refining of ores
containing selenium, in the refining of copper, silver, and gold to
remove the selenium, or from the use of selenium compounds.
1.4
Physical and Chemical Properties
Selenium is a non-metallic
element of the sulfur group. Selenium exists in several allotropic
forms. Three are generally recognized, but as many as six have been
claimed. Selenium can be found as either red in powder form, or black in
vitreous form. Crystalline monoclinic selenium is a deep red;
crystalline hexagonal selenium, the most stable variety, is a
metallic gray. Being a member of the sulfur family, it resembles sulfur
both in its various forms and in its compounds.
The physical and
chemical properties of selenium are listed in Table 1.4.
Table 1.4
Physical and Chemical
Properties of Selenium
|
|
Form 1 |
Form II |
Form III |
|
| Molecular weight |
78.96 g/mole |
78.96 |
78.96 |
| Molecular formula |
Se |
Se |
Se |
| color/ |
bluish-gray |
red |
red amorphous |
| crystalline form |
met hexagonal |
monoclinic prism |
black vitreous |
| specific gravity |
4.81 |
4.50 |
red 4.26 |
| melting point (°C) |
217 |
170-180 |
60-80 |
| boiling point (°C) |
634.9 |
634.8 |
634.8 |
| Solubility (g/100 mL) |
|
|
| cold water |
insoluble |
insoluble |
insoluble |
| hot water |
insoluble |
insoluble |
insoluble |
| H2SO4 |
soluble |
soluble |
------ |
| CHCl3 |
soluble |
------- |
------ |
| alcohol |
insoluble |
------- |
------ |
| CS2 |
very slightly soluble |
------- |
soluble |
| KMO3 |
------- |
soluble |
------ |
| Benzene |
------- |
------- |
soluble |
The red amorphous powder turns black
upon standing and vitreous upon heating.
2. Range and Detection Limit
A lower analytical limit, 0.02 µg/mL, was selected for
routine analysis. 3. Precision and Accuracy
Not determined. 4. Interferences
None known. 5. Sampling Procedure
5.1 The sample is collected on a 0.8-µm AA cellulose
membrane filter using a flow rate between 1.5 and 2.0 liters per minute.
Suggested minimum air volume is 100 liters. A sample blank should
also be submitted. (If considerable loose dust is present in the
cassette, a clean filter should be placed over the dust before
sealing.)
5.2 The sample cassettes are plugged, sealed with
OSHA tape, labeled, and sent to the laboratory for
analysis.
5.3 No storage problems are normally anticipated.
Vibration or jolting of samples should be kept to a minimum to avoid
dislodging of dust from the filter. 6. Analytical
Procedure
6.1 Apparatus
Atomic absorption
spectrophotometer equipped with graphite furnace, argon purge system,
and deuterium arc background corrector.
Chart
recorder.
Glassware.
AA filters (0.8-µm,
cellulose membrane filters 37-mm diam)
2-or 3-piece filter
cassettes Personal sampling pump (capable of sampling between 1.0 and
2.0 liters per minute)
6.2 Reagents
HCl,
reagent grade
HNO3, reagent grade
A
certified aqueous standard such as "SPEX" 1,000 ppm standard. 1,000 ppm
Ni solution, prepared as follows:
Dissolve 5.0 g
Ni(NO3)2.6H2O in 100 mL H2O,
add 5 mL KNO3, dilute to 1 1.
Diluting
solution: Twenty AA filters are ashed with 100 mL concentrate
MNO3 and 100 mL of 1,000 ppm Ni solution to a volume of 20 -
40 mL, diluted to 500 mL with deionized water and 2 mL
HCl.
6.3 Standards Preparation
Standards
are prepared to match the matrix of the samples (filter content acid and
nickel concentration) as closely as possible according to the dilution
scheme of Table 6.3.1.
The 0.2, 1, 2, 5, 10 and 20 ppm
"stock solutions" are made by serial dilution of the 1,000 ppm As
stock (with deionized water) as follows in Table 6.3.1.
Table 6.3.1
Stock
Solutions
| Stock Solution |
Standard Solution Used |
mL Used |
Final Volume |
|
| 10 ppm |
1000 ppm |
10 mL |
1000 mL |
| 1.0 ppm |
10 ppm |
100 mL |
1000
mL |
The diluted stock
solutions should be prepared just before using them to prepare the
working standards as outlined in Table 6.3.2.
When
preparing the working standards from the stock solutions, all
dilutions are made with the diluting solution.
Table 6.3.2
Working Standard
Solutions
|
| STD (PPM) |
mL Stock Used |
Stock Concentration (ppm) |
Final Volume |
|
| 0.02 |
2 |
1 |
100 mL |
| 0.05 |
5 |
1 |
100 mL |
| 0.1 |
10 |
1 |
100 mL |
| 0.2 |
20 |
1 |
100 mL |
| 0.5 |
5 |
1 |
100 mL |
| 1.0 |
10 |
10 |
100 mL |
| 2.0 |
20 |
10 |
100 mL |
|
6.4. Sample
Preparation
Note: All glassware must be rinsed with 1:1
HN03 and deionized water prior to use. Phillips beakers
used for the digestion are refluxed with 1:1 nitric acid and rinsed
with deionized water before use.
Place filter in 125 mL
Phillips beaker, add 5 mL of 1000 ppm Ni solution and 5 µL
concentrated HNO3 and ash to approximately 1-2 mL volume.
After sample has cooled, add 2 drops HCl and swirl contents (no
additional heating is done).
Quantitatively transfer sample
to 25 mL volume flask, dilute to volume with deionized water, and mix.
Additional dilutions for samples over 2 ppm Se are made with the
diluting solution.
6.5 Sample Analysis
The
analysis for selenium is performed using a graphite furnace.
Instrumental parameters are as follows:
Selenium
Conditions
|
|
Dry |
= 90T, 40H, 150F |
|
|
Char |
= 900T, 502, 30H, 100F |
|
|
Atomize |
= 2650T, 0R, 8H, 15F |
| Wavelength: |
196.0 nm |
Chart |
= Range 10 |
| Slit 4 |
|
EDL Power |
=6 1/2 watts |
|
|
(STDS prepared same as
As) |
Injection volume =
10 µL The 2.0 ppm standard should give a near full scale deflection
using these conditions. The entire series of standards should be run at
the beginning and the end of the analysis. A standard should be run
after every fourth or fifth sample in the sample
range.
6.6 Calculations
A linear
regression of standard ppm vs standard peak height is performed using
the 0SHA Automatic AA program. The sample results are calculated based
on sample peak heights; a function of sample
absorption.
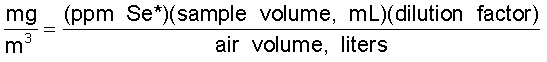
*blank
corrected
|

