1. General Discussion
1.1 Background
1.1.1 History
The determination
of toluene in air has consistently been one of the top analyses
performed at the OSHA Salt Lake Technical Center (SLTC) for the last
25 years. It is based on a pioneering method developed by Otterson and
Guy to determine airborne solvent vapors. (Ref. 5.1) Samples were
collected by drawing air through glass tubes packed with 4 inches of
20/40 mesh activated charcoal, then desorbed with an appropriate
solvent and analyzed by GC. Further developmental work was done to
incorporate a flame-sealed tube containing two 1-inch sections of
activated charcoal. (Ref. 5.2) Procedures for a multitude of solvents
were subsequently evaluated through NIOSH based on a standardized
"NIOSH charcoal tube" consisting of two sections of coconut shell
activated charcoal contained in a 7-cm flame sealed glass tube. The
front section contains 100 mg and the back section 50 mg of charcoal.
There are at least three different methods for toluene using this
sampling tube that utilize carbon disulfide (CS2) as the
desorption solvent. (Refs. 5.3-5.5)
In the work presented
here, tests were done to validate procedures for charcoal tubes as
well as Anasorb® 747 tubes. Additional tests were done so
the new method can also be used for determinations of peak and ceiling
exposures to toluene. Also, the recommend sampling rate was reduced to
50 mL/min to allow samples to be taken for as long as 240 minutes,
which makes it convenient to assess an all-day exposure to a worker by
taking only two samples. The most significant change was in the
solvent used to desorb the samples.
When air is drawn through
activated charcoal tubes, a significant amount of water may be
collected by adsorption along with the analytes of interest. (Ref.
5.6) The amount of water collected is dependent on the water content
of the air and the volume of air sampled. Water is not very soluble in
CS2, thus when samples containing excessive amounts of
water are desorbed with this commonly used solvent, the desorbed water
can form a separate layer. Only the CS2 layer is analyzed
by GC, so if an analyte of interest is appreciably soluble in water,
it will partition into the aqueous layer and the concentration of the
analyte may be grossly under determined. This problem does not occur
for toluene because being fairly nonpolar, it is very soluble in
CS2 and only partially soluble in water. But toluene is
frequently used with a mixture of solvents in workplaces, some of
which may be very soluble in water. It is often desirable to analyze
the mixture of solvents simultaneously from the same sample, so a
solution to this problem was to use a desorption solvent that would
result in a homogeneous solution that includes water after the samples
are desorbed and one that would also desorb the analytes of interest
with high efficiency.
A desorption solvent mixture consisting
of 60/40 (v/v) N,N-dimethylformamide/carbon disulfide
(DMF/CS2) was successfully used in some recently validated
SLTC methods for low molecular weight alcohols (Refs. 5.7-5.9) and was
chosen for this present work. One milliliter of this solvent will
assimilate up to 50 mg of water at room temperature and desorbs a wide
variety of solvents with high efficiency.
It is imperative that
an internal standard procedure be used in the analysis to compensate
for the water that is desorbed and put into solution. For instance, if
an external standard method is used and a sample that contains 45 mg
of water is desorbed with 1.00 mL of 60/40 DMF/CS2, an
uncorrectable error of approximately 4.5% is introduced. The amount of
water (in this case 45 mg or 0.045 mL) that was adsorbed by the
adsorbent is not determined in the analysis, so a correction for the
dilution of the sample by the desorbed water can not be made. In this
case, the final volume of the desorption solution is approximately
1.045 mL because of the desorbed water, not 1.00 mL. The necessary
correction is automatically made when an internal standard procedure
is used. Not only does the internal standard procedure compensate for
any desorbed water, it also compensates for the volume of any other
solvents that are also desorbed, which in some cases could be quite
significant.
Recently, SLTC decided to evaluate diffusive
samplers for possible alternative sampling devices for solvent vapors
and gases. Extensive work was done to help determine the evaluation
tests needed to validate this type of sampler for use by OSHA. (Ref.
5.10) The 3M Company markets two different samplers that are widely
used for the determination of airborne solvent vapors. The 3500
Organic Vapor Monitor (OVM) has a single carbon disk, while the 3520
OVM has a secondary disk used as a backup sampler. By consensus
decision, in order to have a common sampler and based on the
aforementioned work done at SLTC, the 3520 OVM will be evaluated for
all methods utilizing a 3M monitor, even though in many cases the 3500
OVMs may suffice. Also, by consensus decision, the SKC
575-002 Passive Sampler will be the first sampler
considered for all methods utilizing an SKC Passive Sampler. Utilizing
the same basic methodology that was used to evaluate the adsorbent
tubes, the 3M 3520 OVM and SKC 575-002 Passive Sampler
were validated for use in the determination of toluene in air. These
diffusive samplers can also collect significant amounts of water (Ref.
5.11), so 60/40 DMF/CS2 is also used for the desorption
solvent. Samples are desorbed with 2.00 mL instead of 1.00 mL that is
used for adsorbent tube samples.
Under very controlled
conditions in the laboratory, it was found that both the 3M and SKC
diffusive samplers could be used to reliably sample for as short as 1
minute, which would make them useful to determine peak exposures. But
in real world situations, mostly because the samplers begin sampling
as soon as they are removed from their sealed containers, it was felt
that there could be too much chance for introduction of significant
sampling errors. Thus the shortest recommended sampling time for
toluene using diffusive samplers was determined to be 10 minutes,
making them valuable tools to assess ceiling exposures, provided
diligent sampling techniques are used.
1.1.2 Toxic effects (Ref
5.12) (This section is for information only and should not be taken as
the basis of OSHA policy.)
Toluene is a central nervous system
depressant. Exposure to very high levels (15,000 to 30,000 ppm) for a
short time may cause mental confusion, loss of coordination, and
unconsciousness. Exposures to 200 ppm for 8 hours caused mild fatigue,
weakness, confusion, lacrimation, and paresthesias (sensation of
pricking, tingling, or creeping) of the skin. Exposures to 600 ppm for
8 hours produced additional effects including euphoria, headache,
dizziness, dilated pupils, and nausea while at 800 ppm for 8 hours the
symptoms were more pronounced and also included nervousness, muscular
fatigue, and insomnia that persisted for days.
In another
study, exposure to 100 ppm for 6 hours resulted in eye and nose
irritation, and in some cases, headaches, dizziness, and a feeling of
intoxication, but no significant differences in performance on some
neurobehavioral tests were observed. No symptoms were noted at 10 or
40 ppm exposures.
Long-term inhalational abuse by glue-sniffers
resulted in chronic organic brain dysfunction associated with cerebral
and cerebellar atrophy. Some studies of workers chronically exposed to
toluene suggested minor abnormalities on neuropsychological testing,
but a recent study of 43 rotogravure printers exposed to average
concentrations of 117 ppm for an average of 22 years failed to
demonstrate significant clinical neuropsychological differences
compared to a control group of unexposed workers.
Exposure to
toluene does not result in the hematopoietic effects caused by
benzene. Most of the toluene introduced in the body by inhalation is
metabolized to benzoic acid, which is conjugated with glycine in the
liver to form hippuric acid. The hippuric acid is then excreted in the
urine.
Repeated or prolonged skin exposure to toluene causes
skin drying, fissuring, and dermatitis. Liquid splashed in the eyes of
two workers caused transient corneal damage and conjunctival
irritation with complete recovery within 48 hours.
Recent
inhalation studies on rats exposed to levels of 600 to 1200 ppm and
mice exposed to 120, 600, or 1200 ppm for two years found no evidence
of carcinogenic activity.
1.1.3 Workplace
exposure
Toluene is used in the manufacture of benzoic acid,
benzaldehyde, explosives, dyes and many other organic compounds. It is
also used as a solvent for paints, lacquers, gums, and resins and as a
thinner for inks, perfumes, and dyes. It is used in the extraction of
various principles from plants. It is a component in gasoline and is
also used as a gasoline additive. (Ref. 5.13)
1.1.4 Physical
properties (Ref. 5.13 unless otherwise noted)
| CAS number: |
108-88-3 |
| molecular weight: |
92.14 |
| boiling point: |
110.6°C |
| melting point: |
-95°C |
| appearance: |
colorless liquid |
| density: |
0.866 g/mL at 20°C |
| molecular formula: |
C7H8 |
| vapor pressure: |
2.92 kPa (21.9 mmHg) at
20°C (Ref. 5.14) |
| flash point: |
40°F (4.4°C) (closed
cup) |
| odor: |
benzene-like |
| explosive limits: |
1.27-7% in air (Ref.
5.15) |
| solubility: |
very slightly soluble in
water [0.067% (w/w) in water at 23.5°C]; miscible with alcohol,
chloroform, ether, acetone, glacial acetic acid, carbon
disulfide. |
| synonyms: |
methylbenzene; toluol;
phenylmethane; Methacide. |
| structural formula: |
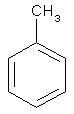 |
The analyte air concentrations throughout this method
are based on the recommended sampling and analytical parameters. TWA
target concentration samples are based on 240 minutes, ceiling samples on
10 minutes and peak samples on 1 minute of sampling/diffusive sampler
exposure. Air concentrations listed in ppb and ppm are referenced to 25°C
and 101.3 kPa (760 mmHg).
1.2 Limit defining parameters
1.2.1 Detection limit of the
analytical procedure
The detection limit of the analytical
procedure is 2.60 pg. This is the amount of toluene that will give an
instrument response that is significantly different from the
background response of a reagent blank. (Sections 4.1 and
4.2)
1.2.2 Detection limit of the overall procedure
The
detection limits of the overall procedure are 246 ng per sample (5.4
ppb or 20.5 µg/m³), 344 ng per sample (7.6
ppb or 28.7 µg/m³), 657 ng per sample (25
ppb or 93 µg/m³) and 904 ng per sample (67
ppb or 253 µg/m³) for charcoal tubes,
Anasorb® 747 tubes, 3M 3520 OVMs and SKC
575-002 samplers respectively. These are the amounts of
toluene spiked on the respective samplers that will give instrument
responses that are significantly different from the background
responses of respective sampler blanks. (Sections 4.1 and
4.3)
1.2.3 Reliable quantitation limit
The reliable
quantitation limits are 820 ng per sample (18.1 ppb or 68.3 µg/m³), 1146 ng per sample (25.4 ppb or 95.5 µg/m³), 2190 ng per sample (82 ppb or 309 µg/m³) and 3012 ng per sample (224 ppb or 844
µg/m³) for charcoal tubes,
Anasorb® 747 tubes, 3M 3520 OVMs and SKC
575-002 samplers respectively. These are the amounts of
toluene spiked on the respective samplers that will give signals that
are considered the lower limits for precise quantitative measurements.
(Section 4.4)
1.2.4 Precision (analytical procedure)
The
precisions of the analytical procedure, measured as the pooled
relative standard deviations from standards over concentration ranges
equivalent to 0.5 to 2 times the TWA target concentration, are 0.76%,
0.94% and 0.76% for the adsorbent tubes, 3M 3520 OVMs and SKC
575-002 samplers respectively. (Section 4.5)
1.2.5
Precision (overall procedure
1.2.5.1 Adsorbent tubes
samples
The precisions of the overall procedure at the 95%
confidence level from the ambient temperature storage tests for TWA,
ceiling and peak samples for charcoal tubes and Anasorb®
747 tubes are given in Table 1.2.5.1. The TWA samples are 240-min
samples taken from 200-ppm atmospheres, the ceiling samples are
10-min samples taken from 300-ppm atmospheres and the
peak samples are 1-min samples taken from 500-ppm atmospheres.
(Section 4.6)
Table
1.2.5.1
Precision of the Overall Procedure at the
95%
Confidence Interval for Adsorbent Tubes
|
| Adsorbent |
TWA Samples |
Ceiling Samples |
Peak Samples |
|
Charcoal
Anasorb® 747 |
±10.8%
±10.1% |
±10.2%
±10.1% |
±10.3%
±10.5% |
|
1.2.5.2 Diffusive
samplers
The precisions of the overall procedure at the 95%
confidence level from the ambient temperature storage tests for TWA
and ceiling samples for 3M 3520 OVMs and SKC 575-002
samplers are given in Table 1.2.5.2. The TWA samples are 240-min
samples taken from 200-ppm atmospheres and the ceiling samples are
10-min samples taken from 300-ppm atmospheres. There
are different values given for each sampler for each of the levels,
depending on whether the sampling site conditions are known. The
possible cases would be when both, either, or neither temperature or
atmospheric pressure are known. If the temperature is not known, the
sampling site temperature is assumed to be 22.2±15°C (72±27°F) and a
variability of ±7.7% is included. If the atmospheric pressure is not
known, a variability ±3% is included. (Section 4.6)
Table
1.2.5.2
Precision of the Overall Procedure at the 95%
Confidence Interval
for Diffusive Samplers When Sampling
Site Temperature (T)
or Atmospheric Pressure (P) are Known
or Unknown
|
| Sampler |
TWA Samples |
Ceiling
Samples |
|
3M 3520
OVM
both T&P
known
only T
known
only P
known
neither T nor P
known
SKC 575-002
Sampler
both T&P
known
only T
known
only P
known
neither T nor P known |
±14.1%
±15.3%
±20.6%
±21.5%
±18.0%
±18.9%
±23.5%
±24.3% |
±15.5%
±16.6%
±21.7%
±22.4%
±18.6%
±19.5%
±23.9%
±24.7% |
| 1.2.6
Recovery
The recovery of toluene from TWA samples used in
19-day storage tests remained above 99.9%, 99.5%, 102.5% and 97.3% for
charcoal tubes, Anasorb® 747 tubes, 3M 3520 OVMs and SKC
575-002 samplers respectively when the samples were
stored at ambient temperatures. The recovery of toluene from ceiling
samples used in 15-day storage tests remained above
97.8%, 97.5%, 100.0% and 94.5% for charcoal tubes, Anasorb®
747 tubes, 3M 3520 OVMs and SKC 575-002 samplers
respectively when the samples were stored at ambient temperatures. The
recovery of toluene from peak samples used in 15-day
storage tests remained above 94.8% and 95.2% for charcoal tubes and
Anasorb® 747 tubes respectively when the samples were
stored at ambient temperatures. (Section 4.7)
1.2.7
Reproducibility
Six samples at the TWA target concentration for
each of the four different samplers that were collected from
controlled test atmospheres, along with a draft copy of this
procedure, were submitted to an SLTC service branch for analysis. The
charcoal tube and the 3M 3520 OVM samples were analyzed 20 days after
generation and the Anasorb® 747 tube and SKC
575-002 samples were analyzed 53 days after generation.
All samples were stored at room temperature. No individual sample
result deviated from its theoretical value by more than the precisions
reported in Section 1.2.5. (Section 4.8)
2. Sampling Procedure
2.1 Apparatus
2.1.1 Adsorbent tube samples
2.1.1.1 Samples are collected using
personal sampling pumps that have been calibrated, with sampling
devices attached, to within ±5% at the recommended flow rate of 50
mL/min. The sampling devices consist of adsorbent tubes that are
contained in commercially available tube holders (such as SKC Inc.,
Fullerton, CA, Catalog No. 222-3-1). The devices are connected to
the pumps with flexible, non-crimpable
tubing.
2.1.1.2 Samples are collected with 7-cm × 4-mm i.d. ×
6-mm o.d. glass sampling tubes packed with two sections of either
coconut shell charcoal or Anasorb® 747.
Anasorb® 747 is a beaded activated carbon. SKC adsorbent
tubes were used in this evaluation. Charcoal tubes (Catalog No.
226-01) contain 100 mg of adsorbent in the front section and 50 mg
in the back section. Anasorb® 747 tubes (Catalog No.
226-81A) contain 140 mg of adsorbent in the front section and 70 mg
in the back section. The adsorbent sections are held in place with
glass wool plugs and are separated by urethane foam plugs. The ends
of the glass sampling tubes are heat sealed. Lot 120 charcoal and
Lot 299 Anasorb® 747 tubes were used for this
evaluation. 2.1.2 Diffusive
samplers
2.1.2.1 Samples are collected with
either 3M (3M Occupational Health and Safety Products Division, St.
Paul, MN) 3520 Organic Vapor Monitors (OVMs) or SKC
575-002 Passive Samplers (SKC, Inc., Fullerton, CA).
The 3M 3520 OVMs are badges containing two activated charcoal disks.
The secondary disk collects contaminant when the capacity of the
primary disk has been exceeded. The SKC 575-002 Passive
Samplers are badges that contain 500 mg of Anasorb® 747
adsorbent. Lot 5163009 3M OVMs and Lot 263 SKC 575-002
Samplers were used in this evaluation.
2.1.2.2 A thermometer
to determine the sampling site air temperature.
2.1.2.3 A
barometer to determine the sampling site atmospheric
pressure. 2.2
Reagents
None required
2.3 Technique
2.3.1 Adsorbent tubes
2.3.1.1 Immediately before sampling,
break off the ends of the adsorbent tube. All tubes should be from
the same lot.
2.3.1.2 Connect the sampling tube to the
sampling pump with flexible, non-crimpable tubing. It
is desirable to utilize a sampling tube holder that shields the
employee from the sharp, jagged end of the sampling tube. Position
the tube so that sampled air first passes through the larger
adsorbent section.
2.3.1.3 Air being sampled should not pass
through any hose or tubing before entering the sampling
tube.
2.3.1.4 To avoid channeling, place the sampling tube
vertically in the employee's breathing zone. Position the sampler so
it does not impede work performance or safety.
2.3.1.5 After
sampling for the appropriate time, immediately remove the sampling
tube and seal it with plastic caps. Wrap each sample lengthwise with
a Form OSHA-21 seal.
2.3.1.6 Submit at least one blank
sampling tube with each sample set. Blanks should be handled in the
same manner as samples, except no air is drawn through
them.
2.3.1.7 Record sample volumes (in liters of air),
sampling times (minutes) and sampling rate (mL/min) for each sample
on Form OSHA-91A.
2.3.1.8 Also list any compounds that could
be considered potential interferences, especially solvents, that are
being used in the sampling area.
2.3.1.9 Ship any bulk
sample(s) in a container separate from the air samples.
2.3.2 3M OVMs (In general, follow the
manufacturer's instructions supplied with the samplers.)
2.3.2.1 The monitors come
individually sealed in small metal cans. When ready to begin
sampling, remove the plastic lid from the can and lift up on the
revealed ring. Pull back on the ring to open the can. Discard the
metal top of the can and remove the monitor. CAUTION- The monitor
immediately begins to sample when the can is
unsealed.
2.3.2.2 Keep the two closure caps with attached
port plugs, cup and Teflon® tubes in the can for later
use. Close the can with the plastic lid.
2.3.2.3 Record the
start time on the back of the monitor or on Form
OSHA-91A.
2.3.2.4 Attach the monitor to the worker near
his/her breathing zone with the white face forward. Assure that the
area directly in front of the sampler is unobstructed throughout the
sampling period. Do not remove the white
film and ring from the monitor until the sampling period is
terminated.
2.3.2.5 At the end of the sampling period, detach
the monitor from the worker and remove the white film and retaining
ring. Immediately snap a closure cap onto the primary (top) section
of the monitor (where the white film and ring were removed). It is
critical that this step be done as quickly as possible because the
sampling rate is more than 5 times faster without the white film in
place, which can be an important consideration, especially for
short-term sampling. Assure that the attached port plugs are placed
firmly into the port holes. The white film and ring can be
discarded. Record the stop time on the back of the monitor or on
Form OSHA-91A.
2.3.2.6 The following steps should be
performed in a low background area for a set of monitors as soon as
possible after sampling.
2.3.2.7 Ready a blank by removing
the white film and ring and attaching a closure cap onto an unused
monitor.
2.3.2.8 For each monitor (one at a time), separate
the primary (top) and secondary (bottom) sections of the monitor
using the edge of a coin as a pry.
2.3.2.9 Securely snap a
cup onto the bottom of the primary section.
2.3.2.10 Snap a
closure cap onto the secondary section of the monitor and assure
that the attached port plugs are placed firmly into the port
holes.
2.3.2.11 Return the sampler sections with closure caps
and cup in place to the metal can which contains the
Teflon® tubes (which will be used by the laboratory).
Close the can with the plastic lid, and wrap it with a Form OSHA-21
seal.
2.3.2.12 Verify that the sampling times are properly
recorded on Form OSHA-91A for each sample. Also identify blank
samples on this form.
2.3.2.13 Record the room temperature
and atmospheric pressure (station pressure) of the sampling site on
Form OSHA-91A.
2.3.2.14 List any compounds that could be
considered potential interferences, especially solvents, that are
being used in the sampling area.
2.3.2.15 Submit the monitors
(contained in the metal cans) to the laboratory for analysis as soon
as possible.
2.3.2.16 Ship any bulk sample(s) in a container
separate from the air samples. 2.3.3 SKC 575-002 Samplers (In general,
follow the manufacturer's instructions supplied with the
samplers.)
2.3.3.1 Remove the sampler (enclosed
in a clear inner package) from the envelope. Keep the O-ring,
press-on cover, cover retainer, port plugs and Teflon®
tube in the envelope for later use.
2.3.3.2 Remove the
sampler from the clear inner package when ready to begin sampling.
CAUTION- The monitor immediately begins to sample when it is removed
from this package.
2.3.3.3 Record the start time on the
sampler label or on Form OSHA-91A.
2.3.3.4 Attach the sampler
to the worker near his/her breathing zone with the perforations in
the sampler facing out. Assure that the area directly in front of
the sampler is unobstructed throughout the sampling
period.
2.3.3.5 At the end of the sampling period,
immediately detach the sampler from the worker and attach the cover
with the O-ring in place onto the sampler using the cover retainer.
Make sure the O-ring is forming a proper seal around the entire
circumference of the sampler. Record the stop time on sampler label
or on Form OSHA-91A.
2.3.3.6 Prepare a blank by removing an
unused sampler from its clear package and immediately attaching a
cover with the O-ring in place onto it.
2.3.3.7 Wrap each
sampler with a Form OSHA-21 seal.
2.3.3.8 Verify that the
sampling times are properly recorded on Form OSHA-91A for each
sample. Also identify blank samples on this form.
2.3.3.9
Record the room temperature and atmospheric pressure (station
pressure) of the sampling site on Form OSHA-91A.
2.3.3.10
List any compounds that could be considered potential interferences,
especially solvents, that are being used in the sampling
area.
2.3.3.11 Submit the samplers to the laboratory for
analysis as soon as possible. Include all port plugs and
Teflon® tubes which will be used in the laboratory
analyses.
2.3.3.12 Ship any bulk sample(s) in a container
separate from the air samples. 2.4 Sampler capacity (adsorbent tubes) and sampler
rate/capacity (diffusive samplers)
2.4.1 Charcoal tubes
The
sampling capacity of the front section of charcoal sampling tubes was
tested by sampling from a dynamically generated test atmosphere of
toluene at 401.6 ppm (1513 mg/m³). The samples were collected at a
nominal flow rate of 50 mL/min and the relative humidity of the
atmosphere was 73% at 29.1°C. The average 5% breakthrough volume was
determined to be 16.8 L (25.4 mg or 336 min) from three
determinations. (Section 4.9.1)
2.4.2 Anasorb® 747
tubes
The sampling capacity of the front section of
Anasorb® 747 sampling tubes was tested by sampling from a
dynamically generated test atmosphere of toluene at 401.6 ppm (1513
mg/m³). The samples were collected at a nominal flow rate of 50 mL/min
and the relative humidity of the atmosphere was 73% at 29.1°C. The
average 5% breakthrough volume was determined to be 20.6 L (31.1 mg or
412 min) from three determinations. (Section 4.9.2)
2.4.3 3M
3520 OVMs
The sampling rate and capacity of 3M 3520 OVMs were
determined by taking samples from a dynamically generated test
atmosphere of toluene (nominal concentration of 400 ppm or 1507 mg/m³)
for increasing time intervals. A sampling rate of 29.54 mL/min (at 760
mmHg, 25°C) and capacity of greater than 32 mg per sample (>21.2 L
or >718 min) were obtained from this test. (Section
4.9.3)
2.4.4 SKC 575-002 Samplers
The
sampling rate and capacity of SKC 575-002 Samplers were
determined by taking samples from a dynamically generated test
atmosphere of toluene (nominal concentration of 400 ppm or 1507 mg/m³)
for increasing time intervals. A sampling rate of 14.89 mL/min (at 760
mmHg, 25°C) and capacity of greater than 16 mg per sample (>10.6 L
or >712 min) were obtained from this test. (Section 4.9.4)
2.5 Desorption efficiency
2.5.1 Charcoal tubes
2.5.1.1 The average desorption
efficiency from charcoal tubes over the range of 0.5 to 2 times the
TWA target concentration is 99.0%. (Section 4.10.1.1)
2.5.1.2
The desorption efficiency at 0.05, 0.1 and 0.2 times the target
concentration was found to be 97.4, 98.2% and 98.4% respectively.
(Section 4.10.1.1)
2.5.1.3 Desorbed samples remain stable for
at least 24 h. (Section 4.10.1.2) 2.5.2 Anasorb® 747 tubes
2.5.2.1 The average desorption
efficiency from Anasorb® 747 tubes over the range of 0.5
to 2 times the TWA target concentration is 99.1%. (Section
4.10.2.1)
2.5.2.2 The desorption efficiency at 0.05, 0.1 and
0.2 times the target concentration was found to be 97.3, 98.1% and
99.1% respectively. (Section 4.10.2.1)
2.5.2.3 Desorbed
samples remain stable for at least 24 h. (Section 4.10.2.2)
2.5.3 3M OVMs
2.5.3.1 The average desorption
efficiency from 3M OVMs over the range of 0.5 to 2 times the TWA
target concentration is 98.1%. (Section 4.10.3.1)
2.5.3.2 The
desorption efficiency at 0.05, 0.1 and 0.2 times the target
concentration was found to be 98.4, 98.2% and 98.0% respectively.
(Section 4.10.3.1)
2.5.3.3 Desorbed samples remain stable for
at least 24 h. (Section 4.10.3.2) 2.5.4 SKC 575-002 Samplers
2.5.4.1 The average desorption
efficiency from SKC 575-002 Samplers over the range of
0.5 to 2 times the TWA target concentration is 97.0%. (Section
4.10.4.1)
2.5.4.2 The desorption efficiency at 0.05, 0.1 and
0.2 times the target concentration was found to be 98.0, 98.1% and
97.6% respectively. (Section 4.10.4.1)
2.5.4.3 Desorbed
samples remain stable for at least 24 h. (Section 4.10.4.2)
2.6 Recommended air volume
and sampling rate
2.6.1 When using adsorbent tubes for
TWA (long-term) samples, sample up to 12 L of air at 50 mL/min (up to
240 min). When using diffusive samplers, sample for as long as 240
minutes.
2.6.2 When using adsorbent tubes for ceiling samples,
sample greater than 0.5 L of air at 50 mL/min (greater than 10 min).
When using diffusive samplers, sample for greater than 10
minutes.
2.6.3 When using adsorbent tubes for peak samples,
sample at least 0.05 L of air at 50 mL/min (at least 1 min). The use
of diffusive samplers is not recommended for peak
samples.
2.6.4 When short-term samples are collected, the air
concentrations equivalent to the reliable quantitation limits becomes
larger. For example, the reliable quantitation limits for charcoal
tubes become 0.43 ppm (1.6 mg/m³) for 10-min samples and
4.3 ppm (16 mg/m³) for 1-min samples. 2.7 Interferences (sampling)
2.7.1 The presence of other
contaminants in the sampled air can potentially reduce the capacity of
all four samplers to collect toluene. Also, the sampling rates of
diffusive samplers could possibly be altered. Interference studies
were performed by sampling for 240 minutes from a test atmosphere (10%
RH, 26°C, 654.8 mmHg) containing 396 ppm of toluene with 50 ppm of
2-butanone (MEK), 20 ppm of 4-methyl-2-pentanone (MIBK),
20 ppm of 1-butanol, 30 ppm of isobutyl acetate and 30 ppm of xylene.
The presence of these compounds, which may represent typical
substances that may be collected with toluene, did not have a
significant effect on sample results using any of the samplers.
(Section 4.11.1)
2.7.2 Short-term sampling interference studies
were performed for all four samplers by sampling for 1 minute from a
test atmosphere (10% RH, 25°C, 654.3 mmHg) containing 495 ppm of
toluene with 50 ppm of 2-butanone (MEK), 20 ppm of
4-methyl-2-pentanone (MIBK), 20 ppm of 1-butanol, 30 ppm
of isobutyl acetate and 30 ppm of xylene. The presence of these
compounds, which may represent typical substances that may be
collected with toluene, did not have a significant effect on sample
results using any of the samplers. (Section 4.11.2)
2.7.3 A
reverse diffusion study for the diffusive samplers and a stripping
study for the adsorbent tubes was performed by sampling a 402 ppm
atmosphere of toluene (78% RH, 23.5°C, 649.2 mmHg) for 120 minutes
with six of each samplers. Three samplers from each set were
additionally subjected to 120 minutes of the same atmosphere without
the toluene present to determine if any of the collected toluene
diffused off of the diffusive samplers and also whether it was
stripped off of the adsorbent tubes. Upon analysis of the samples, the
average recovery of the removed samplers versus the average recovery
of the samplers that were additionally exposed to the atmosphere
without toluene was within 90% for all samplers, indicating that
reverse diffusion and stripping are not significant. (Section
4.11.3)
2.7.4 The effects of sampling from relatively dry
atmospheres was investigated by sampling from a 403.2-ppm toluene
atmosphere (9% RH, 25.3°C, 654.5 mmHg) for 240 minutes and by sampling
from a 499-ppm atmosphere (9% RH, 26.1°C, 653.9 mmHg) for 1 minute
with all four samplers. Sampling from dry atmospheres did not have a
significant effect on results using any of the samplers. (Section
4.11.4)
2.7.5 The effects from sampling from atmospheres
containing low concentrations of toluene was investigated by sampling
from a 19.8-ppm toluene atmosphere (74% RH, 26.0°C, 651.4 mmHg) for
240 minutes and from a 49.3-ppm atmosphere (73% RH,
27.8°C, 650.7 mmHg) for 1 minute with all four samplers.
Sampling low concentrations of toluene for 1 or 240 minutes did not
have a significant effect on sample results using any of the samplers.
(Section 4.11.5)
2.7.6 Suspected interferences should be
reported to the laboratory with submitted samples.
2.8 Safety precautions
(sampling)
2.8.1 Attach the sampling equipment to
the employee so that it will not interfere with work performance or
safety.
2.8.2 Follow all safety procedures that apply to the
work area being sampled. 3. Analytical Procedure
3.1 Apparatus
3.1.1 A GC equipped with a flame
ionization detector. For this evaluation, a
Hewlett-Packard 6890 Series Gas Chromatograph equipped
with an Automatic Liquid Sampler was used.
3.1.2 A GC column
capable of separating toluene from the desorption solvent, internal
standard and any interferences. A 30-m × 0.32-mm i.d. fused silica
XTI-5 (bonded 5% phenyl - 95% dimethylsiloxane) capillary column with
a 1.0-µm df from Restek Corporation
(Bellefonte, PA) was used in this evaluation.
3.1.3 An
electronic integrator or some other suitable means of measuring peak
areas. A Waters MillenniumTM 2020 Networking Computer
System was used in this evaluation.
3.1.4 Two-milliliter vials
with Teflon®-lined caps.
3.1.5 A dispenser capable
of delivering 1.0 mL of desorption solvent to prepare standards and
samples. If a dispenser is not available, a 1.0-mL volumetric pipet
may be used.
3.1.6 A sampler rack (SKC Cat. No. 226-04-5) and a
specialized shaker (SKC Cat. No. 226D-03-1) to facilitate the
desorption of SKC 575-002 Samplers.
3.2 Reagents
3.2.1 Toluene (CAS 108-88-3), reagent
grade. Baxter Burdick & Jackson B&J BrandTM High
Purity Solvent, Lot BK583, was used in this evaluation.
3.2.2
N,N-Dimethylformamide (DMF) [CAS 68-12-2],
chromatographic grade. EM Science OmniSolv®, Lot 36130, was
used in this evaluation.
3.2.3 Carbon disulfide
(CS2) [CAS 75-15-0], chromatographic grade. EM Science
OmniSolv®, Lot 35200, was used in this
evaluation.
3.2.4 A suitable internal standard, reagent grade.
Supelco Neat EPA Standard, Lot LA59304, ethylbenzene (CAS 100-41-4)
was used in this evaluation.
3.2.5 The desorption solvent
consists of 60/40 (v/v) DMF/CS2 containing 1.0 milliliter
of internal standard per liter of solution (1µL/mL).
3.2.6 GC grade nitrogen, air and
hydrogen. 3.3 Standard
preparation
3.3.1 Prepare standards by injecting
microliter amounts of toluene into vials containing 1.0 mL (for
adsorbent tubes) or 2.0 mL (for diffusive samplers) of desorption
solvent delivered from the same dispenser used to desorb samples. For
example, inject 6.00 µL of toluene into a
vial containing 1.0 mL of desorption solvent. Assuming the density of
toluene is 0.866 g/mL (which is dependent on the temperature of the
toluene), this standard contains 5196 µg of
toluene per sample for adsorbent tube samples.
3.3.2 Bracket
sample concentrations with standard concentrations. If upon analysis,
sample concentrations fall outside the range of prepared standards,
prepare and analyze additional standards to ascertain the linearity of
instrument response or dilute high samples with desorption solvent and
reanalyze the diluted samples. 3.4 Sample preparation
3.4.1 Adsorbent tube samples
3.4.1.1 Transfer each section of
adsorbent from the sampling tubes to separate labeled vials. Discard
the glass tubes, urethane foam plugs and glass wool
plugs.
3.4.1.2 Add 1.0 mL of desorption solvent to each vial
using the same dispenser as used for preparation of
standards.
3.4.1.3 Immediately cap the vials.
3.4.1.4
Allow the adsorbent sections to desorb for 30 minutes. Periodically
apply gentle agitation to the vials during the desorption
period. 3.4.2 3M 3520 OVMs (In
general, follow the manufacturer's instructions supplied with the
samplers.)
3.4.2.1 Remove each sampler section
from its individual metal can, along with the sections of
Teflon® tubing. Assure that the closure caps are firmly
snapped to the primary and secondary sections of all the samplers.
Also assure that all cap plugs are firmly seated in the cap ports.
Any deviations must be noted.
3.4.2.2 Prepare one section of
sampler at time by temporarily removing the cap plugs from the ports
and adding 2.0 mL of desorption solvent through the center port.
This is most easily done by dispensing two 1.0-mL aliquots of
desorption solvent using a dispenser. Immediately replace the plugs
in the ports.
3.4.2.3 Allow the sampler sections to desorb
for 30 minutes. Periodically apply gentle agitation to the sampler
sections during the desorption period.
3.4.2.4 Transfer the
solution from each sampler section by removing both plugs from the
ports, inserting a decanting spout (a small section of
Teflon® tubing) into the rim port and pouring the liquid
through the spout into a labeled autosampler vial. Immediately cap
each vial. 3.4.3 SKC
575-002 Samplers (In general, follow the manufacturer's
instructions supplied with the samplers.)
3.4.3.1 Cut off the ends of the two
protruding tubes of each sampler with a razor blade or sharp
knife.
3.4.3.2 Slowly add 1.0 mL of desorption solvent
through one of the protruding tubes (ports). After about 30 seconds,
slowly add another 1.0 mL of desorption solvent.
3.4.3.3
Immediately insert plugs into the ports.
3.4.3.4 Mount the
samplers in the sampler rack (SKC Cat. No. 226-04-5) of a
specialized shaker (SKC Cat. No. 226D-03-1) and shake the samplers
for 1 hour.
3.5.2.5 According to the manufacturer of the
sampler, do not leave the desorbed sample in the sampler. Transfer
each desorbed sample by removing the plugs from the sampler ports,
firmly inserting the tapered end of a supplied Teflon®
tube into the outer port and carefully pouring the solution through
the Teflon® tube into a labeled autosampler vial.
3.5 Analysis
3.5.1 GC conditions
| zone temperatures: |
column- 60°C
(isothermal)
injector- 250°C
detector- 275°C |
| gas flows: |
hydrogen (carrier)- 2.5
mL/min (43 kPa head pressure)
nitrogen (makeup)- 50
mL/min
hydrogen (flame)- 38 mL/min
air- 450 mL/min |
| signal range: |
0 |
| injection volume: |
1.0 µL (with a 100:1 split) |
| column: |
30-m × 0.32-mm i.d. fused
silica, XTI-5 1.0-µm df |
| retention times: |
toluene- 5.2
min
ethylbenzene- 9.8 min (internal
standard)
(CS2- 1.7 min, DMF- 6.1
min) |
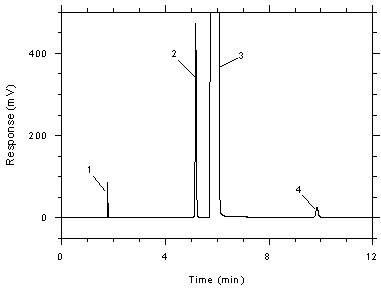
Figure
3.5.1.1 Chromatogram of a standard near the TWA target
concentration for the adsorbent tubes. Key: (1) CS2,
(2) toluene, (3) DMF, (4)
ethylbenzene. |
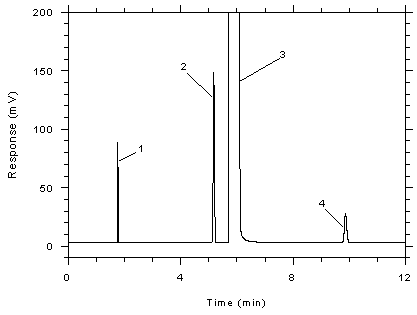
Figure
3.5.1.2 Chromatogram of a standard near the TWA target
concentration for 3M 3520 OVMs. Key: (1) CS2, (2)
toluene, (3) DMF, (4) ethylbenzene. |
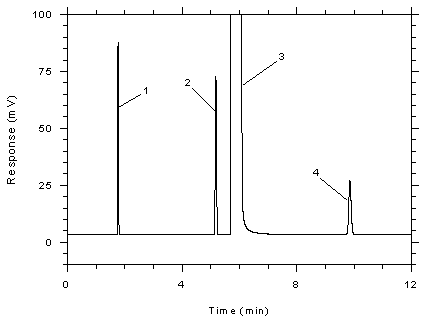
Figure
3.5.1.3 Chromatogram of a standard near the TWA target
concentration for SKC 575-002 samplers. Key: (1)
CS2, (2) toluene, (3) DMF, (4)
ethylbenzene. |
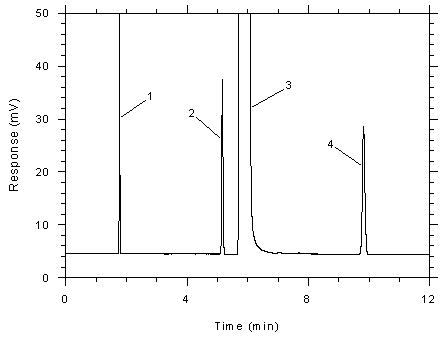
Figure
3.5.1.4 Chromatogram of a standard near the ceiling
concentration for the adsorbent tubes. Key: (1) CS2,
(2) toluene, (3) DMF, (4)
ethylbenzene. |
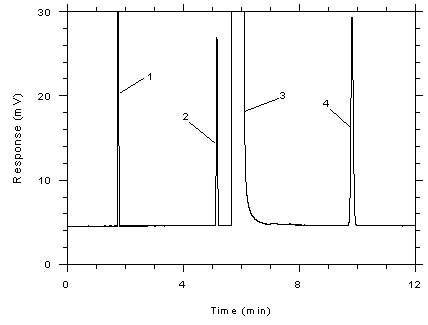
Figure
3.5.1.5 Chromatogram of a standard near the ceiling
concentration for 3M 3520 OVMs. Key: (1) CS2, (2)
toluene, (3) DMF, (4) ethylbenzene. |
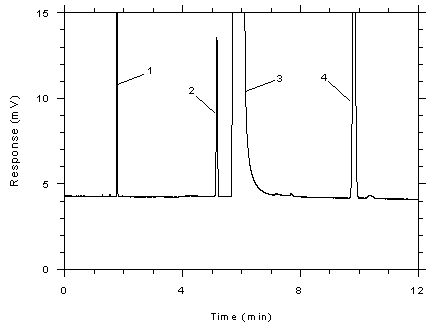
Figure
3.5.1.6 Chromatogram of a standard near the ceiling
concentration for SKC 575-002 samplers. Key: (1)
CS2, (2) toluene, (3) DMF, (4)
ethylbenzene. |
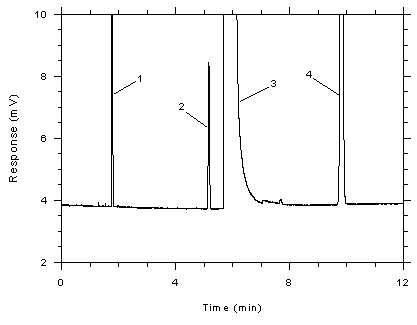
Figure
3.5.1.7 Chromatogram of a standard near the peak target
concentration for adsorbent tubes. Key: (1) CS2, (2)
toluene, (3) DMF, (4) ethylbenzene. |
3.5.2 Peak areas are measured by an integrator or other
suitable means.
3.5.3 An internal standard (ISTD) calibration
method is used. A calibration curve is prepared by analyzing standards
and plotting micrograms of toluene per sample versus ISTD-corrected
area counts of the toluene peaks. Sample concentrations must be
bracketed by standards.
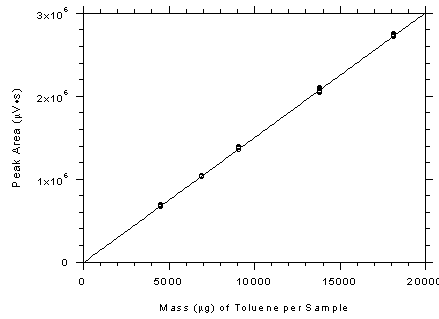
Figure
3.5.3.1 Calibration curve for adsorbent tubes constructed from
the data in Table 4.5. The equation of the line is Y =
150.7X-15400. |
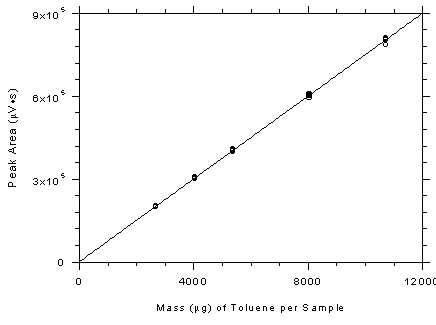
Figure
3.5.3.2 Calibration curve for 3M 3520 OVMs constructed from the
data in Table 4.5.2. The equation of the line is Y =
74.93X+1609. |
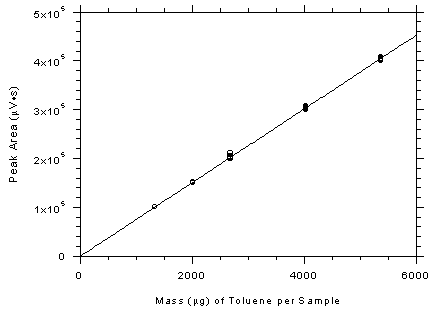
Figure
3.5.3.3 Calibration curve for SKC 575-002 samplers
constructed from the data in Table 4.5.3. The equation of the
line is Y = 75.57X-549.7. | 3.6 Interferences (analytical)
3.6.1 Any compound that produces a
response on a flame ionization detector and has the same general
retention time of toluene or the internal standard is a potential
interference. Possible interferences should be reported to the
laboratory with submitted samples by the industrial hygienist. These
interferences should be considered before samples are
desorbed.
3.6.2 GC parameters (i.e. column and column
temperature) may be changed to possibly circumvent
interferences.
3.6.3 The desorption efficiency from wet
samplers was investigated by spiking samplers with amounts of toluene
equivalent to the mass that would be collected for 240 minutes from
atmospheres containing 200 ppm. Before being spiked with toluene,
humid air (~80% RH, 25°C) had been drawn through the adsorbent tubes
at 50 mL/min for 240 minutes. Similarly, the diffusive samplers had
been exposed to the humid atmosphere for 240 minutes. The desorption
efficiencies were comparable to those reported in Section 2.5.
(Section 4.12)
3.6.4 When necessary, the identity or purity of
an analyte peak may be confirmed with additional analytical data.
(Section 4.13) 3.7
Calculations
3.7.1 Adsorbent tube
samples
The toluene concentration for samples is obtained from
the appropriate calibration curve in terms of micrograms of toluene
per sample, uncorrected for desorption efficiency. The air
concentration is calculated using the following formulae. The back
(50-mg) section is analyzed primarily to determine if there was any
breakthrough from the front section during sampling. If a significant
amount of analyte is found on the back section (e.g., greater than 25%
of the amount found on the front section), this fact should be
reported with sample results. If any analyte is found on the back
section, it is added to the amount found on the front section. This
total amount is then corrected by subtracting the total amount (if
any) found on the blank.
mg/m³ = (µg
of toluene per sample)/((L of air sampled)(desorption
efficiency))
| where: |
desorption efficiency = 0.990 for
Charcoal and 0.991 for Anasorb® 747 Tubes
L of air
sampled = [(sampling time, min)(sampling rate,
mL/min)]/1000 |
ppm =
(mg/m³)(24.46)/(molecular weight of analyte) =
(mg/m³)(0.2655)
| where: |
24.46 is the molar volume at 25°C
and 101.3 kPa (760 mmHg)
and the molecular weight of toluene
= 92.14 |
3.7.2 3M
3520 OVMs and SKC 575-002 Samplers
The toluene
concentration for samples is obtained from the appropriate calibration
curve in terms of micrograms of toluene per sample, uncorrected for
desorption efficiency. The air concentration is calculated using the
following formulae. For the 3M OVMs, the back section is analyzed
primarily to determine if there was any breakthrough from the front
section during sampling. If a significant amount of analyte is found
on the back section (e.g., greater than 25% of the amount found on the
front section), this fact should be reported with sample results. If
any analyte is found on the back section, the amount found is
multiplied by 2.2 (as per manufacturer's instructions) and then added
to the amount found on the corresponding front section. This total
amount is then corrected by subtracting the total amount (if any)
found on the blank.
mg/m³ = (µg of
toluene per sample)/((L of air sampled)(desorption
efficiency))
| where: |
desorption efficiency = 0.981 for
3M OVMs and 0.970 for SKC 575-002 Samplers
L of
air sampled = [(sampling time, min)(sampling rate,
mL/min)]/1000
Sampling rate (3M OVMs) =
(29.54)(760/P)(T/298.2)1.5
Sampling rate (SKC
575-002 Samplers) =
(14.89)(760/P)(T/298.2)1.5
P = the sampling site
pressure (mmHg)
T = the sampling site temperature
(K) |
ppm =
(mg/m³)(24.46)/(molecular weight of analyte) =
(mg/m³)(0.2655)
| where: |
24.46 is the molar volume at 25°C
and 101.3 kPa (760 mmHg)
and the molecular weight of toluene
= 92.14 |
If the
sampling site temperature is not provided, assume that it is 22.2°C
(295.4 K, 72°F). If the sampling site atmospheric
pressure is not given, calculate an approximate value based on the
sampling site elevation from the following equation, which is derived
from the data presented in Ref. 5.16.
P = (3.887 ×
10-7)(E2) B (2.7468 × 10-2)(E) +
760
| Where: |
P = the calculated approximate
atmospheric pressure (mmHg)
E = the sampling site elevation
(ft) |
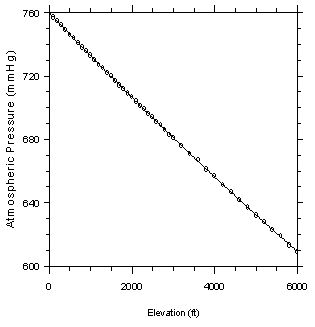
Figure 3.7.2
Plot of atmospheric pressure versus altitude. Data taken from
Reference 5.16. | 3.8 Safety precautions (analytical)
3.8.1 Adhere to the rules set down in
your Chemical Hygiene Plan.
3.8.2 Avoid skin contact and
inhalation of all chemicals.
3.8.3 Wear safety glasses and a
lab coat at all times while in the lab area.
4. Backup Data
4.1 Determination of detection
limits
Detection limits (DL), in general, are defined as the
amount (or concentration) of analyte that gives an instrument response
(YDL) that is significantly different (three standard
deviations (SDBR) from the background response
(YBR).
The
direct measurement of YBR and SDBR in
chromatographic methods is typically inconvenient and difficult because
YBR is usually extremely low. Estimates of these parameters
can be made with data obtained from the analysis of a series of
analytical standards or samples whose responses are in the vicinity of
the background response. The regression curve obtained for a plot of
instrument response versus concentration of analyte will usually be
linear. Assuming SDBR and the precision of data about the
curve are similar, the standard error of estimate (SEE) for the
regression curve can be substituted for SDBR in the above
equation. The following calculations derive a formula for
DL:
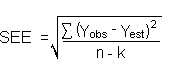
| Yobs |
= observed response |
| Yest |
= estimated response from regression
curve |
| n |
= total no. of data points |
| k |
= 2 for a linear regression
curve |
At point
YDL on the regression curve
| YDL = A(DL) +
YBR |
A = analytical sensitivity
(slope) |
therefore
Substituting 3(SEE) +
YBR for YDL gives
4.2 Detection limit
of the analytical procedure (DLAP)
The DLAP is measured as the
mass of analyte introduced into the chromatographic column. Ten
analytical standards were prepared in equal descending increments with
the highest standard containing 4325 ng of toluene per mL. This standard
produces a peak approximately 10 times the baseline noise of a reagent
blank when a 1-µL injection with a 1:100 split
is made onto the GC column. Standards, plus a reagent blank, were
analyzed and the data obtained were used to determine the required
parameters (A and SEE) for the calculation of the DLAP. Values of 17.66
and 15.3 were obtained for A and SEE respectively. The DLAP was
calculated to be 2.60 pg.
Table
4.2
DLAP for Toluene
|
concentration
(ng/mL) |
mass on
column
(pg) |
peak area
(µV·s) |
|
0.000
432.5
865.0
1298
1730
2162
2595
3028
3460
3892
4325 |
0.00
4.325
8.650
12.98
17.30
21.62
25.95
30.28
34.60
38.92
43.25 |
0
92.7
167
226
338
368
456
547
641
696
761 |
|
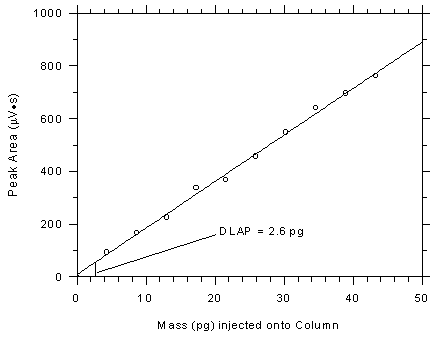
Figure 4.2
Plot of data from Table 4.2 to determine the DLAP. The equation of
the line is Y = 17.66X + 8.40. |
4.3 Detection limit of the overall procedure
(DLOP)
The DLOP is measured as mass per sample and expressed as
equivalent air concentrations, based on the recommended sampling
parameters. Ten samplers of each type were spiked with equal descending
increments of toluene such that the highest sampler loadings were 4325
ng/sample for the adsorbent tubes and 8650 ng/sample for the 3M 3520
OVMs and SKC samplers. (The diffusive samplers were spiked with twice
the amounts of toluene compared to adsorbent tubes because they are
desorbed with 2 mL of solvent versus 1 mL for the adsorbent tubes.)
These are the amounts, when spiked on the samplers, that would produce
peaks approximately 10 times the baseline noise for sample blanks. These
spiked samplers, plus blanks, were analyzed with the recommended
analytical parameters, and the data obtained used to calculate the
required parameters (A and SEE) for the calculation of the DLOPs. Values
of 0.1768 and 14.5, 0.1745 and 20.0, 0.0831 and 18.2, and 0.0943 and
28.4 were obtained for A and SEE for charcoal tubes, Anasorb®
747 tubes, 3M 3520 OVMs and SKC 575-002 samplers
respectively. The DLOPs were calculated to be 246 ng per sample (5.4 ppb
or 20.5 µg/m³), 344 ng per sample (7.6 ppb or
28.7 µg/m³), 657 ng per sample (25 ppb or 93
µg/m³) and 904 ng per sample (67 ppb or 253
µg/m³) for charcoal tubes, Anasorb®
747 tubes, 3M 3520 OVMs and SKC 575-002 samplers
respectively.
Table
4.3.1
Detection Limit of the Overall Procedure
for Charcoal
Tubes
|
mass
(ng)
per sample |
peak
area
(µV·s) |
|
0.0
432.5
865.0
1298
1730
2162
2595
3028
3460
3892
4325 |
0
90.1
162
244
322
386
491
518
639
686
776 |
|
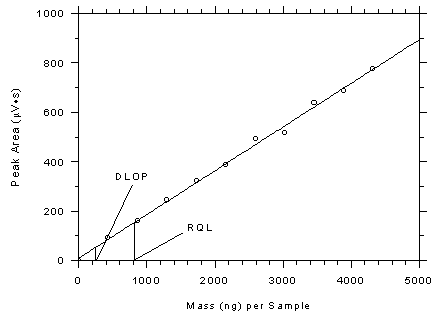
Figure 4.3.1
Plot of data from Table 4.3.1 to determine the DLOP/RQL for
charcoal tubes. The equation of the line is Y = 0.1768X +
9.83. |
Table
4.3.2
Detection Limit of the Overall Procedure
for
Anasorb® 747 Tubes
|
mass
(ng)
per sample |
peak
area
(µV·s) |
|
0.0
432.5
865.0
1298
1730
2162
2595
3028
3460
3892
4325 |
0
130
168
254
308
429
488
579
626
694
768 |
|
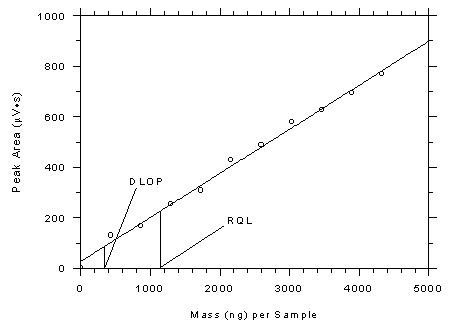
Figure 4.3.2
Plot of data from Table 4.3.2 to determine the DLOP/RQL for
Anasorb® 747 tubes. The equation of the line is Y =
0.1745X + 26.7. |
Table
4.3.3
Detection Limit of the Overall Procedure
for 3M 3520
OVMs
|
mass
(ng)
per sample |
peak
area
(µV·s) |
|
0.0
865
1730
2595
3460
4325
5190
6055
6920
7785
8650 |
0
91.1
146
209
304
370
455
546
599
646
700 |
|
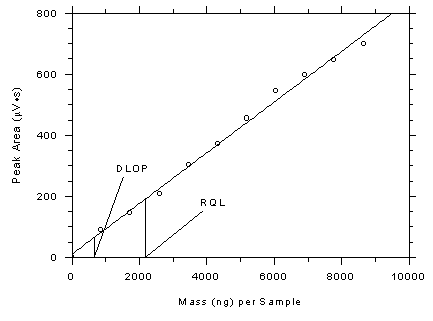
Figure 4.3.3
Plot of data from Table 4.3.3 to determine the DLOP/RQL for 3M
3520 OVMs. The equation of the line is Y = 0.0831X +
10.4. |
Table
4.3.4
Detection Limit of the Overall Procedure
for SKC
575-002 Samplers
|
mass
(ng)
per sample |
peak
area
(µV·s) |
|
0.0
865
1730
2595
3460
4325
5190
6055
6920
7785
8650 |
0
95.1
195
223
307
423
556
588
639
730
813 |
|
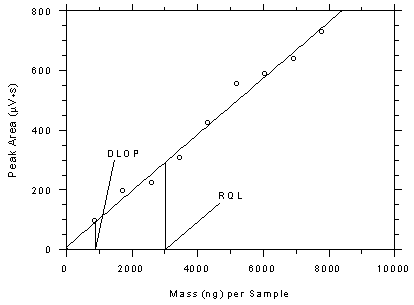
Figure 4.3.4
Plot of data from Table 4.3.4 to determine the DLOP/RQL for SKC
575-002 samplers. The equation of the line is Y =
0.0943X + 8.59. |
4.4
Reliable quantitation limit (RQL)
The RQL is considered the lower
limit for precise quantitative measurements. It is determined from the
regression line data obtained for the calculation of the DLOP (Section
4.3). The RQL is defined as the amount of analyte that gives an
instrument response (YRQL) such that
therefore
The RQLs were
calculated to be 820 ng per sample (18.1 ppb or 68.3 µg/m³), 1146 ng per sample (25.4 ppb or 95.5 µg/m³), 2190 ng per sample (82 ppb or 309 µg/m³) and 3012 ng per sample (224 ppb or 844 µg/m³) for charcoal tubes, Anasorb® 747
tubes, 3M 3520 OVMs and SKC 575-002 samplers respectively.
The recoveries at these levels are 100%, 99%, 94% and 107%
respectively.
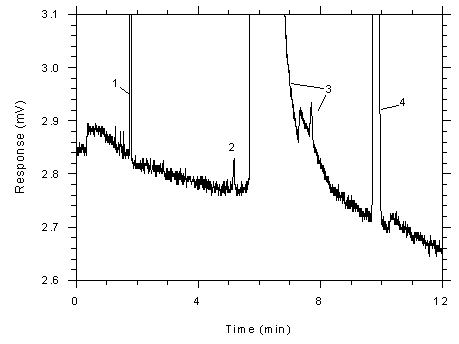
Figure 4.4.1
Chromatogram of a sample (865 ng of toluene per sample) near the
RQL for charcoal tubes (820 ng). Key: (1) CS2, (2)
toluene, (3) DMF and impurities, (4)
ethylbenzene. |
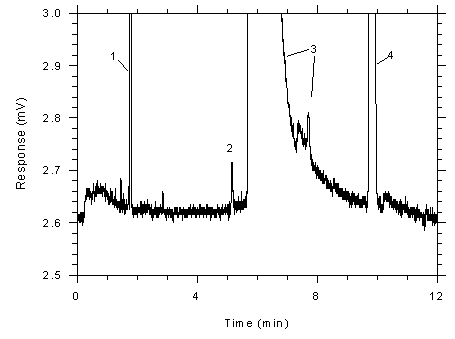
Figure 4.4.2
Chromatogram of a sample (1298 ng of toluene per sample) near the
RQL for Anasorb® (1146 ng). Key: (1) CS2,
(2) toluene, (3) DMF and impurities, (4)
ethylbenzene. |
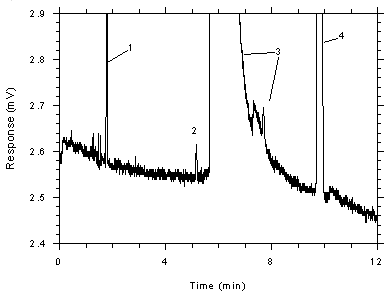
Figure 4.4.3
Chromatogram of a sample (2595 ng of toluene per sample) near the
RQL for 3M 3520 OVMs (2190 ng). Key: (1) CS2, (2)
toluene, (3) DMF and impurities, (4)
ethylbenzene. |
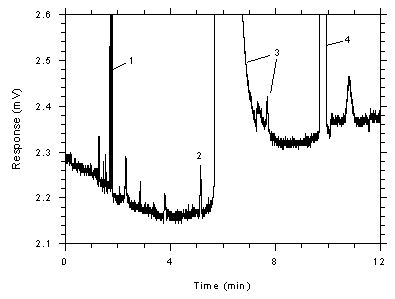
Figure 4.4.4
Chromatogram of a sample (1298 ng of toluene per sample) near the
RQL for SKC 575-002 samplers (3012 ng). Key: (1)
CS2, (2) toluene, (3) DMF and impurities,
(4)ethylbenzene. |
4.5 Precision (analytical method)
The precisions of
the analytical procedure are defined as the pooled relative standard
deviations (RSDP). Relative standard deviations were
determined from six replicate injections of standards at 0.5, 0.75, 1,
1.5 and 2 times the TWA target concentrations. After assuring that the
RSDs satisfy the Cochran test for homogeneity at the 95% confidence
level, the RSDPs were calculated to be 0.76%, 0.94% and 0.76%
for the adsorbent tubes, 3M 3520 OVMs and SKC 575-002
samplers respectively.
Table
4.5.1
Instrument Response for Adsorbent Tubes
|
× target concn
(µg/sample) |
0.5×
4541.2 |
0.75×
6920.0 |
1.0×
9082.5 |
1.5×
13840 |
2.0×
18165 |
|
peak areas
(µV·s)
|
672250
679710
676130
686650
667840
676290 |
1030700
1024500
1033400
1035000
1029400
1033100 |
1388800
1382300
1358700
1375800
1372900
1374800 |
2034600
2055000
2092000
2060800
2093000
2069600 |
2718600
2737100
2744500
2729200
2734000
2744000 |
|
mean
SD
RSD (%) |
676479
6427.5
0.950 |
1031017
3770.1
0.366 |
1375550
10126.2
0.736 |
2067500
22532.0
1.090 |
2734567
9778.9
0.358 |
|
Table
4.5.2
Instrument Response for 3M 3520 OVMs
|
× target concn
(µg/sample) |
0.5×
2681.5 |
0.75×
4022.2 |
1.0×
5363 |
1.5×
8044.5 |
2.0×
10726 |
|
peak areas
(µV·s)
|
204130
200620
202700
198390
201720
201940 |
305410
302040
304170
03280
307530
300290 |
407980
399910
400930
406990
404850
404490 |
605690
595360
601140
608650
606270
606280 |
811630
808570
810300
803260
811770
786360 |
|
mean
SD
RSD (%) |
201584
1949.6
0.967 |
303787
2542.8
0.837 |
404192
3214.82
0.795 |
603899
4849.7
0.803 |
805315
9803.1
1.217 |
|
Table
4.5.3
Instrument Response for SKC 575-002 Samplers
|
× target concn
(µg/sample) |
0.5×
1340.8 |
0.75×
2011.1 |
1.0×
2681.5 |
1.5×
4022.2 |
2.0×
5363 |
|
peak areas
(µV·s)
|
100120
100800
100590
100320
100050
100100 |
151260
150640
149190
151860
150840
152170 |
204130
200620
202700
198390
201720
201940 |
305410
302040
304170
303280
307530
300290 |
407980
399910
400930
406990
404850
404490 |
|
mean
SD
RSD (%) |
100330
304.6
0.304 |
150994
1059.0
0.701 |
201584
1949.6
0.967 |
303787
2542.8
0.837 |
404192
3214.8
0.795 |
|
The Cochran test
for homogeneity:
The
g statistics are 0.410, 0.337 and 0.328 for
the adsorbent tubes, 3M 3520 OVMs and SKC 575-002 samplers
respectively. The critical value of the g statistic at the 95%
confidence level for five variances, each associated with six
observations, is 0.5065. Because the g statistics do not exceed this
value, the RSDs can be considered equal and they can be pooled
(RSDP) to give an estimated RSD for the concentration range
studied.
4.6
Precision (overall procedure)
The precision of the overall
procedure is determined from the storage data in Section 4.7. The
determination of the standard error of estimate (SEER) for a
regression line plotted through the graphed storage data allows the
inclusion of storage time as one of the factors affecting overall
precision. The SEER is similar to the standard deviation,
except it is a measure of dispersion of data about a regression line
instead of about a mean. It is determined with the following
equation:
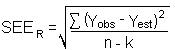
| n |
= |
total no. of data points |
| k |
= |
2 for linear regression |
| k |
= |
3 for quadratic regression |
| Yobs |
= |
observed % recovery at a given
time |
| Yest |
= |
estimated % recovery from the
regression line at the same given
time |
The following
formula is used to determine the total standard error of estimate (SEE)
for adsorbent tubes. An additional 5% for pump error (SP) is added to
the SEER by the addition of variances.
The
following formula is used to determine the total standard error of
estimate (SEE) for diffusive samplers when the sampling site temperature
and pressure are known. SR is the sampling rate variability that has
been determined in separate studies to be 6.4% for 3M OVMs and 8.7% for
SKC samplers. (Refs. 5.10 and 5.17)
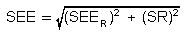 |
The
following formula is used when the sampling site temperature or pressure
are not known. Additional variances are included that account for
uncertainty for uncorrected temperature (ST) and pressure (SPr) effects
on the sampling rate. When the temperature is unknown, it will be
assumed that it is 22.2°C (295.4 K, 72°F) and a value of
7.7% shall be used for ST. This is the maximum variability in the
sampling rate due to temperature over the range of 7.2-37.2°C (22.2±15°C
or 72±27°F). When the atmospheric pressure is unknown, it will be
determined from the estimated elevation of the sampling site. A value of
3% shall be used for SPr to account for pressure fluctuations due to
weather conditions. This is an estimate of the variability due to
weather, based on a study that tracked atmospheric pressure variations
over a year's time at SLTC. (Ref. 5.10)
 |
The
precision at the 95% confidence level is obtained by multiplying the SEE
by 1.96 (the z statistic from the standard normal distribution at the
95% confidence level). The 95% confidence intervals are drawn about
their respective regression lines in the storage graphs, as shown in the
figures in Section 4.7. The SEEs and precisions of the overall procedure
are given in the following tables for adsorbent tubes and diffusive
samplers. All values are based on the ambient storage tests in Section
4.7.
Table
4.6.1
SEEs and Precisions of the Overall Procedure at the 95%
Confidence Interval
for Adsorbent Tubes
|
|
TWA Samples |
Ceiling
Samples |
Peak
Samples |
| Adsorbent |
|
|
SEE |
Precision |
SEE |
Precision |
SEE |
Precision |
|
Charcoal
Anasorb® 747 |
5.51%
5.15% |
±10.8%
±10.1% |
5.20%
5.14% |
±10.2%
±10.1% |
5.25%
5.36% |
±10.3%
±10.5% |
|
Table 4.6.2
SEEs and Precisions of the Overall
Procedure at the 95% Confidence Interval
for Diffusive Samplers
When Sampling Site Temperature (T)
or Atmospheric Pressure (P)
are Known or Unknown
|
|
TWA
Samples |
Ceiling Samples |
| Sampler |
|
|
SEE |
Precision |
SEE |
Precision |
|
3M 3520
OVM
both T&P
known
only T
known
only P
known
neither T nor P known
SKC
575-002 Sampler
both
T&P known
only T
known
only P
known
neither T nor P known |
7.19%
7.79%
10.5%
11.0%
9.18
9.66
12.0%
12.4% |
±14.1%
±15.3%
±20.6%
±21.5%
±18.0%
±18.9%
±23.5%
±24.3% |
7.93%
8.48%
11.1%
11.5%
9.49%
9.95%
12.2%
12.6% |
±15.5%
±16.6%
±21.7%
±22.4%
±18.6%
±19.5%
±23.9%
±24.7% |
|
4.7 Storage
tests
Adsorbent tube storage samples were prepared by sampling at
50 mL/min from controlled test atmospheres that were at approximately
80% RH and at room temperatures ranging from 22-26°C and
atmospheric pressures from 649-656 mmHg. Storage samples for 3M 3520
OVMs and SKC samplers were prepared by exposing them to the same
atmospheres as for the adsorbent tubes. The flow of the atmospheres
through the exposure chamber provided for face velocities on the
diffusive samplers of approximately 0.4 m/s. TWA samples were prepared
by sampling from 200-ppm atmospheres for 240 minutes, ceiling samples
from 300-ppm atmospheres for 10 minutes and peak samples from 500-ppm
atmospheres for 1 minute. Six samples for each set were analyzed
immediately after generation, fifteen were stored in a refrigerator at
0°C and fifteen were stored in a closed drawer at ambient temperatures
of 20-25°C. Only ambient storage tests were done for the
ceiling and peak samples and only adsorbent tube samples were generated
for the peak level. At approximately three-day intervals, three samples
were selected from each of the storage sets and analyzed.
Table
4.7.1
TWA Storage Tests for Charcoal Tubes
|
| time (days) |
refrigerated
storage
recovery (%) |
|
ambient
storage
recovery (%) |
|
0
0
3
6
11
14
19 |
100.6
102.8
99.7
101.7
99.8
99.4
98.1 |
102.0
100.4
99.1
101.3
100.4
101.4
99.5 |
99.9
101.3
101.4
101.3
99.4
99.9
100.8 |
|
100.6
102.8
100.2
100.0
99.5
99.6
100.3 |
102.0
100.4
90.7
99.7
100.1
99.8
99.8 |
99.9
101.3
99.2
100.2
100.1
100.6
100.8 |
|
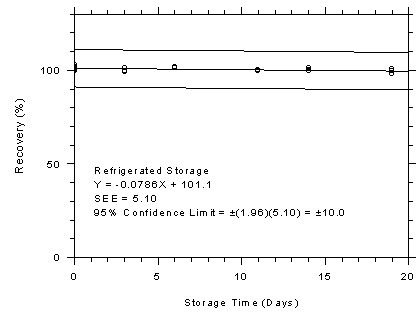
Figure 4.7.1.1
Charcoal tubes refrigerated storage test, 240-minute samples at
200 ppm. |
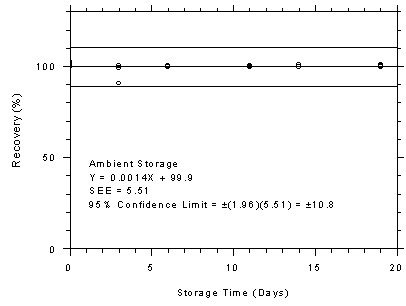
Figure 4.7.1.2
Charcoal tubes ambient storage test, 240-minute samples at 200
ppm. |
Table
4.7.2
TWA Storage Tests for Anasorb® 747 Tubes
|
| time (days) |
refrigerated
storage
recovery (%) |
|
ambient
storage
recovery (%) |
|
0
0
3
6
11
14
19 |
100.2
100.7
100.4
100.2
100.6
103.3
100.9 |
100.5
100.0
100.3
99.6
99.2
101.2
100.6 |
99.8
95.6
99.9
100.6
101.0
103.8
100.3 |
|
100.2
100.7
98.6
99.9
99.2
100.7
99.3 |
100.5
100.0
101.5
99.4
100.1
101.6
101.6 |
99.8
95.6
99.5
100.2
100.4
100.6
101.5 |
|
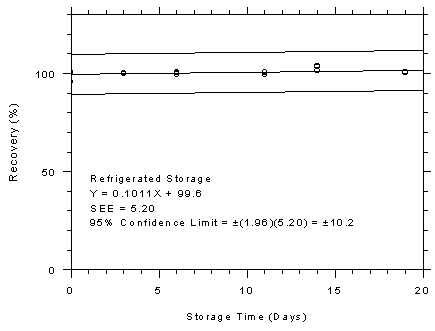
Figure 4.7.2.1
Anasorb® 747 tubes refrigerated storage test,
240-minute samples at 200 ppm. |
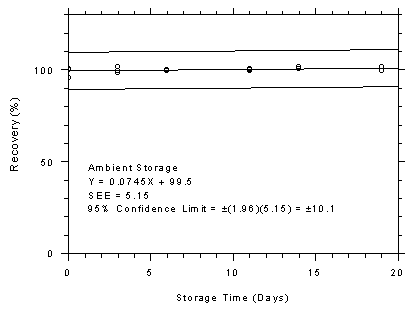
Figure 4.7.2.2
Anasorb® 747 tubes ambient storage test, 240-minute
samples at 200 ppm. |
Table
4.7.3
TWA Storage Tests for 3M 3520 OVMs
|
| time (days) |
refrigerated
storage
recovery (%) |
|
ambient
storage
recovery (%) |
|
0
0
3
6
11
14
19 |
105.0
104.3
101.9
103.9
103.1
104.3
105.2 |
100.7
102.4
99.1
104.6
107.2
104.0
102.6 |
104.4
99.7
101.9
100.9
103.8
101.0
101.4 |
|
105.0
104.3
107.9
98.1
99.0
109.6
111.0 |
100.7
102.4
102.7
105.8
102.9
102.1
107.9 |
104.4
99.7
103.9
104.3
101.3
104.4
104.4 |
|
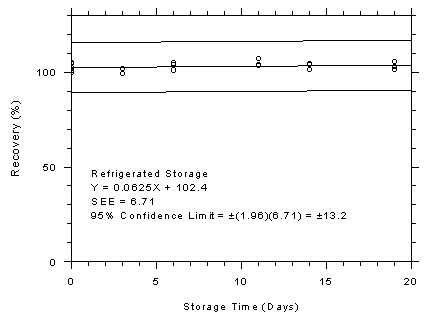
Figure 4.7.3.1
3M 3520 OVMs refrigerated storage test, 240-minute samples at 200
ppm. |
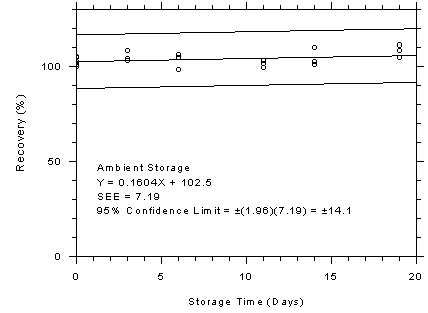
Figure 4.7.3.2
3M 3520 OVMs ambient storage test, 240-minute samples at 200
ppm. |
Table
4.7.4
TWA Storage Tests for SKC 575-002 Samplers
|
| time (days) |
refrigerated
storage
recovery (%) |
|
ambient
storage
recovery (%) |
|
0
0
3
6
11
14
19 |
100.3
99.8
100.1
91.2
101.1
97.4
101.0 |
95.8
97.8
97.3
92.3
95.8
98.8
101.3 |
102.6
96.3
99.2
98.5
93.9
97.8
90.6 |
|
100.3
99.8
97.0
95.9
100.2
100.2
100.2 |
95.8
97.8
94.9
96.5
91.4
102.2
95.7 |
102.6
96.3
94.9
94.8
99.9
100.6
99.9 |
|
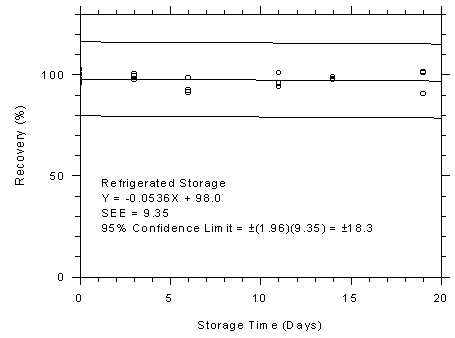
Figure 4.7.4.1
SKC 575-002 samplers refrigerated storage test,
240-minute samples at 200 ppm. |
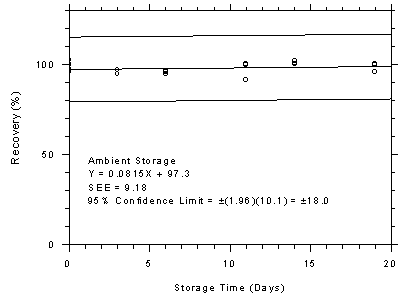
Figure 4.7.4.2
SKC 575-002 samplers ambient storage test, 240-minute
samples at 200 ppm. |
Table
4.7.5
Ceiling Storage Tests for Adsorbent Tubes
|
| time (days) |
Charcoal
recovery (%) |
|
Anasorb® 747
recovery (%) |
|
0
0
3
6
10
12
15 |
97.9
99.2
99.9
97.3
98.5
97.0
98.8 |
99.7
98.6
100.2
94.7
97.1
99.4
98.0 |
101.4
99.7
97.2
98.0
100.0
97.5
98.4 |
|
98.0
100.9
97.4
97.4
97.7
98.0
97.1 |
98.0
100.9
99.7
97.1
98.9
99.2
98.5 |
99.5
98.0
97.7
96.4
96.6
97.6
98.0 |
|
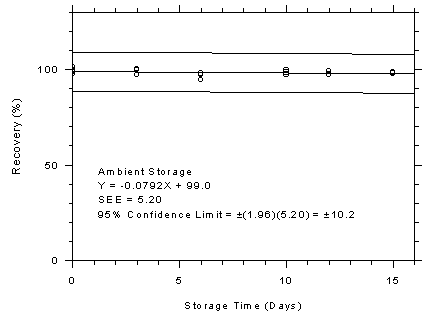
Figure 4.7.5.1
Charcoal tubes ambient storage test, 10-minute
samples at 300 ppm. |
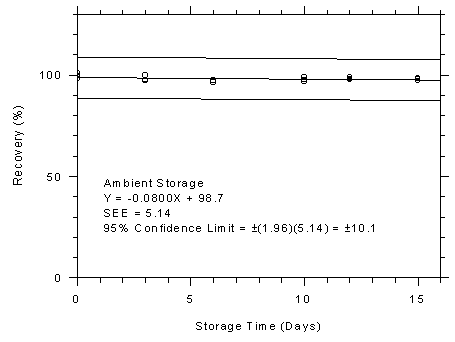
Figure 4.7.5.2
Anasorb® 747 tubes ambient storage test,
10-minute samples at 300-ppm. |
Table
4.7.6
Ceiling Storage Tests for Diffusive Samplers
|
| time (days) |
3M 3520
OVMs
recovery (%) |
|
SKC
575-002 Samplers
recovery (%) |
|
0
0
3
6
10
12
15 |
110.8
102.5
102.7
103.6
101.8
100.0
96.7 |
111.6
101.6
100.8
99.4
102.7
95.6
100.8 |
96.9
101.8
104.4
100.7
111.7
94.2
106.0 |
|
98.8
90.5
97.6
?99.8
93.9
90.2
94.6 |
94.3
94.6
100.7
99.2
96.6
92.4
98.2 |
97.3
87.7
93.5
89.3
92.2
97.2
92.0 |
|
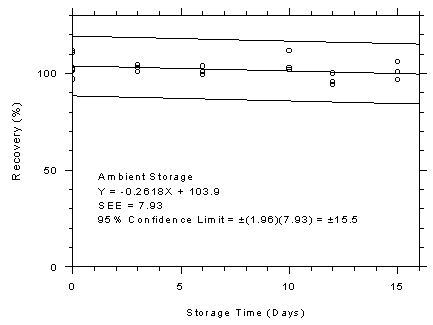
Figure 4.7.6.1
3M 3520 OVMs ambient storage test, 10-minute samples
at 300 ppm. |
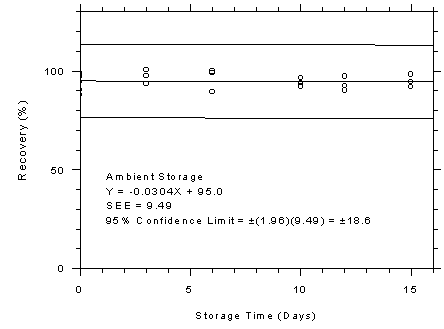
Figure 4.7.6.2
SKC 575-002 samplers ambient storage test,
10-minute samples at 300
ppm. |
Table
4.7.7
Peak Storage Tests for Adsorbent Tubes
|
| time (days) |
Charcoal
Tubes
recovery (%) |
|
Anasorb® 747 Tubes
recovery (%) |
|
0
0
3
6
10
12
15 |
96.1
98.0
95.5
92.0
95.0
95.3
96.6 |
94.9
99.7
96.5
94.4
95.0
95.2
95.9 |
97.8
95.1
93.8
94.1
94.8
95.8
94.3 |
|
95.3
97.5
97.9
95.2
96.4
97.4
90.7 |
98.2
97.5
97.6
95.0
96.5
99.0
96.8 |
99.3
96.8
99.4
95.7
93.0
95.1
96.7 |
|
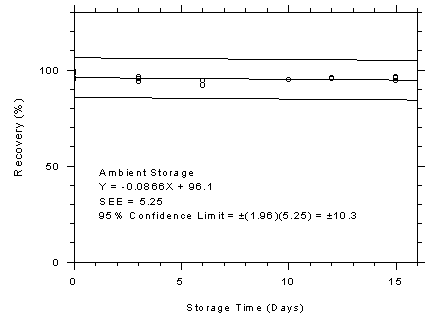
Figure
4.7.7.1 Charcoal tubes ambient storage test, 1-minute samples at
500 ppm. |
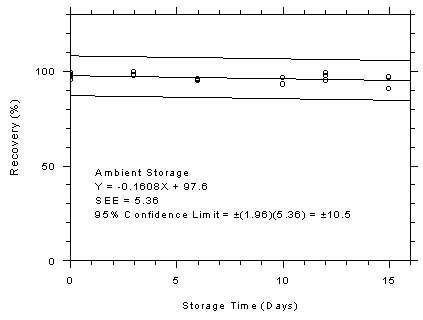
Figure 4.7.7.2
Anasorb® 747 tubes ambient storage test, 1-minute
samples at 500 ppm. |
4.8
Reproducibility
Six TWA reproducibility samples for each of the
four types of samplers were prepared by collecting them from a
controlled test atmosphere similar to that which was used in the
collection of the storage samples in Section 4.7. The samples were
submitted to an SLTC service branch for analysis. The charcoal tube and
3M 3520 OVM samples were analyzed 20 days after generation and the
Anasorb® 747 tube and SKC 575-002 samples were
analyzed 53 days after generation. The samples were stored at room
temperature. No sample result had a deviation greater than the
precisions of the overall procedure determined in Section 4.6, which are
±10.8%, ±10.1%, ±14.1% and ±18.0% for charcoal tubes,
Anasorb® 747 tubes, 3M 3520 OVMs and SKC 575-002
samplers respectively.
Table
4.8.1
Reproducibility Data for Charcoal Tubes
|
sample
no. |
ppm
reported |
ppm
expected |
percent
found |
deviation
(%) |
|
1
2
3
4
5
6 |
183.0
183.1
183.8
180.5
183.0
182.2 |
197.1
197.1
197.1
197.1
197.1
197.1 |
92.8
92.9
93.3
91.6
92.8
92.4 |
-7.2
-7.1
-6.7
-8.4
-7.2
-7.6 |
|
Table
4.8.2
Reproducibility Data for Anasorb® 747 Tubes
|
sample
no. |
ppm
reported |
ppm
expected |
percent
found |
deviation
(%) |
|
1
2
3
4
5
6 |
197.8
197.6
197.4
198.2
194.2
199.1 |
201.1
201.1
201.1
201.1
201.1
201.1 |
98.4
98.3
98.2
98.6
96.6
99.0 |
-1.6
-1.7
-1.8
-1.4
-3.4
-1.0 |
|
Table
4.8.3
Reproducibility Data for 3M 3520 OVMs
|
sample
no. |
ppm
reported |
ppm
expected |
percent
found |
deviation
(%) |
|
1
2
3
4
5
6 |
197.7
193.4
192.6
197.1
195.6
199.1 |
197.1
197.1
197.1
197.1
197.1
197.1 |
100.3
98.1
97.7
100.0
99.2
101.0 |
+0.3
-1.9
-2.3
0.0
-0.8
+1.0 |
|
Table
4.8.4
Reproducibility Data for SKC 575-002
Samplers
|
sample
no. |
ppm
reported |
ppm
expected |
percent
found |
deviation
(%) |
|
1
2
3
4
5
6 |
207.9
213.0
208.1
206.8
191.7
209.7 |
201.1
201.1
201.1
201.1
201.1
201.1 |
103.4
105.9
103.5
102.8
95.3
104.3 |
+3.4
+5.9
+3.5
+2.8
-4.7
+4.3 |
|
4.9 Sampler capacity
and sampling rate
4.9.1 Charcoal tubes
The
sampling capacity of the front section of charcoal sampling tubes was
tested by sampling from a dynamically generated test atmosphere of
toluene at 401.6 ppm (1513 mg/m³). The samples were collected at a
nominal flow rate of 50 mL/min and the relative humidity of the
atmosphere was 73% at 29.1°C. Complete charcoal tubes were placed
in-line behind the front test sections and changed at measured
intervals. The average 5% breakthrough volume was determined to be
16.8 L from three determinations.
Table
4.9.1
Breakthrough of Toluene with
Charcoal Sampling Tubes
|
| smplr tube no. |
air vol (L) |
sampling time (min) |
downstream concn
(mg/m³) |
break-through
(%) |
|
1
2
3
|
5.50
12.56
15.71
18.84
21.99
25.13
5.28
12.77
15.96
19.15
22.34
25.54
5.25
11.97
14.97
17.96
20.95
23.95 |
0-210
210-270
270-330
330-390
390-450
450-510
0-210
210-270
270-330
330-390
390-450
450-510
0-210
210-270
270-330
330-390
390-450
450-510 |
0
0
9.68
410.6
1301
1461
0
0.63
9.75
406.4
1273
1424
0
0
4.65
248.8
1136
1495 |
0
0
0.64
27.1
86.0
96.6
0
0.04
0.65
26.9
84.1
94.1
0
0
0.31
16.4
75.1
98.8 |
|
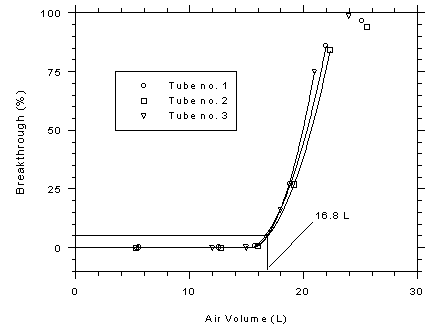
Figure 4.9.1
Determination of the 5% breakthrough volume for charcoal
tubes. |
4.9.2
Anasorb® 747 tubes
The sampling capacity of the
front section of Anasorb® 747 sampling tubes was tested by
sampling from a dynamically generated test atmosphere of toluene at
401.6 ppm (1513 mg/m³). The samples were collected at a nominal flow
rate of 50 mL/min and the relative humidity of the atmosphere was 73%
at 29.1°C. Complete Anasorb® 747 tubes were placed in-line
behind the front test sections and changed at measured intervals. The
average 5% breakthrough volume was determined to be 20.6 L from three
determinations.
Table
4.9.2
Breakthrough of Toluene with
Anasorb® 747
Sampling Tubes
|
| smplr tube no. |
air vol (L) |
sampling time (min) |
downstream concn
(mg/m³) |
break-through
(%) |
|
1
2
3
|
5.06
11.56
14.45
17.34
20.24
23.13
5.65
12.91
16.13
19.36
22.59
25.81
5.28
12.06
15.07
18.09
21.10
24.11 |
0-210
210-270
270-330
330-390
390-450
450-510
0-210
210-270
270-330
330-390
390-450
450-510
0-210
210-270
270-330
330-390
390-450
450-510 |
0
0
0.28
4.67
40.9
198.9
0
0
1.05
26.6
192.3
830.3
0
0
1.73
20.9
100.2
352.5 |
0
0
0.02
0.31
2.70
13.2
0
0
0.07
1.76
12.7
32.2
0
0
0.11
1.38
6.62
14.6 |
|
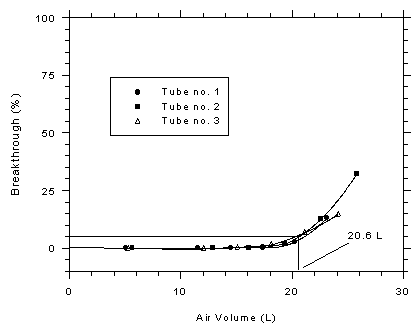
Figure 4.9.2
Determination of the 5% breakthrough volume for
Anasorb® 747 tubes. |
4.9.3 3M 3520 OVMs
The sampling rate and capacity
of 3M 3520 OVMs was determined by taking samples from a dynamically
generated test atmosphere of toluene (nominal concentration of 400 ppm
or 1507 mg/m³) for increasing time intervals. The atmosphere was at
approximately 75% relative humidity, 29°C and 647 mmHg. The flow of
the atmosphere through the exposure chamber provided for face
velocities on the diffusive samplers of approximately 0.4 m/s. The
data obtained are shown in Table 4.9.3 and Figure 4.9.3. Three samples
were taken for each sampling interval. Sampler capacity is exceeded
when the sampling rate decreases rapidly. Because this did not occur
for the time period tested, the capacity is estimated to be greater
than 32 mg. A sampling rate standardized to 760 mmHg and 25°C of 29.54
mL/min was determined to be average sample rate from samples collected
from 7.5 to 240 minutes.
Table
4.9.3
Sampling Rate and Capacity
for Toluene using 3M 3520
OVMs
|
sampling
time
(min) |
sampling
rate
(mL/min) |
RSD
(%) |
|
7.5
15
30
60
120
240
362
481
600
720 |
30.92
29.79
29.37
29.93
28.99
28.25
28.60
27.93
29.61
27.52 |
2.12
2.28
0.71
3.10
0.38
3.68
3.11
0.69
0.84
2.17 |
|
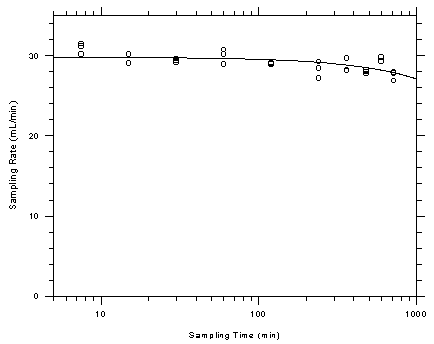
Figure 4.9.3
Determination of sampling rate and capacity for toluene using 3M
3520 OVMs. |
4.9.4
SKC 575-002 Samplers
The sampling rate and
capacity of the SKC 575-002 Sampler were determined by
taking samples from a dynamically generated test atmosphere of toluene
(nominal concentration of 400 ppm or 1507 mg/m³) for increasing time
intervals. The atmosphere was at approximately 76% relative humidity,
26°C and 650 mmHg. The flow of the atmosphere through the exposure
chamber provided for face velocities on the diffusive samplers of
approximately 0.4 m/s. The data obtained are shown in Table 4.9.4 and
Figure 4.9.4. Three samples were taken for each sampling interval.
Sampler capacity is exceeded when the sampling rate decreases rapidly.
Because this did not occur for the time period tested, the capacity is
estimated to be greater than 16 mg. A sampling rate standardized to
760 mmHg and 25°C of 14.89 mL/min was determined to be the average
sample rate from samples collected from 7.5 to 245
minutes.
Table
4.9.4
Sampling Rate and Capacity
for Toluene using SKC
575-002 Samplers
|
sampling
time
(min) |
sampling
rate
(mL/min) |
RSD
(%) |
|
7.5
15
30
60
120
245
360
480
600
720 |
15.25
14.90
14.90
14.78
14.47
15.02
14.90
14.93
15.13
14.67 |
2.13
1.02
1.08
2.48
3.38
1.48
2.19
2.62
1.56
1.55 |
|
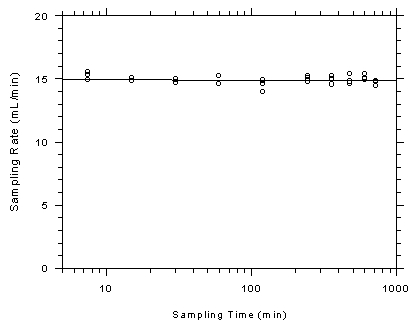
Figure 4.9.4
Determination of sampling rate and capacity for toluene using
SKC 575-002
samplers. | 4.10
Desorption efficiency
4.10.1 Charcoal tubes
4.10.1.1 The desorption efficiency
(DE) of toluene was determined by liquid-spiking 100-mg
portions of charcoal with the analyte at 0.05 to 2 times the TWA
target concentration. These samples were stored overnight at ambient
temperature and then desorbed and analyzed. The average desorption
efficiency over the working range of 0.5 to 2 times the target
concentration is 99.0%.
Table
4.10.1.1
Desorption Efficiency of Toluene from Charcoal
Tubes
|
× target
concn
mass spiked (µg) |
0.05×
451.5 |
0.1×
903.9 |
0.2×
1807.8 |
0.5×
4515.3 |
1.0×
9039.2 |
2.0×
18078 |
|
DE
(%)
|
96.9
97.7
97.9
97.4
97.3
97.4 |
97.8
98.0
98.9
98.4
97.8
98.2 |
98.0
98.9
98.9
98.4
98.6
97.9 |
98.3
98.9
98.4
98.1
98.6
98.0 |
100.2
99.2
99.8
99.3
98.7
98.2 |
98.9
100.4
98.8
99.0
100.4
98.2 |
|
 |
97.4 |
98.2 |
98.4 |
98.4 |
99.2 |
99.3 |
|
4.10.1.2 Stability
of desorbed charcoal tubes samples
The stability of desorbed
samples was investigated by reanalyzing the target concentration
samples 24 h after initial analysis. After the original analysis was
performed three vials were recapped with new septa while the
remaining three retained their punctured septa. The samples were
reanalyzed with fresh standards. The average percent change was
-0.5% for samples that were resealed with new septa and -1.5% for
those that retained their punctured septa.
Table
4.10.1.2
Stability of Desorbed Charcoal Tube Samples
|
| punctured septa replaced |
punctured septa retained |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
|
100.2
99.2
99.8
99.7 |
99.7
99.0
99.1
(averages)
99.3 |
-0.5
-0.2
-0.7
-0.5 |
99.3
98.7
98.2
98.7 |
97.7
97.0
97.0
(averages)
97.2 |
-1.6
-1.7
-1.2
-1.5 |
| 4.10.2
Anasorb® 747 tubes
4.10.2.1 The desorption efficiency
(DE) of toluene was determined by liquid-spiking 140-mg
portions of Anasorb® 747 with the analyte at 0.05 to 2
times the TWA target concentration. These samples were stored
overnight at ambient temperature and then desorbed and analyzed. The
average desorption efficiency over the working range of 0.5 to 2
times the target concentration is 99.1%.
Table
4.10.2.1
Desorption Efficiency of Toluene from
Anasorb® 747 Tubes
|
× target
concn
mass spiked (µg) |
0.05×
451.5 |
0.1×
903.9 |
0.2×
1807.8 |
0.5×
4515.3 |
1.0×
9039.2 |
2.0×
18078 |
|
DE
(%)
|
96.2
98.9
97.6
97.8
95.9
97.4 |
98.2
98.6
98.1
98.1
97.4
98.2 |
99.2
99.0
99.5
99.4
98.8
99.0 |
99.5
99.0
98.7
98.9
98.9
98.9 |
98.9
99.6
99.6
98.3
98.7
99.1 |
97.2
99.6
98.8
99.4
100.7
99.7 |
|
 |
97.3 |
98.1 |
99.2 |
99.0 |
99.0 |
99.2 |
|
4.10.2.2 Stability
of desorbed Anasorb® 747 tube samples
The
stability of desorbed samples was investigated by reanalyzing the
target concentration samples 24 h after initial analysis. After the
original analysis was performed three vials were recapped with new
septa while the remaining three retained their punctured septa. The
samples were reanalyzed with fresh standards. The average percent
change was -0.6% for samples that were resealed with new septa and
-1.5% for those that retained their punctured septa.
Table
4.10.2.2
Stability of Desorbed Anasorb® 747 Tube
Samples
|
| punctured septa replaced |
punctured septa retained |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
|
98.9
99.6
99.6
99.4 |
97.8
99.2
99.4
(averages)
98.8 |
-1.1
-0.4
-0.2
-0.6 |
98.3
98.7
99.1
98.7 |
97.2
97.0
97.5
(averages)
97.2 |
-1.1
-1.7
-1.6
-1.5 |
| 4.10.3 3M
3520 OVMs
4.10.3.1 The desorption efficiency
(DE) of toluene was determined by liquid-spiking
charcoal pads from 3M 3520 OVMs with the analyte at 0.05 to 2 times
the TWA target concentration. These samples were stored overnight at
ambient temperature and then desorbed and analyzed. The average
desorption efficiency over the working range of 0.5 to 2 times the
target concentration is 98.1%.
Table
4.10.3.1
Desorption Efficiency of Toluene from 3M 3520 OVMs
|
× target
concn
mass spiked (µg) |
0.05×
259.5 |
0.1×
519.0 |
0.2×
1038 |
0.5×
2595 |
1.0×
5190 |
2.0×
10380 |
|
DE
(%)
|
99.4
97.9
98.2
98.2
98.6
98.4 |
98.0
98.4
99.1
98.3
98.0
97.5 |
98.1
98.5
98.1
98.4
97.8
97.3 |
98.0
98.3
97.8
99.4
98.0
97.7 |
98.3
97.7
97.8
97.8
98.2
98.5 |
98.0
98.0
98.2
97.8
98.1
98.0 |
|
 |
98.4 |
98.2 |
98.0 |
98.2 |
98.0 |
98.0 |
|
4.10.3.2 Stability
of desorbed 3M 3520 OVM samples
The stability of desorbed
samples was investigated by reanalyzing the target concentration
samples 24 h after initial analysis. After the original analysis was
performed three vials were recapped with new septa while the
remaining three retained their punctured septa. The samples were
reanalyzed with fresh standards. The average percent change was
+0.7% for samples that were resealed with new septa and +0.3% for
those that retained their punctured septa.
Table
4.10.3.2
Stability of Desorbed 3M 3520 OVM Samples
|
| punctured septa replaced |
punctured septa retained |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
|
98.3
97.7
97.8
97.9 |
98.7
98.5
98.7
(averages)
98.6 |
+0.4
+0.8
+0.9
+0.7 |
97.8
98.2
98.5
98.2 |
98.4
98.5
98.6
(averages)
98.5 |
+0.6
+0.3
+0.1
+0.3 |
| 4.10.4 SKC
575-002 Samplers
4.10.4.1 The desorption efficiency
(DE) of toluene was determined by liquid-spiking SKC
575-002 Samplers with the analyte at 0.05 to 2 times
the TWA target concentration. These samples were stored overnight at
ambient temperature and then desorbed and analyzed. The average
desorption efficiency over the working range of 0.5 to 2 times the
target concentration is 97.0%.
Table
4.10.4.1
Desorption Efficiency of Toluene from SKC
575-002 Samplers
|
× target
concn
mass spiked (µg) |
0.05×
129.8 |
0.1×
259.5 |
0.2×
519.0 |
0.5×
1297.5 |
1.0×
2595 |
2.0×
5190 |
|
DE
(%)
|
97.6
97.8
98.0
96.6
99.8
98.2 |
97.4
98.2
99.8
98.4
97.3
97.6 |
97.2
97.0
96.8
98.9
98.1
97.9 |
96.0
97.3
97.2
97.1
96.8
96.6 |
97.4
98.6
97.9
97.7
97.0
97.3 |
97.0
97.1
97.3
96.7
94.7
96.6 |
|
 |
98.0 |
98.1 |
97.6 |
96.8 |
97.6 |
96.6 |
|
4.10.4.2 Stability
of desorbed SKC 575-002 Samplers
The stability
of desorbed samples was investigated by reanalyzing the target
concentration samples 24 h after initial analysis. After the
original analysis was performed three vials were recapped with new
septa while the remaining three retained their punctured septa. The
samples were reanalyzed with fresh standards. The average percent
change was -0.2% for samples that were resealed with new septa and
-0.6% for those that retained their punctured septa.
Table
4.10.4.2
Stability of Desorbed SKC 575-002
Samplers
|
| punctured septa replaced |
punctured septa retained |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
initial
DE
(%) |
DE after
one
day
(%) |
difference |
|
97.4
98.6
97.9
98.0 |
97.6
98.1
97.5
(averages)
97.7 |
+0.2
-0.5
-0.4
-0.2 |
97.7
97.0
97.3
97.3 |
97.4
96.1
96.7
(averages)
96.7 |
-0.3
-0.9
-0.6
-0.6 |
| 4.11 Interferences (sampling)
4.11.1 Interference studies were
performed by sampling for 240 minutes from a test atmosphere (10% RH,
26°C, 654.8 mmHg) containing 396 ppm of toluene with 50 ppm of
2-butanone (MEK), 20 ppm of 4-methyl-2-pentanone (MIBK),
20 ppm of 1-butanol, 30 ppm of isobutyl acetate and 30 ppm of xylene.
The average results for the adsorbent tubes did not deviate from the
theoretical concentration of toluene by more than three standard
deviations of the Day 0 storage samples in Section 4.7 (RSD = 1.07%
for charcoal and 1.93% for Anasorb® 747 tubes). The average
results for the diffusive samplers did not deviate from the
theoretical concentration of toluene by more than three standard
deviations based on the sampling rates determined in Section 4.9.3 for
3M OVMs (RSD = 3.49%) and in Section 4.9.4 for SKC
575-002 samplers (RSD = 2.39%). The presence of these
compounds, which may represent typical substances that may be
collected with toluene, did not have a significant effect on sample
results using any of the samplers.
Table
4.11.1
Recovery (%) from the Atmosphere Described in 4.11.1
for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
102.5
100.9
105.8
102.3
99.7
102.0
102.2 |
98.5
99.2
99.6
100.2
99.6
98.7
99.3 |
107.5
106.9
102.2
105.7
98.1
109.8
105.0 |
96.0
98.7
97.5
100.7
96.1
96.8
97.6 |
|
4.11.2 Short-term
sampling interference studies were performed by sampling for 1 minute
from a test atmosphere (10% RH, 25°C, 654.3 mmHg) containing 495 ppm
of toluene with 50 ppm of 2-butanone (MEK), 20 ppm of
4-methyl-2-pentanone (MIBK), 20 ppm of 1-butanol, 30 ppm
of isobutyl acetate and 30 ppm of xylene. The average results for the
adsorbent tubes did not deviate from the theoretical concentration of
toluene by more than three standard deviations of the Day 0 storage
samples in Section 4.7 (RSD = 1.07% for charcoal and 1.93% for
Anasorb® 747 tubes). The average results for the diffusive
samplers did not deviate from the theoretical concentration of toluene
by more than three standard deviations based on the sampling rates
determined in Section 4.9.3 for 3M OVMs (RSD = 3.49%) and in Section
4.9.4 for SKC 575-002 samplers (RSD =
2.39%).
Table
4.11.2
Recovery (%) from the Atmosphere Described in 4.11.2
for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
96.5
98.1
97.8
100.7
94.8
96.4
97.4 |
95.4
96.6
97.7
99.3
98.8
95.0
97.1 |
113.0
113.0
105.7
111.6
105.7
111.8
110.1 |
103.0
106.3
106.7
102.4
102.8
112.4
105.6 |
|
4.11.3 A reverse
diffusion study for the diffusive samplers and a stripping study for
the adsorbent tubes was performed by sampling a 402 ppm atmosphere of
toluene (78% RH, 23.5°C, 649.2 mmHg) for 120 minutes with six of each
samplers. Three samplers from each set were additionally subjected to
120 minutes of the same atmosphere without the toluene present to
determine if any of the collected toluene diffused off of the
diffusive samplers and also whether it was stripped off of the
adsorbent tubes. Upon analysis of the samples, the average recovery of
the removed samplers versus the average recovery of the samplers that
were additionally exposed to the atmosphere without toluene was within
90% for all samplers, indicating that reverse diffusion and stripping
is not significant. The first three samples in Table 4.11.3 for each
sampler were used to sample the toluene atmosphere for 120 minutes,
while the last three were additionally exposed to a blank atmosphere
for another 120 minutes.
Table
4.11.3
Recovery (%) for the Circumstances Described in 4.11.3
for Each Sampler
(Samples 1-3 used to sample toluene
atmosphere for 120 minutes,
samples 4-6 for an additional 120
minutes of blank atmosphere)
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
 (1-3) (1-3)
4
5
6
 (4-6) (4-6)
 (4-6)/ (4-6)/ (1-3) (1-3) |
99.6
98.2
100.0
99.3
100.4
100.1
100.1
100.2
1.01 |
97.9
98.3
98.2
98.1
97.6
97.1
98.2
97.6
1.00 |
99.7
101.9
94.0
98.5
99.9
99.4
99.5
99.6
1.01 |
108.6
101.0
99.3
103.0
105.0
100.4
98.4
101.3
0.98 |
|
4.11.4 The effects
from sampling from relatively dry atmospheres was investigated by
sampling from a 403.2-ppm toluene atmosphere (9% RH, 25.3°C, 654.5
mmHg) for 240 minutes and from a 499-ppm atmosphere (9% RH, 26.1°C,
653.9 mmHg) for 1 minute with all four samplers. The average results
for the adsorbent tubes did not deviate from the theoretical
concentration of toluene by more than three standard deviations of the
Day 0 storage samples in Section 4.7 (RSD = 1.07% and 1.94% for
240-min and 1-min charcoal tube samples respectively, RSD= 1.93% and
1.38% for 240-min and 1-min Anasorb® 747 tube samples
respectively). The average results for the diffusive samplers did not
deviate from the theoretical concentration of toluene by more than
three standard deviations based on the sampling rates determined in
Section 4.9.3 for 3M OVMs (RSD = 3.49%) and in Section 4.9.4 for SKC
575-002 samplers (RSD = 2.39%).
Table
4.11.4.1
Recovery (%) for 240-minute Samples from a
Dry
403.2-ppm Toluene Atmosphere for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
99.2
98.0
99.3
99.8
99.9
98.8
99.2 |
99.0
98.6
98.5
96.9
98.8
97.1
98.2 |
110.5
101.4
104.0
103.2
98.6
101.7
103.2 |
96.4
99.1
100.1
97.4
102.1
98.0
98.8 |
|
Table
4.11.4.2
Recovery (%) for 1-minute Samples from a
Dry
499-ppm Toluene Atmosphere for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
98.6
98.0
97.7
100.3
97.5
96.9
98.2 |
98.0
99.3
99.0
100.8
100.2
98.1
99.2 |
112.6
113.9
106.7
103.2
109.9
107.6
109.0 |
104.8
106.0
105.7
103.2
105.0
109.4
105.7 |
|
4.11.5 The effects
from sampling from atmospheres containing low concentrations of
toluene was investigated by sampling from a 19.8-ppm toluene
atmosphere (74% RH, 26.0°C, 651.4 mmHg) for 240 minutes and from a
49.3-ppm atmosphere (73% RH, 27.8°C, 650.7 mmHg) for 1
minute with all four samplers. The average results for the adsorbent
tubes did not deviate from the theoretical concentration of toluene by
more than three standard deviations of the Day 0 storage samples in
Section 4.7 (RSD = 1.07% and 1.94% for 240-min and 1-min charcoal tube
samples respectively, RSD= 1.93% and 1.38% for 240-min and 1-min
Anasorb® 747 tube samples respectively). The average
results for the diffusive samplers did not deviate from the
theoretical concentration of toluene by more than three standard
deviations based on the sampling rates determined in Section 4.9.3 for
3M OVMs (RSD = 3.49%) and in Section 4.9.4 for SKC
575-002 samplers (RSD = 2.39%).
Table
4.11.5.1
Recovery (%) for 240-minute Samples from
a
19.8-ppm Toluene Atmosphere for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
97.8
98.7
98.1
99.2
98.4
98.1
98.4 |
100.2
98.5
97.2
98.8
101.4
100.0
99.4 |
104.4
108.1
101.5
94.8
100.3
97.2
101.0 |
97.6
99.2
97.9
99.5
100.3
101.6
99.4 |
|
Table
4.11.5.2
Recovery (%) for 1-minute Samples from a
49.3-ppm
Toluene Atmosphere for Each Sampler
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
97.3
97.7
100.7
90.3
94.4
92.4
95.5 |
107.1
99.6
105.8
103.0
101.2
99.3
102.7 |
112.0
107.2
108.5
110.6
106.8
104.6
108.3 |
111.9
96.2
103.9
104.1
114.0
96.0
104.4 |
| 4.12
Desorption efficiency from wet samplers
The desorption efficiency
from wet samplers was investigated by spiking samplers with amounts of
toluene (9039 µg for the adsorbent tubes, 5190
µg for the 3M OVMs and 2595 µg for the SKC 575-002 samplers)
approximately equivalent to the mass that would be collected for 240
minutes from atmospheres containing 200 ppm. Before being spiked with
toluene, humid air (~80% RH, 25°C) had been drawn through the adsorbent
tubes at 50 mL/min for 240 minutes. Similarly, the diffusive samplers
had been exposed to the humid atmosphere for 240 minutes. The desorption
efficiencies were comparable to those reported in Section
2.5.
Table
4.12
Desorption Efficiency (%) from Wet Samplers
|
sample
no. |
Charcoal
Tubes |
Anasorb®
747
Tubes |
3M
OVMs |
SKC
575-002
Samplers |
|
1
2
3
4
5
6
 |
97.8
97.7
97.5
98.5
98.3
98.5
98.0 |
97.3
98.5
97.8
98.1
98.5
98.3
98.1 |
97.4
97.5
97.6
98.8
100.7
97.5
98.2 |
98.4
97.1
100.1
97.3
97.4
96.2
97.8 |
|
4.13 Qualitative
analysis
Toluene can easily be identified by GC/mass
spectrometry. A typical mass spectrum of toluene is shown in Figure
4.13.
Figure 4.13
Representative mass spectrum of
toluene. | 5.
References
5.1 Otterson, E. J.; Guy, C. U. Transactions of the Twenty-Sixth Annual Meeting of the
American Conference of Governmental Industrial Hygienists,
Philadelphia, PA, 1964; pp 37-46.
5.2 White, L. D.; Taylor, D.
G.; Mauer, P. A.; Kupel, R. E. Am. Ind. Hyg. Assoc.
J., 1970, 31, 225-232.
5.3 NIOSH Manual of Analytical Methods, 2nd ed. Vol. 1;
U.S. Department of Health, Education and Welfare, Public Health Service,
Centers for Disease Control, National Institute for Occupational Safety
and Health; Cincinnati, OH, 1977, Method P&CAM 127, DHEW (NIOSH)
Publication No. 77-157-A.
5.4 NIOSH Manual of
Analytical Methods, 2nd ed. Vol. 3; U.S. Department of Health,
Education and Welfare, Public Health Service, Centers for Disease
Control, National Institute for Occupational Safety and Health;
Cincinnati, OH, 1977, Method S343, DHEW (NIOSH) Publication No.
77-157-C.
5.5 OSHA Analytical Methods
Manual; Vol. 1; U.S. Department of Labor, Occupational Safety and
Health Administration; Directorate for Technical Support, OSHA Salt Lake
Technical Center: Salt Lake City, UT, 1990; Method 7: Organic Vapors;
American Conference of Governmental Industrial Hygienists (ACGIH):
Cincinnati, OH, Publication No. 4542.
5.6 Elskamp, C. J., A Study to Determine (1) How Much Water is Collected by SKC
Lot 120 Activated Charcoal at Various Relative Humidities, (2) How the
Presence of Water Effects the Apparent Desorption Efficiency of
2-Methoxyethanol from Lot 120 Charcoal, and (3) How Effective Anhydrous
Magnesium Sulfate is in Removing Water During the Desorption
Process, OSHA Salt Lake Technical Center, unpublished, Salt Lake
City, UT 84115-1802, January 1994.
5.7 OSHA
Analytical Methods Manual; Vol. 4; U.S. Department of Labor,
Occupational Safety and Health Administration; Directorate for Technical
Support, OSHA Salt Lake Technical Center: Salt Lake City, UT, 1993;
Method 91: Methyl Alcohol; American Conference of Governmental
Industrial Hygienists (ACGIH): Cincinnati, OH, Publication No.
4542.
5.8 OSHA Analytical Methods
Manual; Vol. 4; U.S. Department of Labor, Occupational Safety and
Health Administration; Directorate for Technical Support, OSHA Salt Lake
Technical Center: Salt Lake City, UT, 1993; Method 100: Ethyl Alcohol;
American Conference of Governmental Industrial Hygienists (ACGIH):
Cincinnati, OH, Publication No. 4542.
5.9 Eide, M. "OSHA Method
No. 109, Isopropyl Alcohol"; OSHA Salt Lake Technical Center,
unpublished, Salt Lake City, UT 84115-1802, June 1997.
5.10
Hendricks, W. Development of a Protocol for
Laboratory Testing of Diffusive Samplers, OSHA Salt Lake
Technical Center, unpublished, Salt Lake City, UT 84115-1802, December
1996.
5.11 Elskamp, C. J., The Collection of
Water by 3M and SKC Diffusive Samplers from Humid Air, OSHA Salt
Lake Technical Center, unpublished, Salt Lake City, UT 84115-1802,
January 1997.
5.12 Proctor and Hughes'
Chemical Hazards of the Workplace, 3rd ed.; Hathaway, G. J.;
Proctor, N. H.; Hughes, J. P.; Fischman, M. L., Eds.; Van Nostrand
Reinhold: New York, 1991, pp 546-547.
5.13 The
Merck Index, 12th ed.; Budavari, S., Ed.; Merck & Co.,
Whitehouse Station, NJ, 1996, p 9666.
5.14 Kirk-Othmer Encyclopedia of Chemical Technology, 3rd
ed.; Grayson, M., Ed.; John Wiley & Sons: New York, 1983, Vol. 23;
pp 246-273.
5.15 Hawley's Condensed Chemical
Dictionary, 12th ed.; Revised by Lewis, R. J.; Van Nostrand
Reinhold: New York, 1993; p 1157.
5.16 Nelson, G. O. Gas Mixtures: Preparation and Control; Lewis: Boca
Raton, 1992; Appendix M.
5.17 Hendricks, W., Determination of the Sampling Rate Variation for SKC 575
Series Passive Samplers, OSHA Salt Lake Technical Center,
unpublished, Salt Lake City, UT 84115-1802, April 1998.
|

