![]()
DIMETHYL PHTHALATE (DMP)
DIETHYL PHTHALATE (DEP)
DIBUTYL PHTHALATE (DBP)
DI-2-ETHYLHEXYL PHTHALATE (DEHP)
DI-n-OCTYL PHTHALATE (DNOP)
| Method number: | 104 | |||||||||||||||||||||||||
| Matrix: | Air | |||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Target concentration: |
| |||||||||||||||||||||||||
| OSHA PEL: |
| |||||||||||||||||||||||||
| ACGIH TLV: |
| |||||||||||||||||||||||||
| Procedure: | Samples are collected by drawing known volumes of air
through | |||||||||||||||||||||||||
| Recommended air volume and sampling rate: | 240 L at 1.0 L/min | |||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Reliable quantitation limits: |
| |||||||||||||||||||||||||
| Standard errors of estimate: |
| |||||||||||||||||||||||||
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | |||||||||||||||||||||||||
| Date: August 1994 | Chemist: Yihlin Chan | |||||||||||||||||||||||||
Organic Methods Evaluation Branch
OSHA Salt Lake
Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
1.1 Background
1.1.1 History
Airborne phthalates have been collected in ethylene glycol (Ref.
5.1), on mixed cellulose ester membrane filters (Ref. 5.2), and on
Tenax GC adsorbent (Ref. 5.3). The analytical methods include
GC/FID, GC/MS, GC/ECD, and HPLC/UV. An OSHA stopgap method specifies
collection on
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Dimethyl phthalate (DMP). DMP is of low to moderate toxicity, but when accidentally ingested in large amounts it may cause gastrointestinal irritation, central nervous system depression with coma, and hypotension. It is an irritant to the eyes and the mucous membranes. It is not irritant to the skin and is not absorbed. DMP is not known to cause cancer in humans or animals. (Ref. 5.5)
Diethyl phthalate (DEP). Adverse effects on humans from exposure to DEP have not been reported. DEP has caused death in animals given very high doses by mouth, but brief oral exposures to lower doses caused no harmful effects. The only effect found in animals that ate high doses of DEP for long periods of time was a decrease in weight gain because they ate less food. DEP is not known to cause cancer in humans or animals. DEP does not appear to affect the ability of male animals to sire offspring. However, a decrease occurred in the number of live offspring born to female animals that were exposed to DEP throughout their lives. Some birth defects occurred in newborn rats whose mothers received high doses (approximately 3 g/kg) of DEP by injection during pregnancy. DEP can be mildly irritating when applied to the skin of animals. It can also be slightly irritating when put directly into the eyes of animals. (Ref. 5.6)
Dibutyl phthalate (DBP). Adverse effects on humans from exposure to DBP have not been reported. In animals, eating large amounts of DBP can affect their ability to reproduce. DBP can cause death of unborn animals. In male animals, sperm production can decrease after eating large amounts of DBP. However, when exposure to DBP stops, sperm production seems to return to near normal levels. Exposure to high levels of DBP might cause similar effects in humans as in animals, but this is not known. There is no evidence that DBP causes cancer, but this has not been thoroughly studied. (Ref. 5.7)
Di-2-ethylhexyl phthalate (DEHP). From animal studies,
breathing DEHP does not appear to have serious harmful effects.
Studies in rats have shown that DEHP in the air has no effect on
lifespan or the ability to reproduce. However, eating high doses of
DEHP for a long time resulted in liver cancer in rats and mice. The
U.S. Department of Health and Human Services has determined that
DEHP may reasonably be anticipated to be a carcinogen. (Ref. 5.8)
IARC designated DEHP to Group 2B (possibly carcinogenic to humans)
(Ref. 5.9).
Di-n-octyl phthalate (DNOP) . DNOP may cause irritation to the skin and may cause severe irritation and possible corneal damage to the eyes. Ingestion may cause central nervous system depression with nausea, vomiting, dizziness, weakness, headache, and difficult respiration. A large dose is required to cause death in animals. (Ref. 5.10)
1.1.3 Workplace exposure
DMP is used as a solvent and plasticizer for cellulose acetate
and cellulose
DEP is used as a plasticizer for cellulose ester plastic films
and sheets (photographic, blister packaging, and tape applications)
and molded and extruded articles (consumer articles such as
toothbrushes, automotive components, tool handles, and toys). DEP
was reported as an ingredient in 67 cosmetic formulations at
concentrations ranging from <0.1% to
DBP is used primarily as a specialty plasticizer for nitrocellulose, polyvinyl acetate, and polyvinyl chloride. It has been used in plastisol formulations for carpet back coating and other vinyl compounds. DBP has also been used as an adjusting agent for lead chromate pigments, as a concrete additive, as an insect repellant for the impregnation of clothing, as a solvent for perfume oils, and as a stabilizer in rocket propellants. DBP is widespread in the environment and has been identified at low levels in air, water, and soil. Therefore, humans may be exposed to DBP by inhalation of air or by ingestion of water or food containing DBP. Individuals who manufacture or use specialty plasticizers would have the highest potential for exposure to DBP. No data were located on typical exposure levels in the workplace. (Ref. 5.7)
DEHP is principally used as a plasticizer in the production of
polyvinyl chloride (PVC) and vinyl chloride resins. Estimates are
that at least 95% of the DEHP produced ends up in these uses. PVC is
flexible and is used in many common items such as toys, vinyl
upholstery, shower curtains, adhesives, coatings, and as components
of paper and paperboard. PVC is also used to produce disposable
medical examination and surgical gloves, the flexible tubing used to
administer parenteral solutions, and the tubing used in hemodialysis
treatment.
DNOP is used as a plasticizer in the production of polyvinyl chloride and vinyl chloride resins. Occupational exposure may occur in the workplace where this compound is used. No data on the extent of workplace exposure were found. (Ref. 5.10)
1.1.4 Physical properties and other descriptive information (Ref. 5.11)
Dimethyl phthalate
| CAS no.: | 131-11-3 |
| synonyms: | 1,2-benzenedicarboxylic acid, dimethyl ester;
phthalic acid, dimethyl ester; dimethyl
|
| structural formula: | 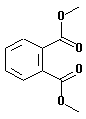 |
| molecular wt: | 194.19 |
| boiling point: | 284°C |
| melting point: | 0 - 2°C |
| appearance: | colorless to pale yellow oily liquid |
| odor: | slight aromatic odor |
| specific gravity: | 1.1905 |
| vapor pressure: | less than 1.3 Pa (0.01 mmHg) at 25°C |
| flash point: | 146°C (closed-cup) |
| solubility: | soluble in benzene, alcohol, ether, chloroform; slightly soluble in mineral oil; practically insoluble in petroleum ether and other paraffin hydrocarbons |
Diethyl phthalate
| CAS no.: | 84-66-2 |
| synonyms: | diethyl |
| structural formula: | 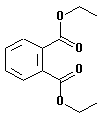 |
| molecular wt: | 222.24 |
| boiling point: | 298°C |
| melting point: | -41°C |
| appearance: | colorless liquid |
| odor: | odorless |
| specific gravity: | 1.1175 |
| vapor pressure: | 1.9 kPa (14 mmHg) at 163°C, 0.22 Pa (1.65×10-3 mmHg) at 25°C |
| flash point: | 140°C (open cup) |
| solubility: | soluble in alcohol, ether, acetone, benzene; moderately soluble in aliphatic solvents |
Dibutyl phthalate
| CAS no.: | 84-74-2 |
| synonyms: | phthalic acid, dibutyl ester; di-n-butyl
phthalate; butyl phthalate; o-benzenedicarboxylic acid,
dibutyl ester; dibutyl |
| structural formula: | 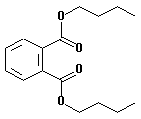 |
| molecular wt: | 278.35 |
| boiling point: | 340°C |
| melting point: | -35°C |
| appearance: | colorless to faint yellow oily liquid |
| odor: | weak aromatic odor |
| specific gravity: | 1.047 |
| vapor pressure: | less than 1.3 Pa (0.01 mmHg) at 20°C |
| flash point: | 157°C (closed-cup); 171°C (open cup) |
| solubility: | soluble in acetone, alcohol, ether, benzene, and other common organic solvents |
Di-(2-ethylhexyl) phthalate
| CAS no.: | 117-81-7 |
| synonyms: | bis-(2-ethylhexyl) phthalate;
|
| structural formula: | 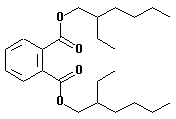 |
| molecular wt: | 390.6 |
| boiling point: | 384°C |
| melting point: | -55°C |
| appearance: | colorless to pale yellow oily liquid |
| odor: | almost odorless |
| specific gravity: | 0.981 |
| vapor pressure: | 0.18 kPa (1.32 mmHg) at 200°C |
| flash point: | 215°C (open cup) |
| solubility: | soluble in hexane, mineral oil |
Di-n-octyl phthalate
| CAS no.: | 117-84-0 |
| synonyms: | phthalic acid, dioctyl ester;
o-benzenedicarboxylic acid, dioctyl ester;
|
| structural formula: | 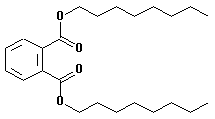 |
| molecular wt: | 390.6 |
| boiling point: | 220°C at 0.67 kPa (5 mmHg) |
| melting point: | -30°C |
| appearance: | light-colored liquid |
| odor: | odorless |
| specific gravity: | 0.9861 |
| vapor pressure: | less than 27 Pa (0.2 mmHg) at 150°C |
| flash point: | 209°C (closed-cup) |
| solubility: | soluble in mineral oil, dimethyl sulfoxide, ethanol, benzene |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters.
1.2 Limit defining parameters
1.2.1 Detection limit of the analytical procedure
The detection limits of the analytical procedure are 0.16, 0.13, 0.10, 0.09, and 0.10 ng for DMP, DEP, DBP, DEHP, and DNOP, respectively. These are the amounts of analytes that will give responses that are significantly different from the background responses of reagent blanks. (Sections 4.1 and 4.2)
1.2.2 Detection limit of the overall procedure
The detection limits of the overall procedure are 6.5, 4.8, 2.4, 3.9, and 3.3 µg per sample (27, 20, 10, 16, and 14 µg/m3) for DMP, DEP, DBP, DEHP, and DNOP, respectively. These are the amounts of analyte spiked on the sampler that will give responses that are significantly different from the background responses of sampler blanks. (Sections 4.1 and 4.3)
1.2.3 Reliable quantitation limit
The reliable quantitation limits are 21.7, 16.2, 8.1, 13.1, and 10.9 µg per sample (90, 68, 34, 55, and 45 µg/m3) for DMP, DEP, DBP, DEHP, and DNOP, respectively. These are the amounts of analyte spiked on a sampler that will give signals that are considered the lower limits for precise quantitative measurements. (Section 4.4)
1.2.4 Precision (analytical procedure)
The precisions of the analytical procedure, measured as the pooled relative standard deviations over a concentration range equivalent to 0.5 to 2 times the target concentration, are 0.35%, 0.54%, 0.45%, 1.15%, and 1.57% for DMP, DEP, DBP, DEHP, and DNOP, respectively. (Section 4.5)
1.2.5 Precision (overall procedure)
The precisions of the overall procedure at the 95% confidence
level for the ambient temperature
1.2.6 Recovery
The recovery of phthalates from samples used in 15-day storage tests remained above 99.6%, 93.1%, 99.1%, 99.8%, and 99.6% for DMP, DEP, DBP, DEHP, and DNOP, respectively, when the samples were stored at ambient temperature. (Section 4.7)
1.2.7 Reproducibility
Twelve samples collected from controlled test atmospheres of mixed phthalates, and a draft copy of this procedure, were submitted to an SLTC organic service branch for analysis. The samples were analyzed after 13 days of storage at ambient temperature. No individual sample result deviated from its theoretical value by more than the precisions reported in Section 1.2.5. (Section 4.8)
2. Sampling Procedure
2.1 Apparatus
2.1.1 A personal sampling pump, calibrated to ±5% of the recommended flow rate with the sampling device attached.
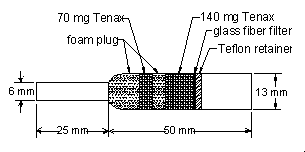
2.1.2
2.2 Reagents
None required.
2.3 Technique
2.3.1 Attach the sampler to the sampling pump with a piece of flexible tubing and place it in the worker's breathing zone. Air should enter the larger end of the tube.
2.3.2 Air should not pass through any hose or tubing before entering the sampling tube.
2.3.3 After sampling replace the plastic caps. Wrap each sample
with a Form
2.3.4 Record air volume for each sample.
2.3.5 Submit at least one blank with each set of samples. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.6 List any compounds that could be considered potential interferences.
2.4 Sampler capacity
Sampling capacity is determined by measuring how much air can be sampled before breakthrough occurs. Breakthrough is considered to occur when the effluent from the sampler contains a concentration of analyte that is 5% of the upstream concentration (5% breakthrough). The sampler capacity for DMP was determined to be over 305 L at a sampling rate of 1.0 L/min with DMP concentration of 10 mg/m3 (2 times the target concentration). The sampler capacities for the other four phthalates exceeded 300 L. (Section 4.9)
2.5 Desorption efficiency
2.5.1 The average desorption efficiencies for phthalates from the
2.5.2 The desorption efficiencies at 0.05, 0.1, and 0.2 times the target concentration (TC) are listed below. (Section 4.10.1)
| Table 2.5.2 | |||||
| Desorption efficiencies (%) at
0.05, 0.1, and 0.2 times the target concentration | |||||
|
| |||||
| DMP | DEP | DBP | DEHP | DNOP | |
|
| |||||
| 0.05× TC | 91.3 | 99.9 | 101.4 | 98.3 | 99.4 |
| 0.1 × TC | 91.4 | 98.8 | 97.6 | 95.5 | 92.2 |
| 0.2 × TC | 95.1 | 100.2 | 100.1 | 99.8 | 94.9 |
|
| |||||
2.5.3 Desorbed samples remain stable for at least 24 h. (Section 4.10.2)
2.6 Recommended air volume and sampling rate
2.6.1 For TWA samples, the recommended air volume is 240 L at 1.0 L/min.
2.6.2 For STEL samples, the recommended air volume is 15 L at 1.0 L/min.
2.6.3 With
2.7 Interferences (sampling)
2.7.1 Generally the presence of other organic contaminants in the air will reduce the capacity of the sampler to collect these phthalates.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
2.8.1 The sampling equipment should be attached to the worker in such a manner that it will not interfere with work performance or safety.
2.8.2 All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
3.1 Apparatus
3.1.1 A GC equipped with an FID. A
3.1.2 A GC column capable of separating DMP, DEP, DBP, DEHP,
DNOP, the internal standard, and any interferences. A
3.1.3 An electronic integrator or other suitable means of measuring detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4 Glass vials, 4.5-mL, with
3.1.5 A dispenser capable of delivering 4.0 mL of desorbing solvent.
3.2 Reagents
3.2.1 Dimethyl phthalate. Dimethyl phthalate, 99%, was obtained from Aldrich.
3.2.2 Diethyl phthalate. Diethyl phthalate, 99%, was obtained from Kodak.
3.2.3 Dibutyl phthalate.
3.2.4
3.2.5
3.2.6 Toluene. Toluene, Optima grade, was obtained from Fisher.
3.2.7
3.2.8 Desorbing solvent with internal standard. Dissolve 0.36 mL
of
3.3 Standard preparation
3.3.1 Prepare stock standards by diluting weighed amounts of phthalate in desorbing solvent.
3.3.2 Prepare analytical standards by diluting the stock standards with desorbing solvent. For each phthalate, a 300 µg/mL standard solution corresponds to the target concentration.
3.3.3 Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4 Sample preparation
3.4.1 Transfer the glass fiber filter, Tenax resin of the front section, and the middle foam plug to a WISP vial.
3.4.2 Transfer the Tenax resin of the back section and the back foam to another WISP vial.
3.4.3 Add 4.0 mL of the desorbing solvent to each vial.
3.4.4 Cap the vials and shake them on a mechanical shaker for 30 min.
3.5 Analysis
3.5.1 GC conditions
| column: | HP-1 (5 m, 0.53-mm i.d., 2.65-µm film) | |
| zone temp: | column | 1 min at 75°C, ramp to 270°C at
|
| injector | 270°C | |
| detector | 275°C | |
| gas flow: | column (He) | 5.53 mL/min |
| auxiliary (N2) | 30 mL/min | |
| hydrogen | 32 mL/min | |
| air | 395 mL/min | |
| split vent | 53 mL/min (split ratio 10:1) | |
| injection volume: | 1 µL | |
| retention times: | DMP | 6.0 min |
| DEP | 7.1 min | |
| 1-phenyldodecane | 9.3 min (ISTD) | |
| DBP | 9.6 min | |
| DEHP | 12.9 min | |
| DNOP | 13.8 min | |
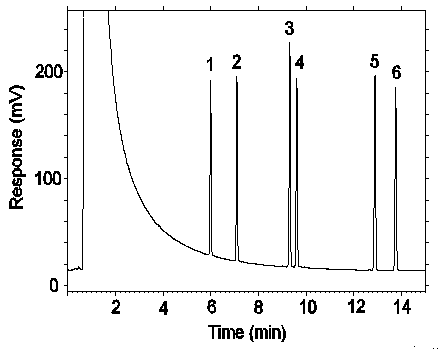
Figure 3.5.1. Chromatogram at target concentration. Key: 1 = DMP,
2 = DEP, 3 =
3.5.2 Measure peak areas by an electronic integrator or other suitable means.
3.5.3 Use an internal standard (ISTD) calibration method. Prepare a calibration curve by plotting micrograms per sample versus ISTD-corrected response of standards. Bracket the samples with analytical standards.
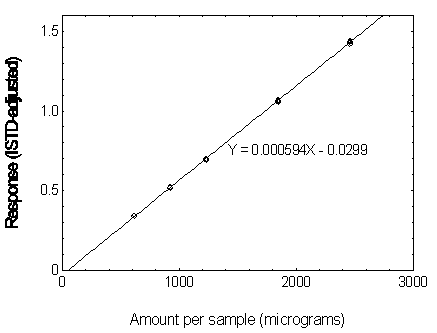
Figure 3.5.3.1 Calibration curve of DMP
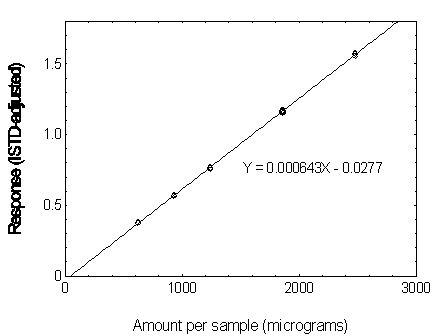
Figure 3.5.3.2. Calibration curve of DEP.
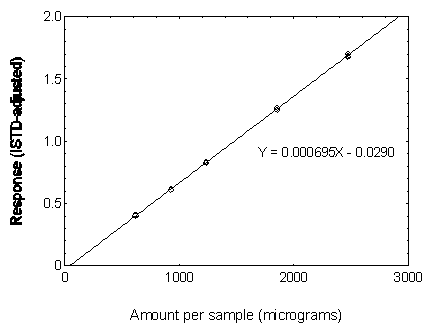
Figure3.5.3.3. Calibration curve of DBP.
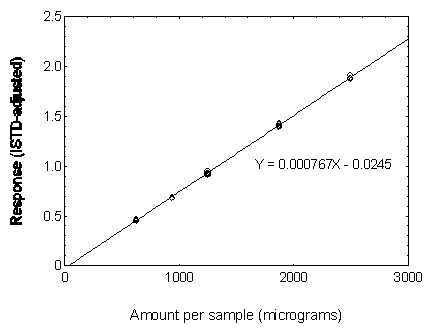
Figure 3.5.3.4. Calibration curve of DEHP.
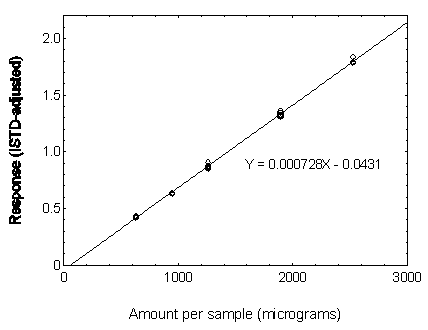
Figure 3.5.3.5. Calibration curve of DNOP.
3.6 Interferences (analytical)
3.6.1 Any compound that produces an FID response and has a similar retention time as any of the analytes or internal standard is a potential interference. If any potential interferences were reported, they should be considered before samples are desorbed. Generally, chromatographic conditions can be altered to separate an interference from the analyte.
3.6.2 When necessary, the identity or purity of an analyte peak may be confirmed with additional analytical data (Section 4.11).
3.7 Calculations
The amount (in micrograms) of a phthalate per sample is obtained from the appropriate calibration curve. The back section is analyzed primarily to determine the extent of breakthrough. If any analyte is found on the back section, it is added to the amount found on the front section. This total amount is then corrected by subtracting the total amount (if any) found in the blank. The air concentration is calculated using the following formula.
| mg/m3 = | micrograms of phthalate per
sample
liters of air sampled × desorption efficiency |
3.8 Safety precautions (analytical)
3.8.1 Adhere to the rules set down in your Chemical Hygiene Plan.
3.8.2 Avoid skin contact and inhalation of all chemicals.
3.8.3 Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
4.1 Determination of detection limits
Detection limits (DL), in general, are defined as the amount (or concentration) of analyte that gives a response (YDL) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
YDL - YBR = 3(SDBR)
The direct measurement of YBR and SDBR in chromatographic methods is typically inconvenient and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of analytical standards or samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for DL:
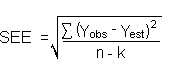
| Yobs | = | observed response |
| Yest | = | estimated response from regression curve |
| n | = | total no. of data points |
| k | = | 2 for a linear regression curve |
At point YDL on the regression curve
| YDL = A(DL) + YBR | A = analytical sensitivity (slope) |
therefore
| DL = | (YDL - YBR)
A |
Substituting 3(SEE) + YBR for YDL gives
| DL = | A |
4.2 Detection limit of the analytical procedure (DLAP)
The DLAP is measured as the mass of analyte actually introduced into the chromatographic column. Ten analytical standards whose concentrations were equally spaced from 0 to 12.5 µg/mL were prepared. The standard containing 12.5 µg/mL represented approximately 10 times the baseline noise for all analytes. These solutions were analyzed with the recommended analytical parameters (1 µL injection with 10:1 split). The data obtained were used to determine the required parameters (A and SEE) for the calculation of the DLAP. These parameters and the calculated DLAP's for the five phthalates are listed below.
| Table 4.2.1 | |||||
| Summary of the calculated A, SEE, and DLAP | |||||
|
| |||||
| DMP | DEP | DBP | DEHP | DNOP | |
|
| |||||
| A(ng-1) | 0.0211 | 0.0260 | 0.0247 | 0.0232 | 0.0201 |
| SEE | 0.00115 | 0.00110 | 0.000812 | 0.000725 | 0.000677 |
| DLAP (ng) | 0.16 | 0.13 | 0.10 | 0.09 | 0.10 |
|
| |||||
| Table 4.2.2 | ||
| Detection Limit of the Analytical
Procedure for DMP | ||
|
| ||
| concentration | mass on column | ISTD-adjusted |
| (µg/mL) | (ng) | (response) |
|
| ||
| 0.00 | 0.000 | 0.000000 |
| 1.23 | 0.123 | 0.000000 |
| 2.46 | 0.246 | 0.006172 |
| 3.69 | 0.369 | 0.007495 |
| 4.92 | 0.492 | 0.009049 |
| 6.15 | 0.615 | 0.011572 |
| 7.38 | 0.738 | 0.013412 |
| 8.61 | 0.861 | 0.018499 |
| 9.84 | 0.984 | 0.019438 |
| 11.07 | 1.107 | 0.022577 |
| 12.30 | 1.230 | 0.025851 |
|
| ||
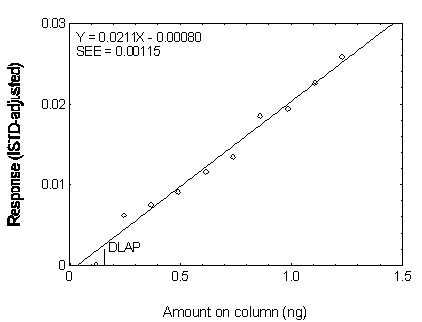
Figure 4.2.2. Plot of the data for determining the
DLAP of DMP.
| Table 4.2.3 | ||
| Detection Limit of the Analytical
Procedure for DEP | ||
|
| ||
| concentration | mass on column | ISTD-adjusted |
| (µg/mL) | (ng) | (response) |
|
| ||
| 0.00 | 0.000 | 0.000000 |
| 1.24 | 0.124 | 0.003659 |
| 2.48 | 0.248 | 0.008365 |
| 3.72 | 0.372 | 0.011870 |
| 4.96 | 0.496 | 0.014416 |
| 6.20 | 0.620 | 0.015966 |
| 7.44 | 0.744 | 0.020705 |
| 8.68 | 0.868 | 0.023028 |
| 9.92 | 0.992 | 0.025402 |
| 11.16 | 1.116 | 0.031727 |
| 12.40 | 1.240 | 0.032579 |
|
| ||
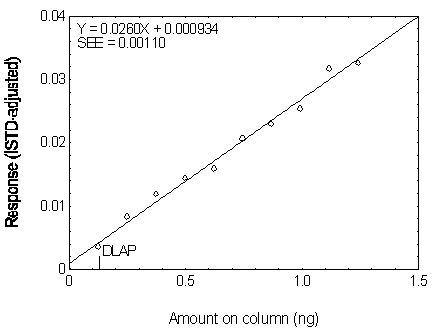
Figure 4.2.3. Plot of the data used for determining
the DLAP of DEP.
| Table 4.2.4 | ||
| Detection Limit of the Analytical
Procedure for DBP | ||
|
| ||
| concentration | mass on column | ISTD-adjusted |
| (µg/mL) | (ng) | (response) |
|
| ||
| 0.00 | 0.000 | 0.000000 |
| 1.24 | 0.124 | 0.003495 |
| 2.47 | 0.247 | 0.006206 |
| 3.71 | 0.71 | 0.009197 |
| 4.94 | 0.494 | 0.012034 |
| 6.18 | 0.618 | 0.014716 |
| 7.41 | 0.741 | 0.020491 |
| 8.65 | 0.865 | 0.021137 |
| 9.88 | 0.988 | 0.023602 |
| 11.12 | 1.112 | 0.027755 |
| 12.36 | 1.236 | 0.030736 |
|
| ||
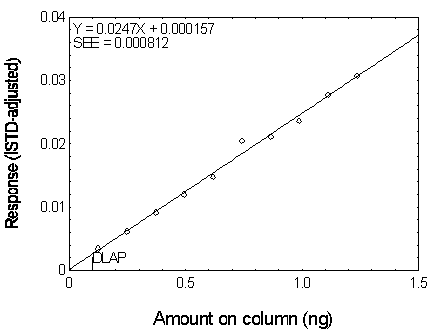
Figure 4.2.4. Plot of the data used for determining
the DLAP of DBP.
| Table 4.2.5 | ||
| Detection Limit of the Analytical
Procedure for DEHP | ||
|
| ||
| concentration | mass on column | ISTD-adjusted |
| (µg/mL) | (ng) | (response) |
|
| ||
| 0.00 | 0.000 | 0.009830 |
| 1.25 | 0.125 | 0.012161 |
| 2.49 | 0.249 | 0.015005 |
| 3.74 | 0.374 | 0.016568 |
| 4.99 | 0.499 | 0.020997 |
| 6.23 | 0.623 | 0.022298 |
| 7.48 | 0.748 | 0.025840 |
| 8.73 | 0.873 | 0.029510 |
| 9.97 | 0.997 | 0.031756 |
| 11.22 | 1.122 | 0.035372 |
| 12.47 | 1.247 | 0.038701 |
|
| ||
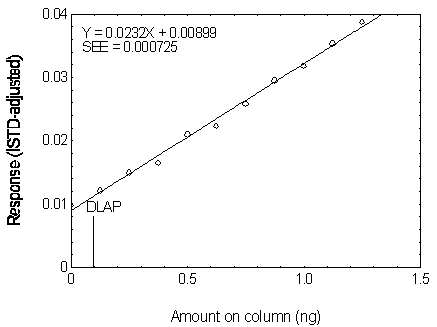
Figure 4.2.5. Plot of the data used for determining
the DLAP of DEHP.
| Table 4.2.6 | ||
| Detection Limit of the Analytical
Procedure for DNOP | ||
|
| ||
| concentration | mass on column | ISTD-adjusted |
| (µg/mL) | (ng) | (response) |
|
| ||
| 0.00 | 0.000 | 0.016174 |
| 1.26 | 0.126 | 0.017594 |
| 2.53 | 0.253 | 0.020140 |
| 3.79 | 0.379 | 0.022811 |
| 5.05 | 0.505 | 0.024236 |
| 6.31 | 0.631 | 0.028774 |
| 7.58 | 0.758 | 0.030987 |
| 8.84 | 0.884 | 0.033952 |
| 10.10 | 1.010 | 0.035165 |
| 11.36 | 1.136 | 0.038195 |
| 12.63 | 1.263 | 0.040625 |
|
| ||
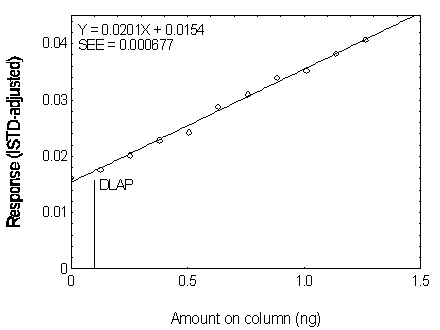
Figure 4.2.6. Plot of the data used for determining
the DLAP of DNOP.
4.3 Detection limit of the overall procedure (DLOP)
The DLOP is measured as mass per sample and expressed as equivalent air concentration, based on the recommended sampling parameters. Ten OVS-Tenax samplers were spiked with amounts of phthalates equally spaced from 0 to 50 µg/sample. The latter amount, when spiked on a sampler, would produce a peak approximately 10 times the baseline noise for a sample blank. These spiked samples were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. The parameters obtained and the calculated DLOP's for the five phthalates are listed below.
| Table 4.3.1 | |||||
| Summary of the calculated A, SEE, and DLOP | |||||
|
| |||||
| DMP | DEP | DBP | DEHP | DNOP | |
|
| |||||
| A (µg-1) | 0.000498 | 0.000631 | 0.000616 | 0.000605 | 0.000491 |
| SEE | 0.00108 | 0.00102 | 0.000499 | 0.000790 | 0.000536 |
| DLOP (µg) | 6.5 | 4.8 | 2.4 | 3.9 | 3.3 |
|
| |||||
| Table 4.3.2 | |
| Detection Limit of the Overall
Procedure for DMP | |
|
| |
| mass per sample | ISTD-adjusted |
| (µg) | response |
|
| |
| 0.00 | 0.000000 |
| 4.92 | 0.003303 |
| 9.84 | 0.006687 |
| 14.76 | 0.005820 |
| 19.68 | 0.009595 |
| 24.60 | 0.011740 |
| 29.52 | 0.014777 |
| 34.44 | 0.015651 |
| 39.36 | 0.019574 |
| 44.28 | 0.023076 |
| 49.20 | 0.025336 |
|
| |
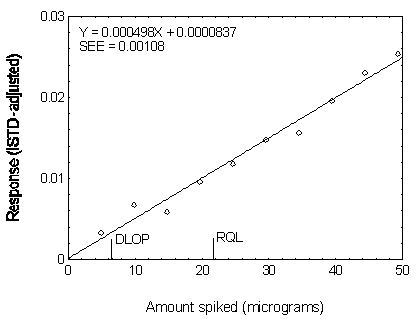
Figure 4.3.2. Plot of data used to determine the DLOP
and RQL of DMP.
| Table 4.3.3 | |
| Detection Limit of the Overall
Procedure for DEP | |
|
| |
| mass per sample | ISTD-adjusted |
| (µg) | response |
|
| |
| 0.00 | 0.000000 |
| 4.96 | 0.005735 |
| 9.92 | 0.009298 |
| 14.87 | 0.010539 |
| 19.83 | 0.013962 |
| 24.79 | 0.017733 |
| 29.75 | 0.018743 |
| 34.71 | 0.023453 |
| 39.66 | 0.026416 |
| 44.62 | 0.030439 |
| 49.58 | 0.032467 |
|
| |
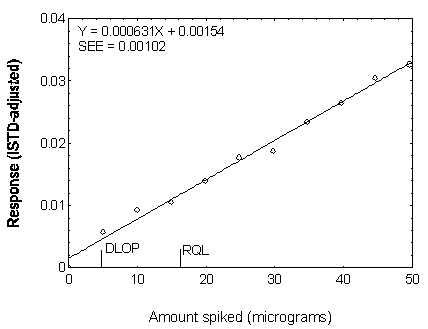
Figure 4.3.3. Plot of data used to determine the DLOP
and RQL of DEP.
| Table 4.3.4 | |
| Detection Limit of the Overall
Procedure for DBP | |
|
| |
| mass per sample | ISTD-adjusted |
| (µg) | response |
|
| |
| 0.00 | 0.000000 |
| 4.94 | 0.003247 |
| 9.88 | 0.006310 |
| 14.83 | 0.009043 |
| 19.77 | 0.012165 |
| 24.71 | 0.014531 |
| 29.65 | 0.017447 |
| 34.59 | 0.020963 |
| 39.54 | 0.023689 |
| 44.48 | 0.027926 |
| 49.42 | 0.030969 |
|
| |
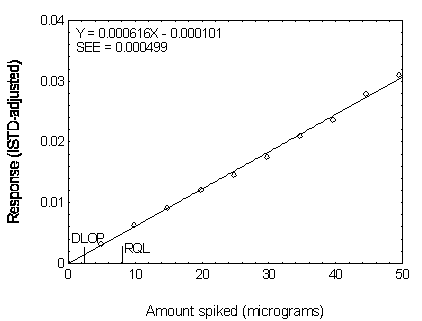
Figure 4.3.4. Plot of data used to determine the DLOP
and RQL of DBP.
| Table 4.3.5 | |
| Detection Limit of the Overall
Procedure for DEHP | |
|
| |
| mass per sample | ISTD-adjusted |
| (µg) | response |
|
| |
| 0.00 | 0.008518 |
| 4.99 | 0.010614 |
| 9.97 | 0.014936 |
| 14.96 | 0.017956 |
| 19.94 | 0.020824 |
| 24.93 | 0.022502 |
| 29.92 | 0.024855 |
| 34.90 | 0.030300 |
| 39.89 | 0.032849 |
| 44.87 | 0.035537 |
| 49.86 | 0.038496 |
|
| |
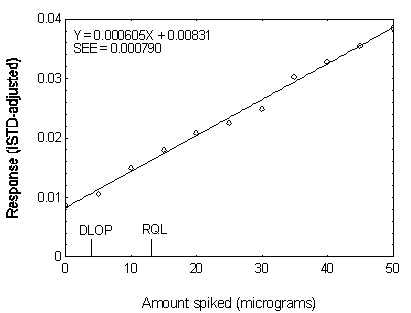
Figure 4.3.5 Plot of data used to determine the DLOP
and RQL of DEHP.
| Table 4.3.6 | |
| Detection Limit of the Overall
Procedure for DNOP | |
|
| |
| mass per sample | ISTD-adjusted |
| (µg) | response |
|
| |
| 0.00 | 0.015581 |
| 5.05 | 0.018904 |
| 10.10 | 0.020513 |
| 15.15 | 0.023587 |
| 20.20 | 0.025651 |
| 25.25 | 0.027891 |
| 30.30 | 0.030478 |
| 35.35 | 0.034282 |
| 40.40 | 0.035045 |
| 45.45 | 0.037866 |
| 50.50 | 0.041035 |
|
| |
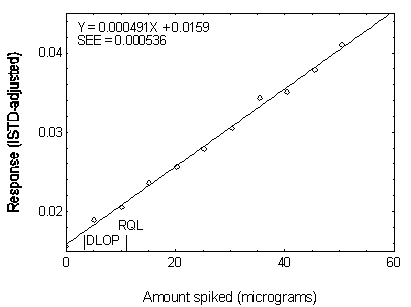
Figure 4.3.6. Plot of data used to determine the DLOP
and RQL of DNOP.
4.4 Reliable quantitation limit
The RQL is considered the lower limit for precise quantitative measurements. It is determined from the regression line data obtained for the calculation of the DLOP (Section 4.3), providing at least 75% of the analyte is recovered. The RQL is defined as the amount of analyte that gives a response (YRQL) such that
YRQL - YBR = 10(SDBR)
therefore
| RQL = | A |
The calculated RQL's for the five phthalates, together with the recoveries at these levels, are listed below. The recoveries are above 75%.
| Table 4.4.1 | |||||
| Summary of the RQL's and the recoveries | |||||
|
| |||||
| DMP | DEP | DBP | DEHP | DNOP | |
|
| |||||
| RQL (µg/sample) | 21.7 | 16.2 | 8.1 | 13.1 | 10.9 |
| RQL (µg/m3) | 90 | 68 | 34 | 55 | 45 |
| Recovery (%) | 100.1 | 99.4 | 100.9 | 103.3 | 100.7 |
|
| |||||
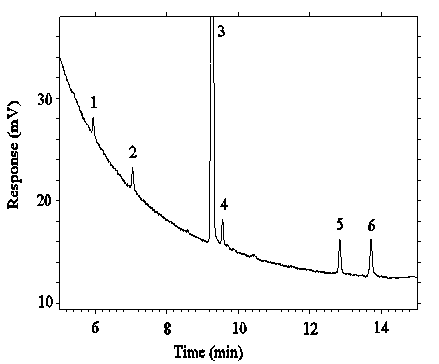
Figure 4.4.1. Chromatogram of the RQL for DMP.
Key:
1 = DMP, 3 = ISTD. 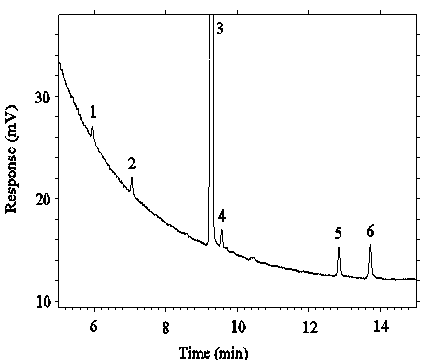
Figure 4.4.2. Chromatogram of the RQL's for DEP and
DEHP.
Key: 2 = DEP, 3 = ISTD, 5 = DEHP. 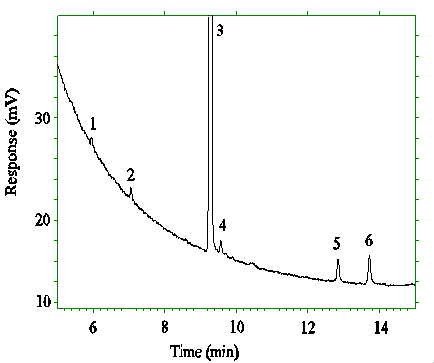
Figure 4.4.3. Chromatogram of teh RQL's for DBP and
DNOP.
Key: 3 = ISTD, 4 = DBP, 6 = DNOP.
4.5 Precision (analytical method)
The precision of the analytical procedure is defined as the pooled relative standard deviation (RSDP). Relative standard deviations were determined from six replicate injections of analytical standards at 0.5, 0.75, 1, 1.5, and 2 times the target concentration. After assuring that the RSDs satisfy the Cochran test for homogeneity at the 95% confidence level, RSDP was calculated.
| Table 4.5.1 | |||||
| Instrument Response to DMP | |||||
|
| |||||
| × target concn µg/mL |
0.5× 153.75 |
0.75× 230.63 |
1× 307.50 |
1.5× 461.25 |
2× 615.00 |
|
| |||||
| ISTD-adjusted response |
0.339180 0.339222 0.341304 0.339692 0.340345 0.338556 |
0.518724 0.518855 0.516464 0.519998 0.518792 0.518545 |
0.694646 0.695280 0.697158 0.694935 0.699107 0.692083 |
1.06782 1.05593 1.06663 1.05802 1.06191 1.06777 |
1.43746 1.42405 1.43872 1.43437 1.43922 1.43860 |
SD RSD (%) |
0.339716 0.000979 0.29 |
0.518563 0.001151 0.22 |
0.695535 0.002389 0.34 |
1.06301 0.00520 0.49 |
1.43540 0.00583 0.41 |
|
| |||||
| Table 4.5.2 Instrument Response to DEP | |||||
|
| |||||
| × target concn µg/mL |
0.5× 154.94 |
0.75× 232.41 |
1× 309.88 |
1.5× 464.81 |
2× 619.75 |
|
| |||||
| ISTD-adjusted response |
0.374911 0.374138 0.378598 0.373550 0.373774 0.372888 |
0.570068 0.569987 0.569111 0.568564 0.570238 0.571293 |
0.763569 0.764417 0.762847 0.763365 0.766802 0.7610260 |
1.17061 1.15667 1.16755 1.15892 1.16228 1.16811 |
1.57033 1.55567 1.57204 1.56837 1.57259 1.57311 |
SD RSD (%) |
0.374643 0.002049 0.55 |
0.569877 0.000948 0.17 |
0.763671 0.001905 0.25 |
1.16402 0.00558 0.48 |
1.56869 0.00661 0.42 |
|
| |||||
| Table 4.5.3 Instrument Response to DBP | |||||
|
| |||||
| × target concn µg/mL |
0.5× 154.44 |
0.75× 231.66 |
1× 308.88 |
1.5× 463.31 |
2× 617.75 |
|
| |||||
| ISTD-adjusted response |
0.405228 0.404333 0.404576 0.404915 0.403932 0.405790 |
0.611808 0.611966 0.612680 0.611583 0.612455 0.611139 |
0.825268 0.822788 0.831174 0.833438 0.830629 0.832469 |
1.26829 1.25226 1.25298 1.25158 1.25108 1.24944 |
1.68342 1.68432 1.70201 1.67908 1.70102 1.70313 |
SD RSD (%) |
0.404796 0.000663 0.16 |
0.611939 0.000566 0.09 |
0.829294 0.004269 0.51 |
1.25427 0.00697 0.56 |
1.69216 0.01100 0.65 |
|
| |||||
| Table 4.5.4 Instrument Response to DEHP | |||||
| × target concn µg/mL |
0.5× 155.81 |
0.75× 233.72 |
1× 311.63 |
1.5× 467.44 |
2× 623.25 |
|
| |||||
| ISTD-adjusted response |
0.464074 0.467006 0.452057 0.458669 0.464892 0.465609 |
0.678952 0.682591 0.686014 0.682044 0.683300 0.682931 |
0.955317 0.933266 0.914818 0.923775 0.911533 0.935958 |
1.40557 1.42112 1.39206 1.42917 1.42689 1.39226 |
1.88144 1.91779 1.87967 1.88589 1.88077 1.88146 |
SD RSD (%) |
0.462051 0.005669 1.23 |
0.682639 0.002274 0.33 |
0.929111 0.016079 1.73 |
1.41118 0.01688 1.20 |
1.88784 0.01483 0.79 |
|
| |||||
| Table 4.5.5 Instrument Response to DNOP | |||||
| × target concn µg/mL |
0.5× 157.81 |
0.75× 236.72 |
1× 315.63 |
1.5× 473.44 |
2× 631.25 |
|
| |||||
| ISTD-adjusted response |
0.428794 0.435110 0.418855 0.423316 0.431818 0.434664 |
0.630011 0.633303 0.639090 0.635316 0.634379 0.635068 |
0.906980 0.872334 0.853098 0.862667 0.849245 0.877654 |
1.32827 1.34664 1.31105 1.35927 1.35852 1.31242 |
1.78854 1.83870 1.78651 1.79472 1.78838 1.78544 |
SD RSD (%) |
0.428760 0.006516 1.52 |
0.634528 0.002955 0.47 |
0.870330 0.020982 2.41 |
1.33603 0.02191 1.64 |
1.79705 0.02066 1.15 |
|
| |||||
The Cochran test for homogeneity requires the calculation of the g statistics according to the following formula:
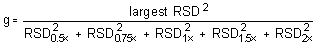
The g statistics obtained were: 0.3692, 0.3750, 0.4117, 0.4482, and 0.4696, for DMP, DEP, DBP, DEHP, and DNOP, respectively. Since these g statistics do not exceed the critical value of 0.5065, the RSDs within each phthalate can be considered equal and they can be pooled (RSDP) to give an estimated RSD for the concentration range studied.
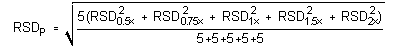
The pooled relative standard deviations are: 0.36%, 0.40%, 0.45%, 1.15%, and 1.57%, for DMP, DEP, DBP, DEHP, and DNOP, respectively.
4.6 Precision (overall procedure)
The precision of the overall procedure is determined from the storage data in Section 4.7. The determination of the standard error of estimate (SEER) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEER is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
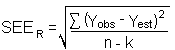
| n | = | total no. of data points |
| k | = | 2 for linear regression |
| k | = | 3 for quadratic regression |
| Yobs | = | observed % recovery at a given time |
| Yest | = | estimated % recovery from the regression line at the same given time |
An additional 5% for pump error (SP) is added to the SEER by the addition of variances to obtain the total standard error of estimate.

The precision at the 95% confidence level is obtained by
multiplying the standard error of estimate (with pump error included)
by 1.96 (the
4.7 Storage test
Storage tests were conducted in three batches: DMP, DEP/DNOP, and
DBP/DEHP. Storage samples were prepared from the controlled test
atmospheres of the appropriate phthalate or phthalate mixtures.
| Table 4.7.1 Storage Test for DMP | |||||||
| time (days) |
percent recovery (ambient) |
percent recovery
(refrigerated) | |||||
|
| |||||||
| 0 0 1 5 8 12 15 |
104.5 99.5 97.6 98.0 97.7 98.6 108.5 |
98.3 100.0 97.4 105.2 95.7 102.4 101.3 |
106.1 91.6 105.2 104.5 106.2 104.1 113.0 |
104.5 99.5 98.1 96.9 88.5 97.9 97.9 |
98.3 100.0 104.3 95.4 104.5 89.7 112.4 |
106.1 91.6 104.6 104.1 107.0 97.2 110.5 | |
|
| |||||||
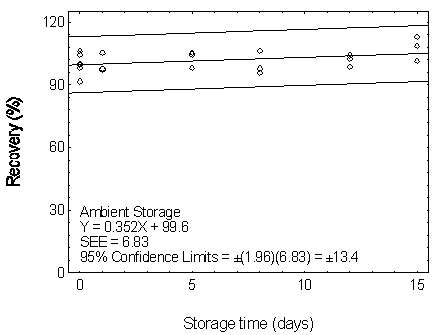
Figure 4.7.1.1. Ambient storage test for DMP.
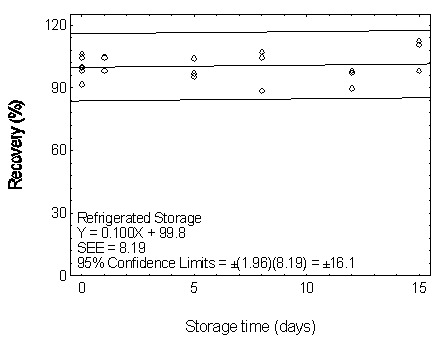
Figure 4.7.1.2. Refrigerated storage test for DMP.
| Table 4.7.2 | |||||||
| Storage Test for DEP | |||||||
|
| |||||||
| time (days) | percent recovery | percent recovery | |||||
| (ambient) | (refrigerated) | ||||||
|
| |||||||
| 0 | 97.6 | 94.6 | 95.7 | 97.6 | 94.6 | 95.7 | |
| 0 | 104.5 | 104.9 | 102.7 | 104.5 | 104.9 | 102.7 | |
| 3 | 94.6 | 93.6 | 102.7 | 90.5 | 101.3 | 102.2 | |
| 6 | 92.9 | 100.2 | 93.5 | 91.9 | 94.6 | 104.4 | |
| 9 | 89.1 | 91.0 | 98.1 | 86.8 | 98.7 | 99.2 | |
| 13 | 90.6 | 94.3 | 99.6 | 92.6 | 90.9 | 100.7 | |
| 15 | 92.1 | 91.2 | 100.3 | 93.2 | 104.5 | 105.3 | |
|
| |||||||
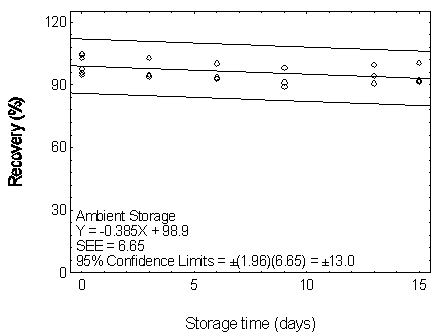
Figure 4.7.2.1. Ambient storage test for DEP.
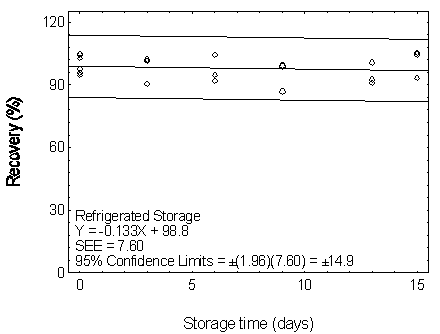
Figure 4.7.2.2. Refrigerated storage test for DEP.
| Table 4.7.3 | |||||||
| Storage Test for DBP | |||||||
|
| |||||||
| time (days) | percent recovery | percent recovery | |||||
| (ambient) | (refrigerated) | ||||||
|
| |||||||
| 0 | 99.1 | 99.5 | 98.3 | 99.1 | 99.5 | 98.6 | |
| 0 | 103.7 | 102.6 | 96.9 | 103.7 | 102.3 | 96.9 | |
| 4 | 100.5 | 101.8 | 101.1 | 103.1 | 101.1 | 101.9 | |
| 6 | 95.8 | 96.7 | 102.7 | 98.7 | 99.0 | 102.1 | |
| 8 | 100.3 | 101.1 | 99.7 | 99.9 | 101.6 | 102.0 | |
| 12 | 101.7 | 99.2 | 104.3 | 93.8 | 97.9 | 101.3 | |
| 15 | 97.4 | 99.1 | 96.4 | 102.2 | 98.3 | 106.0 | |
|
| |||||||
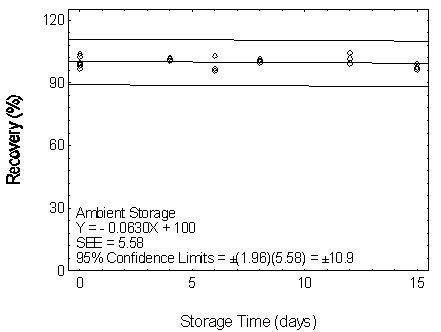
Figure 4.7.3.1. Ambient storage test for DBP.
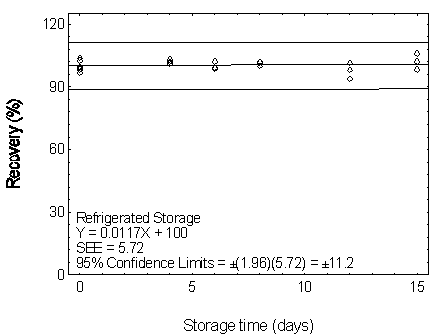
Figure 4.7.3.2. Refrigerated storage test for DBP.
| Table 4.7.4 | |||||||
| Storage Test for DEHP | |||||||
|
| |||||||
| time (days) | percent recovery | percent recovery | |||||
| (ambient) | (refrigerated) | ||||||
|
| |||||||
| 0 | 99.5 | 99.8 | 98.7 | 99.5 | 99.8 | 98.7 | |
| 0 | 102.2 | 102.0 | 97.8 | 102.2 | 102.0 | 197.8 | |
| 4 | 100.6 | 102.9 | 102.8 | 104.8 | 99.8 | 102.0 | |
| 6 | 95.7 | 97.4 | 101.0 | 97.8 | 98.3 | 102.0 | |
| 8 | 100.0 | 101.5 | 98.6 | 99.8 | 100.5 | 101.5 | |
| 12 | 98.1 | 101.9 | 104.2 | 105.9 | 100.8 | 102.4 | |
| 15 | 101.6 | 101.4 | 104.5 | 96.5 | 98.1 | 94.5 | |
|
| |||||||
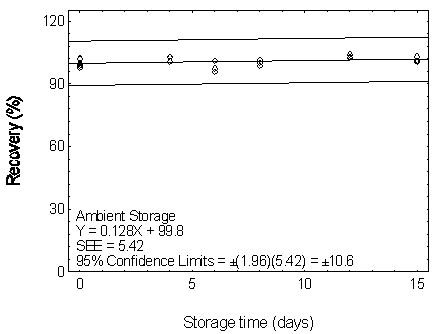
Figure 4.7.4.1. Ambient storage test for DEHP.
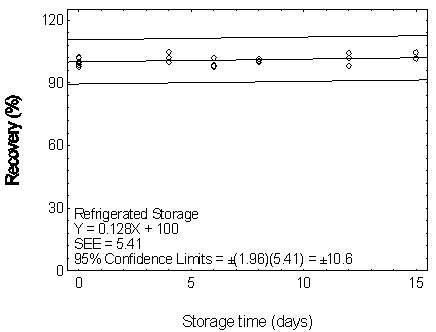
Figure 4.7.4.2. Refrigerated storage test for DEHP.
| Table 4.7.5 | |||||||
| Storage Test for DNOP | |||||||
|
| |||||||
| time (days) | percent recovery | percent recovery | |||||
| (ambient) | (refrigerated) | ||||||
|
| |||||||
| 0 | 101.7 | 101.7 | 101.6 | 101.7 | 101.7 | 101.6 | |
| 0 | 98.7 | 99.7 | 96.6 | 98.7 | 99.7 | 96.6 | |
| 3 | 101.7 | 103.7 | 100.1 | 104.4 | 99.0 | 98.9 | |
| 6 | 99.7 | 94.8 | - | 98.8 | 99.2 | 96.3 | |
| 9 | 99.9 | 99.9 | 95.5 | 98.9 | 98.4 | 98.5 | |
| 13 | 102.1 | 100.0 | 98.7 | 103.5 | 102.2 | 99.9 | |
| 15 | 100.5 | 101.9 | 98.3 | 100.0 | 96.3 | 98.8 | |
|
| |||||||
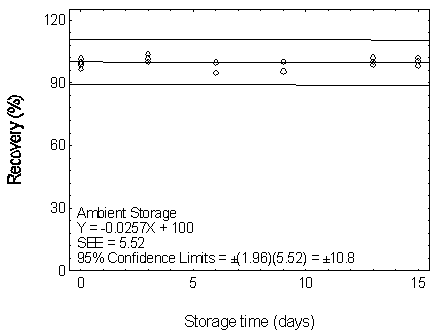
Figure 4.7.5.1.
Ambient storage test for DNOP.
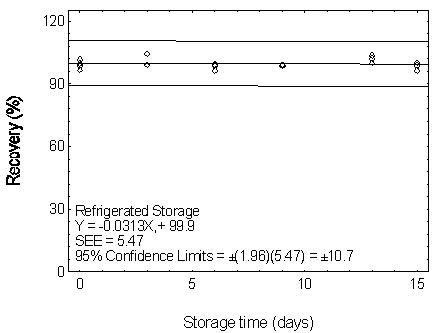
Figure 4.7.5.2.
Refrigerated storage test for DNOP.
4.8 Reproducibility
Reproducibility samples were prepared from controlled test atmospheres of mixed phthalates. They were prepared in two batches: DMP/DEP and DBP/DEHP/DNOP. The samples were submitted to an SLTC service branch for analysis. The samples were analyzed after being stored for 13 days at ambient temperature. No sample result had a deviation greater than the precisions of the overall procedure determined in Section 4.7, which are ±13.4%, ±13.0%, ±10.9%, ±10.6%, and ±10.8% for DMP, DEP, DBP, DEHP, and DNOP, respectively.
| Table 4.8.1 Reproducibility Data for DMP | |||
| µg expected | µg found | percent found | percent deviation |
|
| |||
| 787 788 785 780 804 782 |
756 775 757 774 819 770 |
96.1 98.4 96.4 99.2 101.9 98.5 |
-3.9 -1.6 -3.6 -0.8 +1.9 -1.5 |
|
| |||
| Table 4.8.2 Reproducibility Data for DEP | |||
| µg expected | µg found | percent found | percent deviation |
|
| |||
| 695 696 693 688 710 690 |
655 676 650 680 713 668 |
94.2 97.1 93.8 98.8 100.4 96.8 |
-5.8 -2.9 -6.2 -1.2 +0.4 -3.2 |
|
| |||
| Table 4.8.3 Reproducibility Data for DBP | |||
| µg expected | µg found | percent found | percent deviation |
|
| |||
| 1323 1328 1329 1307 1375 1334 |
1412 1425 1408 1380 1446 1412 |
106.7 107.3 105.9 105.6 105.2 105.8 |
+6.7 +7.3 +5.9 +5.6 +5.2 +5.8 |
|
| |||
| Table 4.8.4 Reproducibility Data for DEHP | |||
| µg expected | µg found | percent found | percent deviation |
|
| |||
| 1367 1372 1373 1351 1421 1379 |
1428 1436 1418 1392 1462 1422 |
104.5 104.7 103.3 103.0 102.9 103.1 |
+4.5 +4.7 +3.3 +3.0 +2.9 +3.1 |
|
| |||
| Table 4.8.5 Reproducibility Data for DNOP | |||
| µg expected | µg found | percent found | percent deviation |
|
| |||
| 1374 1379 1381 1358 1429 1386 |
1495 1448 1427 1396 1472 1395 |
108.8 105.0 103.3 102.8 103.0 100.6 |
+8.8 +5.0 +3.3 +2.8 +3.0 +0.6 |
|
| |||
4.9 Sampler capacity
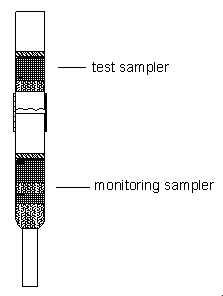
The sampler capacity was assessed by sampling from a dynamically
generated test atmosphere of phthalate at 2 times the target
concentration and at 25°C and 80% RH. The test atmosphere of phthalate
was generated by pumping a
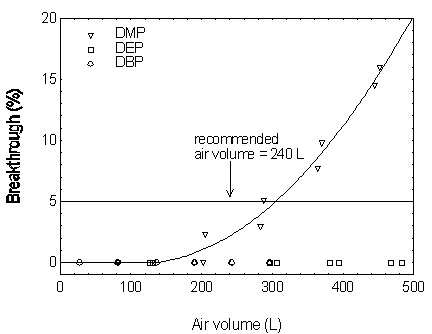
Figure 4.9.1. Breakthrough curves for DMP, DEP, and
DBP. 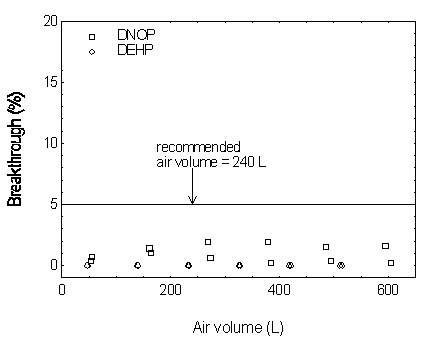
Figure 4.9.2. Breakthrough curves for DEHP and DNOP.
4.10 Desorption efficiency and stability of desorbed samples
4.10.1 Desorption efficiency
The desorption efficiencies (DE) of phthalates were determined by
| Table 4.10.1.1 Desorption Efficiency for DMP | ||||||
| × target conc (µg) |
0.05× 61.5 |
0.1× 123.0 |
0.2× 246.0 |
0.5× 615 |
1.0× 1230 |
2.0× 2460 |
|
| ||||||
| DE (%) |
90.1 90.3 97.7 89.2 89.9 90.5 |
90.8 91.4 91.5 95.1 89.7 89.9 |
94.3 94.1 94.0 98.8 94.5 95.1 |
97.4 98.9 100.1 96.5 97.6 97.9 |
98.0 99.5 98.6 98.6 98.5 97.9 |
98.5 98.5 98.6 99.0 98.4 98.9 |
| 91.3 | 91.4 | 95.1 | 98.1 | 98.5 | 98.6 | |
|
| ||||||
| Table 4.10.1.2 Desorption Efficiency for DEP | ||||||
| × target conc (µg) |
0.05× 62.0 |
0.1× 93.0 |
0.2× 247.9 |
0.5× 619.8 |
1.0× 1239.5 |
2.0× 2479 |
|
| ||||||
| DE (%) |
100.0 101.8 96.2 97.5 103.2 100.9 |
98.2 98.2 98.2 100.9 101.5 96.0 |
102.7 98.8 98.1 99.8 101.9 99.9 |
101.1 99.5 101.9 98.1 99.8 101.2 |
98.5 100.1 99.0 98.5 99.4 98.3 |
98.6 98.6 98.4 99.0 98.1 98.7 |
| 99.9 | 98.8 | 100.2 | 100.3 | 99.0 | 98.6 | |
|
| ||||||
| Table 4.10.1.3 Desorption Efficiency for DBP | ||||||
| × target conc (µg) |
0.05× 61.8 |
0.1× 123.6 |
0.2× 247.1 |
0.5× 617.8 |
1.0× 1235.5 |
2.0× 2471 |
|
| ||||||
| DE (%) |
115.8 98.1 98.1 104.9 97.5 93.9 |
97.8 97.4 96.1 96.1 101.8 96.3 |
98.9 101.9 98.2 101.4 102.1 98.1 |
101.7 100.0 102.5 100.7 100.3 101.2 |
98.8 99.5 99.2 98.9 99.3 99.2 |
99.1 99.2 99.0 99.7 99.0 99.4 |
| 101.4 | 97.6 | 100.1 | 101.1 | 99.2 | 99.2 | |
|
| ||||||
| Table 4.10.1.4 Desorption Efficiency for DEHP | ||||||
| × target conc (µg) |
0.05× 62.3 |
0.1× 124.7 |
0.2× 249.3 |
0.5× 623.3 |
1.0× 1246.5 |
2.0× 2493 |
|
| ||||||
| DE (%) |
108.5 95.7 95.4 95.9 97.6 96.6 |
95.2 96.0 95.3 94.7 94.9 96.9 |
100.4 101.0 100.3 99.3 98.9 98.8 |
98.7 99.4 101.7 97.9 97.4 98.3 |
98.8 98.7 98.9 100.4 100.5 99.5 |
100.5 100.4 99.8 99.5 99.7 100.5 |
| 98.3 | 95.5 | 99.8 | 98.9 | 99.5 | 100.1 | |
|
| ||||||
| Table 4.10.1.5 Desorption Efficiency for DNOP | ||||||
| × target conc (µg) |
0.05× 63.1 |
0.1× 126.3 |
0.2× 252.5 |
0.5× 631.3 |
1.0× 1262.5 |
2.0× 2525 |
|
| ||||||
| DE (%) |
109.4 100.1 96.6 96.4 97.4 96.8 |
92.0 91.5 92.5 93.1 91.6 92.7 |
95.7 95.3 95.2 94.9 94.3 94.2 |
95.5 96.5 99.3 95.1 94.5 95.2 |
97.4 97.5 97.9 100.2 100.4 98.6 |
102.1 101.9 100.5 100.2 100.5 101.8 |
| 99.4 | 92.2 | 94.9 | 96.0 | 98.7 | 101.2 | |
|
| ||||||
4.10.2 Stability of desorbed samples
The stability of the desorbed samples was investigated by reanalyzing the target concentration samples 24 h after initial analysis. After the original analysis was performed three vials were recapped with new septa while the remaining three retained their punctured septa. The samples were reanalyzed with fresh standards.
| Table 4.10.2.1 Stability of desorbed samples for DMP | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 98.0 99.5 98.6 98.7 |
99.0 99.5 99.0 (averages) 99.2 |
+1.0 0.0 +0.4 +0.5 |
98.6 98.5 97.9 98.3 |
99.0 99.4 99.0 (averages) 99.1 |
+0.4 +0.9 +1.1 +0.8 |
|
| |||||
| Table 4.10.2.2 Stability of extracted samples for DEP | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 98.5 100.1 99.0 99.2 |
99.8 99.9 99.7 (averages) 99.8 |
+1.3 -0.2 +0.7 +0.6 |
98.5 99.4 98.3 98.7 |
99.5 99.6 99.4 (averages) 99.5 |
+1.0 +0.2 +1.1 +0.8 |
|
| |||||
| Table 4.10.2.3 Stability of extracted samples for DBP | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day(%) |
difference |
|
| |||||
| 98.8 99.5 99.2 99.2 |
98.2 99.0 98.8 (averages) 98.7 |
-0.6 -0.5 -0.4 -0.5 |
98.9 99.3 99.2 99.1 |
98.2 98.8 98.2 (averages) 98.4 |
-0.7 -0.5 -1.0 -0.7 |
|
| |||||
| Table 4.10.2.4 Stability of extracted samples for DEHP | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 98.8 98.7 98.9 98.8 |
96.8 97.7 98.0 (averages) 97.5 |
-2.0 -1.0 -0.9 -1.3 |
100.4 100.5 99.5 100.1 |
96.9 97.6 97.2 (averages) 97.2 |
-3.5 -2.9 -2.3 -2.9 |
|
| |||||
| Table 4.10.2.5 Stability of extracted samples for DNOP | |||||
| punctured septa replaced | punctured septa retained | ||||
| initial DE (%) |
DE after one day (%) |
difference | initial DE (%) |
DE after one day (%) |
difference |
|
| |||||
| 97.4 97.5 97.9 97.6 |
95.7 96.4 97.7 (averages) 96.6 |
-1.7 -1.1 -0.2 -1.0 |
100.2 100.4 98.6 99.7 |
96.1 96.3 95.9 (averages) 96.1 |
-4.1 -4.1 -2.7 -3.6 |
|
| |||||
4.11 Qualitative analysis
The GC/MS of phthalates can be obtained by using GC conditions
similar to those given in Section 3.5. A
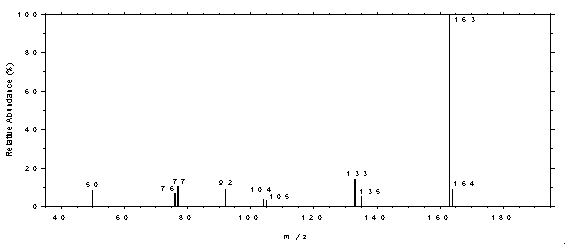
Figure 4.11.1. Mass
spectrum of DMP. 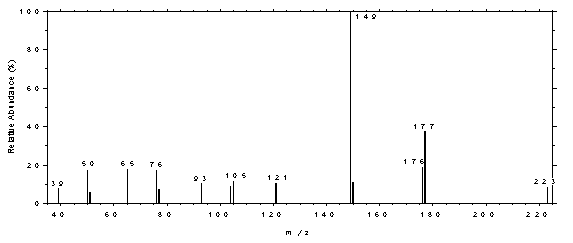
Figure 4.11.2. Mass
spectrum of DEP. 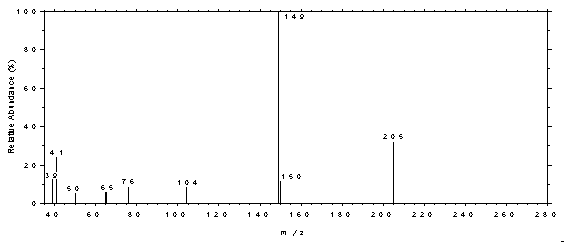
Figure 4.11.3. Mass
spectrum of DBP. 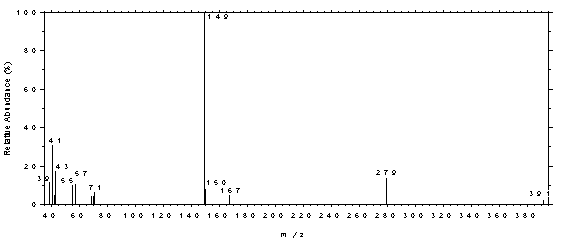
Figure 4.11.4. Mass
spectrum of DEHP. 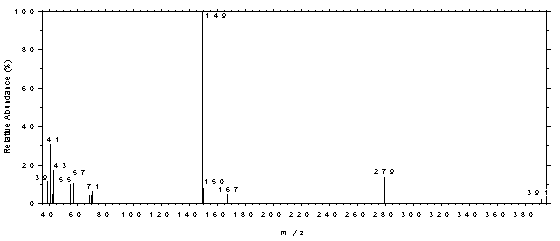
Figure 4.11.5. Mass
spectrum of DNOP.
5. References
5.1. Thomas, G. H., "Quantitative Determination and Confirmation of
Identity of Trace Amounts of Dialkyl Phthalates in Environmental
Samples", Environmental Health Perspectives, No. 3, pp
5.2. "Dibutyl Phthalate and Di(2-ethylhexyl) Phthalate - Method 5020", in: NIOSH Manual of Analytical Methods, 3rd ed., Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health, 1984.
5.3. "Dioctyl Phthalates in Air. Laboratory Method using Tenax Adsorbent Tubes, Solvent Desorption and Gas Chromatography", MDHS Report No. 32, Health and Safety Executive, Her Majesty's Stationery Office, London, England, 1983.
5.4. Eide, M., "Dimethyl Phthalate, Diethyl Phthalate, Dibutyl
Phthalate,
5.5. Clayton, G. D. and F. E. Clayton, Patty's Industrial Hygiene and Toxicology, 3rd ed., Vol. IIA, p. 2343, John Wiley & Sons, New York, 1981.
5.6. Toxicological Profile for Diethyl Phthalate, U. S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 1993.
5.7. Toxicological Profile for
5.8. Toxicological Profile for Di(2-ethylhexyl) Phthalate, U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 1993.
5.9. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 29, 257 (1982), Suppl. 7, 62, World Health Organization, International Agency for Research on Cancer, Lyon, France, 1987.
5.10. Material Safety Data Sheets, Dimethyl Phthalate, Diethyl
Phthalate,
5.11. Material Safety Data Sheets, Dimethyl Phthalate, Diethyl
Phthalate,
Footnote (1) The air volume for each sampling period was adjusted to 2 times the target concentrations. The air volume of the
(Back to text)