![]()
| Method no.: | 97 |
| Matrix: | Air |
| Target concentration: | 1 ppm (2 mg/m3) (skin) |
| Procedure: | Samples are collected by drawing air through sampling tubes containing petroleum-based charcoal which has been coated with hydrobromic acid. The samples are desorbed with toluene and analyzed by GC using an electron capture detector. |
| Recommended air volume and sampling rate: |
6 L at 0.05 L/min |
| Reliable quantitation limit: | 0.73 ppb (1.68 µg/m3) |
| Standard error of estimate at the target concentration: |
7.4% |
| Special requirements: | Store samples under refrigeration when they are not in transit. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: September 1992 | Chemist: Warren Hendricks |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1. Background
- 1.1.1. History
Previous to this method, propargyl alcohol had been collected on
Propargyl alcohol (2-propyn-1-ol) has three reactive sites: a
primary hydroxyl group, a triple bond, and an acetylenic hydrogen
(Ref.
5.3.). These reactive sites make the chemical an ideal candidate
for derivatization. A derivative was quantitatively formed when
propargyl alcohol was liquid spiked on
The propargyl alcohol/HBr derivative has a double bond which makes the presence of cis and trans isomers possible. The analysis of air samples, collected from a controlled test atmosphere, revealed that both isomers were produced, but that the ratio of the isomers was greater than 99:1. The predominant isomer could be the trans isomer (with respect to the bromine atoms), because the cis isomer would be more difficult to form due to steric hinderance.
This method features air sample collection and derivatization of
propargyl alcohol using commercially available sampling tubes
containing
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Propargyl alcohol is a primary skin irritant (but not a
sensitizer), and a severe eye and mucous membrane irritant. It is
toxic by ingestion, inhalation, and skin adsorption. The oral
LD50 for rats is 70 mg/kg, 60 mg/kg for
guinea pigs, and 50 mg/kg for mice. The
1.1.3. Workplace exposure
Propargyl alcohol has been used as a chemical intermediate, a pharmaceutical intermediate, a corrosion inhibitor, a laboratory reagent, a solvent stabilizer, a soil fumigant, and to prevent hydrogen embrittlement of steel. The United States imported 1.85 × 108 g of propargyl alcohol in 1984. (Ref. 5.5.)
No estimate of U.S. production or of the number of workers potentially exposed to propargyl alcohol was found.
1.1.4. Physical properties and other descriptive information (Refs. 5.5. and 5.6. for propargyl alcohol)
| chemical name: | propargyl alcohol |
| CAS no.: | 107-19-7 |
| molecular wt: | 56.07 |
| boiling point: | 114-115° |
| melting point: | -52 to -48° |
| density: | 0.9715 (d420) |
| vapor pressure: | 11.6 mmHg at 20° |
| vapor density: | 1.93 |
| description: | colorless |
| solubility: | soluble in water, benzene, chloroform, 1,2-dichloroethane, ethanol, ether, acetone, dioxane, and tetrahydrofuran; insoluble in aliphatic hydrocarbons |
| synonyms: | |
| structural formula: | HC |
| propargyl alcohol/HBr derivative (following data taken from Fluka's (vendor) catalog and reagent bottle label) | |
| chemical name: | 2,3-dibromo-2-propen-1-ol (cis and trans) |
| CAS no.: | 7228-11-7 |
| boiling point: | 60 to 62° at 0.1 mmHg |
| density: | 2.216 (d420) |
| refractive index: | 1.580 (nD20) |
| molecular weight: | 215.90 |
| synonym: | 2,3-dibromoallyl alcohol |
| structural formula: | BrHC = CBr - CH2OH |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm and ppb are referenced to 25° and 101.3 kPa (760 mmHg). The amounts presented are calculated as propargyl alcohol even though the derivative is the actual species analyzed.
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.8 fg per injection. This is the amount of analyte that will produce a major isomer peak with a height that is approximately 5 times the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 10.08 ng per sample. This is the amount of analyte spiked on the sampling device that, upon analysis, produces a peak similar in size to that of the detection limit of the analytical procedure. This detection limit corresponds to an air concentration of 0.73 ppb (1.68 µg/m3). (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 10.08 ng per sample. This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. This reliable quantitation limit corresponds to an air concentration of 0.73 ppb (1.68 µg/m3). (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The instrument response over concentration ranges representing 0.5 to 2 times the target concentration was linear. (Section 4.4.)
1.2.5. Recovery
The recovery of propargyl alcohol from samples used in the
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.011. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the
1.2.8. Reproducibility
Six samples, prepared by liquid spiking, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 36 days of storage at about 5°. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated within
±5% of the recommended flow rate with the sampling device in line.
2.1.2. Samples are collected with
2.2. Reagents
No reagents are required for sampling.
2.3. Technique
- 2.3.1. Break off both ends of the sampling tubes immediately
before sampling. The holes in the broken ends of the sampling tubes
should be approximately 1/2 the i.d. of the sampling tube. All tubes
should be from the same lot.
2.3.2. Connect the sampling tube to the sampling pump with
flexible tubing. Use a sampling tube holder with a protective tube
shield to cover the sharp, jagged end of the sampling tube. Position
the sampling tube so that sampled air passes through the
2.3.3. Sampled air should not pass through any hose or tubing before entering the sampling tube.
2.3.4. Attach the sampler vertically in the worker's breathing
zone, with the
2.3.5. Remove the sampling tube after sampling for the
appropriate time and seal the sampler with plastic end caps. Wrap
each sample
2.3.6. Submit at least one blank with each set of samples. The blank should be handled the same as the other samples except no air is drawn through it.
2.3.7. Record the sample air volume (in liters of air) for each sample. Note any potential interferences.
2.3.8. Ship any bulk sample separate from air samples.
2.3.9. Submit air samples to the laboratory for analysis as soon as possible after sampling. Store the samples at reduced temperature if delay is unavoidable.
2.4. Sampler capacity
Several sampler capacity studies were performed using controlled test atmospheres and the recommended sampling tubes. The average propargyl alcohol concentration of the test atmospheres was 4.7 µg/L (2 ppm) at 73% relative humidity and 27°. Samples were collected at 0.05 L/min for as long as 7.6 hours and no breakthrough from the front to the back section was observed in any of the test samples. The average propargyl alcohol recovery was 99% of theoretical, after correction for desorption efficiency.
An additional sampler capacity test was performed to determine if the relative humidity of the sampled air had an effect on sampler capacity. The propargyl alcohol concentration of the test atmosphere was 4.8 g/L (2 ppm) at 19% relative humidity and 25°. Samples were collected at 0.05 L/min for as long as 7.1 hours and, again, no breakthrough was observed. The average recovery of these samples was 105% of theoretical.
The recommended air volume (6 L at 0.05 L/min) is a significant
reduction of the air volumes (more than 21 L) shown to be feasible by
the capacity tests. The reduction was made as a precaution against the
available HBr being entirely consumed at the head of the sampling tube
and then propargyl alcohol being collected but not derivatized. This
situation could cause low results, especially for stored samples.
Storage tests confirmed this condition did not occur in samples
collected for 2 h at 0.05 L/min (6-L samples). The analytical method
can detect exceptionally low amounts of the propargyl alcohol/HBr
derivative, therefore, a
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiency of propargyl alcohol
from HBr-coated charcoal over the range of from 0.5 to 2 times the
target concentration is 88.6%. (Section
4.9.1.)
2.5.2. Desorbed samples remain stable for at least 2 days. (Section 4.9.2.)
2.5.3. Desorption efficiencies should be confirmed periodically because differences may occur due to variations between sampling media lots, desorption solvent, and operator technique.
2.6. Recommended air volume and sampling rate
- 2.6.1. Sample 6 L of air at 0.05 L/min for TWA samples.
2.6.2. Sample 0.75 L of air at 0.05 L/min for
2.6.3. The air concentration corresponding to the reliable
quantitation limit becomes larger when
2.7. Interferences (sampling)
- 2.7.1. There are no known interferences with the collection of
propargyl alcohol on HBr-coated charcoal. Any chemical that could
deplete HBr from the sampling tube or prevent the derivatization
reaction from occurring is a potential interference. The derivative
itself, if present in the sampled air, would be an interference.
2.7.2. Suspected interferences should be reported to the laboratory when samples are submitted.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices applicable to the work area.
2.8.3. Wear protective eyeware when breaking the ends of the glass sampling tubes.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with an electron capture detector (ECD). A
3.1.2. A GC column capable of separating the HBr derivative of
propargyl alcohol from the desorbing solvent and potential
interferences. A Supelco
3.1.3. An electronic integrator or other suitable means of measuring detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Sample vials, 2- and
3.1.5. A mechanical tube rotator. A Fisher Roto-Rack was used in this evaluation.
3.2. Reagents
- 3.2.1.
3.2.2. Toluene, reagent grade or better. b&j Brand, High Purity Solvent, Lot AY 377, was used in this evaluation.
3.2.3. Toluene/internal standard solution. The internal standard
solution is prepared by adding an appropriate internal standard to
toluene.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by weighing
3.3.2. Add 2.0 mL of toluene (without the internal standard) to
each of several
3.3.3. Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the adsorbent sections of each sampling tube to
separate
3.4.2. Add 2.0 mL of toluene (without the internal standard) to each vial.
3.4.3. Seal the vials with
3.5. Analysis
- 3.5.1. Dilute both standards and samples by
adding 10 µL of each standard or sample to separate
3.5.2. GC Conditions
| temperatures (°) | ||||
| injector: | 250 | |||
| detector: | 275 | |||
| column: | 40, hold 1 min, program at 10°/min to 220 | |||
| gas flow rates (mL/min) | ||||
| column: | 2.6 (H2) | |||
| split: | 69.0 (H2) | |||
| septum purge: | 3.0 (H2) | |||
| auxiliary: | 53.0 (N2) | |||
| miscellaneous | ||||
| detector: | ECD (Ni-63) | |||
| column: | ||||
| injection size: | 1 µL (28:1 split) | |||
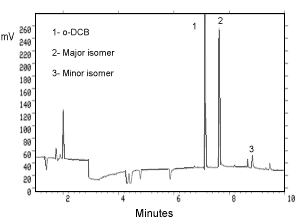 Figure 3.5.2. Chromatogram of a standard. | ||||
| GC retention times (min) | ||||
| 7.1 | ||||
| major isomer: | 7.6 | |||
| minor isomer: | 8.8 | |||
The total GC run time was 19 min as a precaution to clear the column.
3.5.3. Commercial
Determine the area percent ratio of the 2 isomers and use an internal standard method to calibrate the integrator. For example: the concentration of a standard is 14.0 µg/standard, the area of 1 isomer is 1078356, and the area of the other isomer is 97052. The percent ratio of the first isomer is 1078356 / (1078356 + 97052) = 91.7%. Multiply the concentration by 0.917 (14.0 µg/standard × 0.917 = 12.8 µg/sample) and use an internal standard method to calibrate the integrator at 12.8 µg/sample for the major isomer and 1.2 µg/standard (14.0 - 12.8 = 1.2 µg/standard) for the minor isomer.
3.5.4. Add the integrator results for the 2 isomers of the standards together. Construct a calibration curve by plotting micrograms per standard versus summed integrator results for each standard.
3.5.5. Sum the integrator results for the samples. Analysis of air samples may reveal only the presence of the major isomer. The other isomer may not have been formed during sample collection.
3.6. Interferences (analytical)
- 3.6.1. Any compound that gives an ECD response and has a similar
GC retention time as the analytes or the internal standard is a
potential interference. Generally, chromatographic conditions can be
altered to separate an interference.
3.6.2. Retention time on a single column is not proof of chemical identity. Confirmation of suspected identity should be performed by GC/mass spectrometry when necessary.
3.7. Calculations
The analyte amount per sample, micrograms of propargyl alcohol per sample, is obtained from the calibration curve. The back section of the sample is analyzed primarily to determine if there was any breakthrough from the front section during sampling. If a significant amount of analyte is found on the back section (e.g., greater than 25% of the amount found on the front section), this fact should be reported with sample results. If any analyte is found on the back section, it is added to the amount on the front section. The analyte amount is then corrected by subtracting the total amount found in the blank. The air concentration is obtained by using the following equations. The dilution specified in Section 3.5.1. does not have to be included in the calculations if it was performed on both standards and samples.
|
| ||||||||
|
|
3.8. Safety precautions (analytical)
- 3.8.1. Restrict the use of all chemicals to a fume hood.
3.8.2. Avoid skin contact and inhalation of all chemicals.
3.8.3. Wear safety glasses, gloves, and a lab coat at all times while working with chemicals.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
| The injection size recommended in the analytical procedure
(1 µL, 28:1 split) was used in the determination of the
detection limit of the analytical procedure. The detection limit
was 1.8 fg |
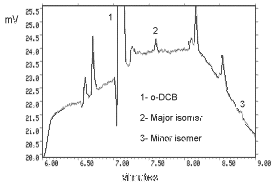 Figure 4.1. Detection limit of the analytical procedure. |
4.2. Detection limit of the overall procedure
4.3. Reliable quantitation limit
| The reliable quantitation limit was determined by analyzing
|
| ||||||||||||||||
4.4. Instrument response to the analyte
The instrument response to the isomers of
|
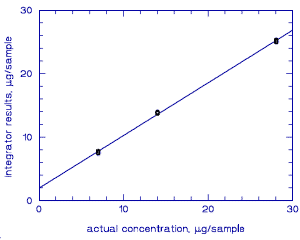 Figure 4.4. Calibration curve for | |||||||||||||||||||||||||||||
4.5. Storage data
Thirty-six samples were collected over 2 days (18 samples each day) from controlled test atmospheres containing an average of 1.0 ppm propargyl alcohol. Each sample was collected for 2 h at 0.05 L/min. The average relative humidity of the controlled test atmospheres was 75% at 27°. Six samples (3 each day) were analyzed immediately after collection. Fifteen samples were stored in a refrigerator at about 5°, and 15 different samples were stored in the dark at about 23°. Every few days, 3 samples from each group were selected and analyzed. The recovery of propargyl alcohol from samples stored at ambient temperature remained above 80.3%.
|
| ||||||||
| days of amb. storage |
% recovery (ambient) |
days of ref. storage |
% recovery (refrigerated) | |||||
|
| ||||||||
| 0 | 88.3 | 89.8 | 84.6 | 0 | 82.6 | 86.4 | 83.2 | |
| 4 | 104.0 | 84.9 | 83.6 | 2 | 88.5 | 85.2 | 84.1 | |
| 7 | 85.1 | 82.9 | 80.7 | 5 | 89.7 | 87.7 | 86.0 | |
| 11 | 87.2 | 83.8 | 83.3 | 8 | 92.6 | 88.2 | 86.4 | |
| 14 | 83.8 | 74.4 | 83.7 | 12 | 86.0 | 88.9 | 82.6 | |
| 18 | 81.8 | 85.0 | 77.5 | 15 | 89.9 | 80.5 | 83.4 | |
|
| ||||||||
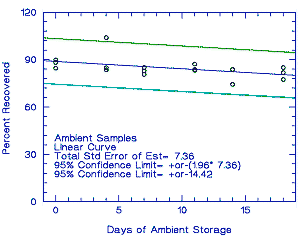 Figure 4.5.1. Ambient temperature storage test. |
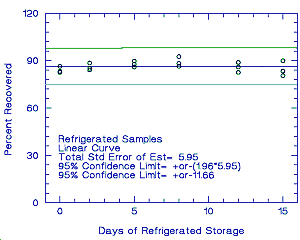 Figure 4.5.2. Refrigerated temperature storage test. |
4.6. Precision (analytical method)
| The precision of the analytical procedure is defined as the pooled coefficient of variation determined from replicate injections of analytical standards representing 0.5, 1, and 2 times the target concentration. The coefficients of variation are calculated from the data in Table 4.4. The pooled coefficient of variation is 0.011. |
| |||||||||||||||||||||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
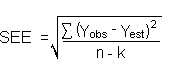
| where | n = k = k = |
total number of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed percent recovery at a given time | |
| Yest = | estimated percent recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility data
| Six samples were prepared by liquid spiking sampling tubes
with |
| |||||||||||||||||||||
4.9. Desorption efficiency and stability of desorbed samples
- 4.9.1. Desorption efficiency
| ||||||||||||||||||||||||||||||
Propargyl alcohol could probably be used to evaluate desorption
efficiency because the derivatization reaction is quantitative.
4.9.2. Stability of desorbed samples
The stability of desorbed samples was verified by reanalyzing the
1.0 times target concentration samples 2 days after the original
analysis. The samples were resealed immediately after the original
analysis and fresh standards were used in the reanalysis. Both the
original desorbed (but not diluted) samples
|
| ||
| initial recovery (percent) |
recovery after 2 days (percent) |
percent change |
|
| ||
| 88.5 87.0 87.9 88.9 90.0 89.0 |
87.3 86.9 88.3 87.9 86.6 87.2 |
-1.2 -0.1 +0.4 -1.0 -3.4 -1.8 |
|
| ||
|
| ||
| initial recovery (percent) | recovery after 2 days (percent) | percent change |
|
| ||
| 88.5 87.0 87.9 88.8 90.0 89.0 |
88.3 86.2 87.4 88.6 88.5 88.4 |
-0.2 -0.8 -0.5 -0.2 -1.5 -0.6 |
|
| ||
5. References
- 5.1. OSHA Computerized Information System
Database, SLCAL Chemical Sampling Information, Propargyl Alcohol, REV
900508, OSHA SLTC, Salt Lake City, UT.
5.2. OSHA Salt Lake Technical Center, Organic Division In-house File for Propargyl Alcohol, Desorption/Extraction Study Form, August 1990, OSHA SLTC, Salt Lake City, UT.
5.3. Hort, E. V. in Kirk-Othmer Encyclopedia
of Chemical Technology, 3rd ed.; Grayson, M. Ed.; John Wiley &
Sons: New York, 1978, Vol. 1; pp
5.4. Documentation of the Threshold Limit Values and Biological Exposure Indices, 5th. ed.; American Conference of Governmental Industrial Hygienists, Inc.: Cincinnati, OH, 1986; p 496.
5.5. OSHA Computerized Information System Database, OSHA Regulated Substances (PEL Standard) Profiles, Propargyl Alcohol, Revision Date: 03/08/88, OSHA SLTC, Salt Lake City, UT.
5.6. OSHA Computerized Information System Database, Occupational Health Services, Inc. (OHS) MSDS File, Propargyl Alcohol, Revision Date: 09/25/91, OSHA SLTC, Salt Lake City, UT.