|
| Method no.: |
077 |
| |
|
| Matrix: |
Air |
| |
|
| Target concentration: |
1.5 mg/m3 |
| |
|
| Procedure: |
Air samples are collected by
drawing known volumes of air through XAD-4 adsorbent tubes.
The samples are desorbed with 2-propanol and analyzed by HPLC
using postcolumn derivatization and an ultraviolet
detector. |
| |
|
Recommended air volume
and
sampling rate: |
15 L at 1.0 L/min |
| |
|
| Reliable quantitation limit: |
0.30 mg/m3 |
| |
|
Standard error of estimate
at
the target concentration:
(Section 4.7.) |
7.41% |
| |
|
| Special
requirements: |
Ship samples at reduced
temperature. Store samples in a freezer upon receipt at the
laboratory. Use clean silanized glassware for standard and
sample preparations. |
| |
|
| Status of method: |
Evaluated method. This method
has been subjected to the established evaluation procedures of
the Organic Methods Evaluation Branch. |
| |
|
| Date: September 1989 |
Chemist: Yihlin
Chan |
| |
|
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
|
1.
General Discussion
1.1. Background
1.1.1. History
Airborne methyl ethyl
ketone peroxide (MEK peroxide) was first determined by
collecting in a midget impinger containing dimethyl
phthalate (DMP). (Ref. 5.1.)
A portion of the solution was treated with
diphenylcarbazide and the absorbance of the resulting
color complex was measured at 565 nm. A bubbler containing
an acid solution of titanium tetrachloride has also been
used. (Ref. 5.2.)
NIOSH Methods P&CAM 331 and 3508 (Refs. 5.3.
and 5.4.)
are based on the former procedure. However, these
colorimetric methods are prone to interference and they do
not differentiate the isomeric forms of MEK peroxide shown
below.
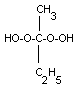
monomer |
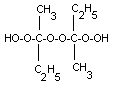
dimer |
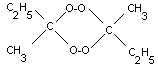
cyclic dimer |
A misconception of the
true identity of MEK peroxide is prevalent in IH
literature. For example, both ACGIH (Ref. 5.5.)
and NIOSH (Refs. 5.3.
and 5.4.)
assign cyclic dimer as the structure for MEK peroxide when
it has been shown conclusively (Refs. 5.6.
and 5.7.)
that the main components of the commonly used industrial
MEK peroxide are monomer and dimer and that the cyclic
dimer is not present.
Preparing analytical
standards of the two isomers was a major obstacle since
MEK peroxide with known concentrations of monomer and
dimer is not available commercially. In this evaluation,
the preparation of monomer and dimer for analytical
standards was accomplished by modifying a procedure
reported in the literature (Ref. 5.8.).
A two-column HPLC method, based on the reaction
between hydroperoxide and triphenylphosphin (Ref. 5.9.),
has been developed (Ref. 5.10.)
to quantitate the MEK peroxide monomer and the dimer
separately. But the method suffers from a long run time as
it requires dual injections and a column wash. An HPLC
method using electrochemlcal detection has been reported,
(Refs. 5.11.
and 5.12.)
but the dissolved oxygen in the sample interfered with
detection of the dimer.
The analytical procedure
of this method was adapted from the literature (Ref. 5.13.).
It is an HPLC/postcolumn derivatization method using a
sodium iodide/acetic acid system where the MEK peroxide
monomer and dimer can be analyzed within a 10-min run
time. XAD-4 adsorbent was selected as the sampling medium
because it has previously been found to be suitable for
collecting airborne MEK peroxide (Ref. 5.10.).
The ACGIH has set a TLV (ceiling) of 0.2 ppm (1.5
mg/m3) for MEK peroxide. This value was
selected for the target concentration. After most of the
work in this evaluation had been done, OSHA published a
new PEL (ceiling) of 0.7 ppm (5 mg/m3). The
sampler of this method easily permits the collection of
MEK peroxide at the new PEL.
1.1.2. Toxic effects
(This section is for information only and should not be
taken as the basis of OSHA policy.)
Following is a
direct quote from Ref. 5.14.
Given orally, by inhalation, or by intraperitoneal
injection, methyl ethyl ketone peroxide causes hyperemia
of the lungs with petechial or gross hemorrhage in mice
and rats. Subacute exposures have been associated with
mild liver damage in rats. In addition, this compound can
be severely irritating to the eyes and skin. In rabbits,
maximum nonirritating strengths were found to be 1.5 and
0.6% for the skin and eyes, respectively.
Mice
given a total dose of (about) 7 mg methyl ethyl ketone
peroxide developed malignant tumors, the first of which
appeared after fifteen months. One subcutaneous sarcoma,
three malignant lymphomas, and a pulmonary adenoma were
noted in 34 of the 50 mice surviving exposure.
Chemical burns of the gastrointestinal tract, as
well as residual scarring and stricture of the esophagus,
were noted in an individual surviving ingestion of two
ounces of a 60% methyl ethyl ketone peroxide solution.
1.1.3. Workplace exposure
Methyl ethyl
ketone peroxide is used widely in the polymer industry for
curing unsaturated polyester resins. According to the
NIOSH National Occupational Exposure Surveys of 1972 and
1982, workers in the following industries (with standard
industrial code) are most likely to be exposed to MEK
peroxide: boat building and repairing (SIC 3732), cut
stone and stone products (SIC 3281), pressed and blown
glass (SIC 3229), terrazzo, tile, marble, mosaic (SIC
1743), household furniture (SIC 2519), and transportation
equipment and supply (SIC 5088).
1.1.4. Physical
properties and other descriptive information (Ref. 5.15.
unless noted otherwise)
| Commercial mixture |
|
| chemical
name: |
methyl ethyl ketone
peroxide |
| CAS no.: |
1338-23-4 |
| synonyms: |
2-butanone peroxide;
MEK peroxide; methyl ethyl ketone hydroperoxide;
NCI-C55447 |
| trade names: |
Butanox 1PT; Butanox M
50; Butanox M 105; Cadox; Chaloxyd
MEKP-HA 1; Chaloxyd
MEKP-LA 1; Esperfoam FR; FR 222;
Hi-Point 90; Hi-Point
PD-1; Kayamek A; Kayamek M; Ketonox;
Lucidol Delta X; Lupersol Delta X 9; Lupersol DNF;
Lupersol DSW; MEK Peroxide; Mekpo; Mepox; Permek G;
Permek N; Quickset Extra; Quickset Super; RCRA Waste
Number U160; Sprayset MEKP; Thermacure; Trigonox M
50 |
| description: |
a colorless to pale
yellow liquid |
| solubility: |
soluble in water |
|
| MEK
peroxide monomer |
|
| chemical name: |
(1-methylpropylidene)bishydroperoxide |
| CAS no.: |
2625-67-4 |
| synonyms: |
2,2-dihydroperoxybutane; MEK
peroxide monomer |
| molecular weight: |
122.12 |
| molecular formula: |
C4H10O4 |
| structural formula: |
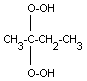 |
| appearance: |
colorless liquid |
| solubility: |
miscible with water,
alcohols, ether (personal observation) |
| derivative: |
bis-p-nitrobenzoate: mp
107.5-109°C (personal observation) |
|
| MEK
peroxide dimer |
| chemical name: |
[dioxybis(1-methylpropylidene)]bishydroperoxide |
| CAS no.: |
126-76-1 |
| synonyms: |
2,2'-dihydroperoxy-2,2'-dibutyl peroxide;
MEK peroxide dimer |
| molecular weight: |
210.23 |
| molecular formula: |
C8H18O6 |
| structural formula: |
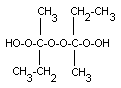 |
| description: |
may exist in two stereo
isomeric forms: dl and meso |
| melting points: |
one isomer: 43-49°C;
the other isomer: liquid at room temperature (mp not
determined) (personal observation) |
| solubility: |
soluble in hexane,
isooctane, methyl t-butyl ether; slightly soluble in
water (personal observation) |
| derivatives: |
bis-p-nitrobenzoate of
one isomer: mp 192-218°C;
bis-p-nitrobenzoate of the other
isomer: sublimes at around 120°C (personal
observation) |
In this method, MEK peroxide from K&K
was used in preparing validation samples. The composition was
26.0% monomer and 20.7% dimer. Unless specifically stated, the
analyte amounts throughout this method are the sum of the
monomer and the dimer. The analyte air concentrations are
based on the recommended sampling and analytical parameters.
1.2. Limit defining parameters
1.2.1. Detection limit of the analytical
procedure
The detection limit of the analytical
procedure is 31 ng per injection. This is the amount of
analytes which gave MEK peroxide monomer and dimer peaks
with heights about 5 times the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is
4.51 µg per sample (0.30 mg/m3). This is the
amount of analyte spiked on the sampling device which
allows recovery of an amount equivalent to the detection
limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The
reliable quantitation limit is 4.51 µg per sample (0.30
mg/m3). This is the smallest amount of MEK
peroxide spiked on the sampling device which can be
quantitated within the requirements of a recovery of at
least 75% and a precision (±1.96 SD) of ±25% or better.
(Section 4.3.)
The reliable quantitation limit and detection limits reported
in the method are based upon optimization of the instrument
for the smallest possible amount of the analyte. When the
target concentration of the analyte is exceptionally higher
than these limits, they may not be attainable at the routine
operating parameters.
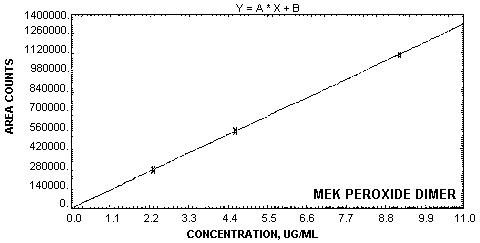
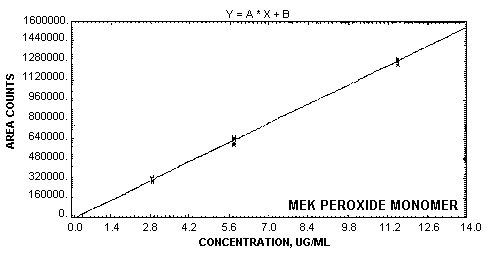
1.2.4. Instrument response to the analyte
The instrument response over the concentration
range of 0.5 to 2 times the target concentration is linear
with a slope of 120700 area counts per µg/mL for MEK
peroxide dimer and 113200 area counts per µg/mL for MEK
peroxide monomer. (Section 4.4.)
1.2.5. Recovery
The recovery of MEK
peroxide from samples used in a 16-day storage test
remained above 92.2% when the samples were stored in a
freezer. (Section 4.5.)
The recovery of an analyte from the collection medium
during storage must be 75% or greater.
1.2.6.
Precision (analytical procedure only)
The pooled
coefficient of variation obtained from replicate
injections of analytical standards at 0.5, 1 and 2 times
the target concentration is 0.027 for MEK peroxide dimer
and 0.035 for MEK peroxide monomer. (Section 4.6.)
1.2.7. Precision (overall
procedure)
The precision at the 95% confidence
level for the refrigerated 16-day storage test is ±14.5%.
(Section 4.7.)
This includes an additional ±5% for sampling error. The
overall procedure must provide results at the target
concentration that are ±25% or better at the 95%
confidence level.
1.2.8. Reproducibility
A
draft copy of this procedure and six samples spiked with
MEK peroxide were given to a chemist unassociated with
this evaluation. The samples were analyzed after 7 days of
storage at about -25°C. No individual sample
result deviated from its theoretical value by more than
the precision reported in Section 1.2.7.
(Section 4.8.)
1.3. Advantages
1.3.1. This sampling and analytical method
provides a simple and convenient means of monitoring
occupational exposure to MEK peroxide.
1.3.2.
Individual isomers of MEK peroxide can be determined.
1.4. Disadvantage
Samples must be
stored in a freezer until analysis. 2. Sampling
Procedure
2.1. Apparatus
2.1.1. A personal sampling pump that can be
calibrated to within ±5% of the recommended flow rate with
the sampling device in line.
2.1.2. Glass sampling
tubes each packed with an 80-mg front section and a 40-mg
back section of XAD-4 adsorbent. XAD-4 sampling tubes from
SKC, Inc. (catalog no. 226-93, Lot 146) were used in this
evaluation. 2.2. Reagents
No sampling
reagents are required.
2.3. Sampling technique
2.3.1. Break open both ends of the sampling
tube so that the holes are at least one-half the inside
diameter of the tube. Attach the tube to the sampling pump
with a piece of flexible tubing such that the front
section is exposed directly to the atmosphere. Attach the
sampler vertically in the worker's breathing zone.
2.3.2. After sampling, seal the tube with plastic
end caps. Wrap the tube lengthwise with an official OSHA
seal (Form 21).
2.3.3. Submit at least one blank
with each set of samples. Handle the blank the same as the
other samples except draw no air through it.
2.3.4. List any potential interferences on the
sample data sheet. 2.4. Sampler capacity
The sampler can be used to collect MEK peroxide at
10 times the target concentration for at least 8 times the
recommended sampling time without breakthrough. (Section 4.9.)
2.5. Desorption efficiency and stability of desorbed
samples
2.5.1. The average desorption efficiency for
MEK peroxide from 80 mg of XAD-4 over the range of 0.5 to
2 times the target concentration was 99.4%. (Section 4.10.)
2.5.2. Desorbed samples remain stable for at least
24 h when stored at room temperature. 2.6.
Recommended air volume and sampling rate
2.6.1. The recommended air volume is 15 L.
2.6.2. The recommended air sampling rate is 1
L/min. 2.7. Interferences (sampling)
MEK peroxide is very labile. Heat, acids, bases, or
trace amounts of metal ions will cause decomposition.
2.8. Safety precautions (sampling)
2.8.1. Attach the sampling equipment to the
worker in such a manner that it will not interfere with
work performance or safety.
2.8.2. Follow all
safety practices applicable to the work area being
sampled. 3. Analytical Procedure
3.1. Apparatus
3.1.1. An HPLC equipped with a UV detector. A
Waters HPLC system consisting of a 600E pump, a 900
photodiode array detector, and a 712 WISP autosampler was
used in this evaluation. For the alternate analytical
conditions, a BAS 200 HPLC equipped with electrochemical
detector was used.
3.1.2. An HPLC column capable
of separating MEK peroxide monomer and dimer from
potential interferences. A 25-cm × 6-mm Bakerbond
cyanopropyl column was used in this evaluation. A 25-cm ×
6-mm Supelco LC18DB column was used in the alternate
analytical conditions.
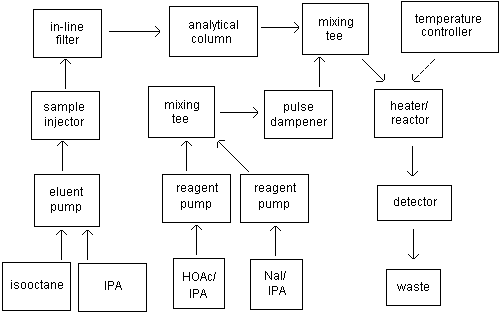
3.1.3. A postcolumn
derivatization system. Two Waters 6000 pumps, pulse
dampeners (part nos. 98060 and 25561), ninhydrin reaction
coil and temperature controller were used in this study. A
schematic of the HPLC/postcolumn system is illustrated in
Figure 3.1.3.
3.1.4. An electronic integrator or other suitable
means of measuring detector response. A Hewlett-Packard
3357 laboratory data system was used in this evaluation.
3.1.5. Four-milliliter vials with
polytetrafluoroethylene (PTFE)-lined caps. New unsilinized
vials were used in this evaluation. If old vials are to be
used, they must be silanized.
3.1.6. Volumetric
flasks and pipets, silanized. Sylon CT from Supelco was
used in silanizing the glassware. The directions supplied
with the reagent were followed. 3.2. Reagents
3.2.1. MEK peroxide solution in DMP. Methyl
ethyl ketone peroxide, 60% in DMP, from K & K
Laboratories was used in this evaluation.
3.2.2.
2-Propanol (IPA) and isooctane, HPLC grade. 2-Propanol,
Optima, and isooctane, Optima, both from Fisher Scientific
Co., were used in this evaluation.
3.2.3. 10%
Acetic acid solution: Dilute 100 mL of glacial acetic acid
to 1 L with 2-propanol.
3.2.4. Sodium iodide
solution: Dissolve 1 g of sodium iodide in 1 L of
2-propanol. 3.3. Standard preparation
3.3.1. Determine the concentration of the
dimer and monomer in the DMP solution by using the pure
dimer and monomer synthesized in Section 4.12.
Although MEK peroxide/DMP solutions are quite stable when
stored in a refrigerator, determine the dimer and monomer
concentrations once every 6 months.
3.3.2. Prepare
stock standards by weighing 3 drops of MEK peroxide/DMP
solution in 10-mL volumetric flasks and diluting to volume
with 2-propanol. Prepare fresh stock standards daily.
3.3.3. Prepare analytical standards by further
diluting stock standards with 2-propanol.
3.3.4.
Prepare a sufficient number of standards to generate
calibration curves. Analytical standard concentrations
must bracket sample concentrations. 3.4. Sample
preparation
3.4.1. Transfer the front section of XAD-4 to
a 4-mL glass vial. Place the back section in a separate
4-mL vial. Discard the glass wool and foam plugs.
3.4.2. Add 2.0 mL of 2-propanol to each vial.
3.4.3. Seal the vials with PTFE-lined caps and
shake them on a mechanical shaker for 1 h. 3.5.
Analysis
3.5.1. Analytical conditions
| column: |
Bakerbond cyanopropyl,
25 cm × 6 mm |
| mobile phase: |
95:5 (v/v)
isoctane/2-propanol |
| flow rate: |
1.0 mL/min |
| postcolumn
derivatization: |
10% acetic acid in
2-propanol at 0.5 mL/min; 1 g/L sodium iodide in
2-propanol at 0.5 mL/min; the reaction coil was
heated to 65°C. |
| injection volume: |
15 µL |
| detector: |
292 nm or 360 nm |
| chromatogram: |
Figure 4.11.1. |
3.5.2. Alternate
analytical conditions
| column: |
Supelco LC18DB, 25 cm ×
6 mm |
| mobile phase: |
45:55 (v/v)
methanol/0.1 M lithium perchlorate |
| flow rate: |
1.0 mL/min |
| injection volume: |
15 µL |
| electrochemical
detector: |
Au/Hg electrode at -550
mV relative to the reference electrode |
| chromatogram: |
Figure 4.11.2. |
| note: |
The two isomers of the
dimer are separated under these
conditions. |
3.5.3. Measure detector response using a
suitable method such as electronic integration.
3.5.4. Prepare a calibration curve using several
standards over a range of concentrations. Bracket the
samples with analytical standards. 3.6. Interferences (analytical)
3.6.1. Any compound having a similar retention
time as MEK peroxide monomer or dimer and capable of
liberating iodine from the sodium iodide/acetic acid
mixture is a potential interference. Generally,
chromatographic conditions can be altered to separate an
interference.
3.6.2. Hydrogen peroxide and
dimethyl phthalate are not interferences.
3.6.3. A
useful means of confirming the MEK peroxide is by
electrochemical detector using reverse phase column
(Section 3.5.2.).
A GC/MS procedure would not be viable because MEK peroxide
is thermally labile. 3.7. Calculations
3.7.1. Prepare separate calibration curves for
MEK peroxide monomer and dimer by plotting detector
responses versus the analytical standard concentrations.
Determine the best-fit lines.
3.7.2. Determine the
concentrations of a sample, in micrograms of MEK peroxide
monomer or dimer per milliliter, by comparing its detector
responses to the calibration curves.
3.7.3.
Perform blank corrections for each section.
3.7.4.
The air concentration of MEK peroxide can be obtained by
using the following equations:
| mg/m3 = |
(A + B)
(C)
(D) (E) |
| where |
A
B
C
D
E |
=
=
=
=
= |
µg/mL of MEK peroxide
dimer
µg/mL of MEK peroxide monomer
desorption
volume (2 mL)
liters of air sampled
desorption
efficiency,
0.994 |
Values in ppm are not calculated because
they are dependent on the dimer/monomer ratio.
3.8. Safety precautions (analytical)
3.8.1. Avoid skin contact and inhalation of
all chemicals.
3.8.2. Restrict the use of all
chemicals to a fume hood.
3.8.3. Wear safety
glasses in all laboratory areas. 4.
Backup Data
4.1. Detection limit of the
analytical procedure
The detection limit of the
analytical procedure is 31 ng per injection. It is based on
a 15-µL injection of a 2.07 µg/mL standard (consisting of
0.92 µg/mL of MEK peroxide dimer and 1.15 µg/mL of MEK
peroxide monomer.) This amount produced MEK peroxide monomer
and dimer peaks whose heights were about 5 times the height
of the baseline noise. A chromatogram of the detection limit
of the analytical procedure is shown in Figure 4.1.
4.2. Detection limit of the
overall procedure
The detection limit of the overall
procedure is 4.51 µg per sample (0.30 mg/m3). Six
vials containing 80 mg of XAD-4 were each liquid spiked with
4.51 µg of MEK peroxide. The samples were desorbed 24 h
later with 2 mL of 2-propanol.
4.3. Reliable quantitation limit
The
reliable quantitation limit is also 4.51 µg per sample (0.30
mg/m3). This was derived from the samples and
data of Table 4.2.
Because the recovery of MEK peroxide from the spiked samples
was greater than 75% and the precision (1.96 SD) was less
than ±25%, the detection limit of the overall procedure and
reliable quantitation limit are the same.
Table 4.3.
Reliable Quantitation
Limit
(based on samples and data of Table 4.2.)
|
|
| %
recovery |
statistics |
|
| 85.4 |
|
| 96.5 |
 |
= |
89.4% |
| 94.9 |
SD |
= |
5.21% |
| 84.9 |
Precision |
= |
±(1.96)(5.21%) |
| 89.8 |
|
= |
±10.2% |
| 85.1 |
|
|
4.4. Instrument response to MEK peroxide
The instrument response to MEK peroxide over the
range of 0.5 to 2 times the target concentration is linear
with a slope of 120700 area counts per µg/mL for the dimer
and 113213 area counts per µg/mL for the monomer. The
responses to MEK peroxide were determined by multiple
injections of standards. The data in Tables 4.4.1. and
4.4.2. are presented graphically in Figures 4.4.1.
and 4.4.2.
Table 4.4.1.
Instrument Response to
MEK Peroxide Dimer |
|
| × target
conc. |
0.5× |
1× |
2× |
| µg/mL |
2.30 |
4.60 |
9.19 |
|
| area counts |
264032 |
539484 |
1106430 |
|
283125 |
559323 |
1114170 |
|
268162 |
544467 |
1107150 |
|
288679 |
568779 |
1105220 |
|
265246 |
566636 |
1091830 |
|
263122 |
563841 |
1106160 |
|
 |
272061 |
557088 |
1105160 |
|
4.5. Storage data
Thirty-six samples
were generated by liquid-spiking 22.62 µg of MEK peroxide
onto XAD-4 adsorbent tubes. Humid air (80% relative humidity
at 24°C) was pulled through the tubes for 15 min at 1 L/min.
Six tubes were analyzed the same day. Fifteen tubes were
stored in a freezer (-25°C) and the other fifteen were
stored in the dark at ambient temperature (about 22°C).
Every few days over a 15-day period, three samples were
selected from each of the two sets and analyzed. Another set
of storage samples were prepared and analyzed over a 16-day
period. The combined results are listed Table 4.5. There was
no significant loss of MEK peroxide in the refrigerated
samples, but those stored at ambient temperature suffered a
significant loss. The storage data are also presented
graphically in Figures 4.5.1.
and 4.5.2.
Table 4.5.
Storage Test |
|
| storage
time |
%
recovery |
%
recovery |
| (days) |
(ambient) |
(refrigerated) |
|
|
|
| 0 |
95.7 |
89.8 |
93.8 |
95.7 |
89.8 |
93.8 |
| 0 |
90.1 |
72.4 |
81.9 |
90.1 |
72.4 |
81.9 |
| 0 |
95.4 |
96.4 |
100.4 |
95.4 |
96.4 |
100.4 |
| 0 |
98.1 |
95.4 |
95.1 |
98.1 |
95.4 |
95.1 |
| 3 |
77.7 |
84.2 |
78.2 |
92.8 |
93.8 |
95.0 |
| 6 |
68.0 |
12.6* |
72.6 |
95.7 |
91.6 |
80.3 |
| 7 |
64.2 |
78.3 |
59.0 |
93.1 |
93.8 |
97.4 |
| 9 |
57.3 |
55.9 |
59.5 |
96.9 |
96.2 |
94.3 |
| 10 |
65.3 |
66.9 |
57.8 |
102.3 |
98.0 |
97.8 |
| 11 |
69.5 |
72.2 |
52.0 |
94.7 |
97.4 |
97.7 |
| 15 |
44.7 |
70.4 |
62.1 |
93.4 |
90.7 |
98.3 |
| 16 |
45.6 |
51.9 |
51.7 |
95.0 |
95.8 |
95.6 |
|
| *
outlier, excluded |
4.6. Precision (analytical
method only)
The precision of the analytical
procedure is 0.027 for MEK peroxide dimer and 0.035 for MEK
peroxide monomer. The precision of the analytical procedure
is defined as the pooled coefficient of variation determined
from replicate injections of MEK peroxide standards at 0.5,
1 and 2 times the target concentration.
Table 4.6.1.
Precision of the Analytical
Method
for MEK Peroxide Dimer
(based on the data
of Table 4.4.1.) |
|
| ×
target conc. |
0.5× |
1× |
2× |
| µg/mL |
2.30 |
4.60 |
9.19 |
|
| SD
(area counts) |
10996 |
12228 |
7286 |
| CV |
0.0404 |
0.0219 |
0.0066 |
|
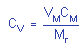 = 0.0268 = 0.0268 |
|
|
Table 4.6.2.
Precision of the Analytical
Method
for MEK Peroxide Monomer
(based on the
data of Table 4.4.2.) |
|
| × target
conc. |
0.5× |
1× |
2× |
| µg/mL |
2.89 |
5.77 |
11.54 |
|
| SD (area
counts) |
12210 |
26203 |
20861 |
| CV |
0.0405 |
0.0414 |
0.0163 |
|
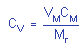 = 0.0347 = 0.0347 |
|
|
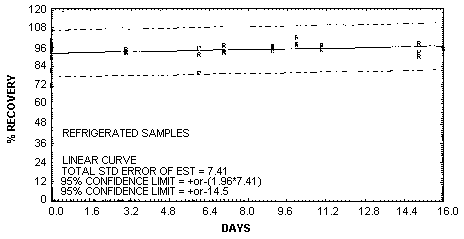
4.7. Precision (overall procedure)
The precision of the overall procedure is determined
from the storage data. The determination of the standard
error of estimate (SEE) for a regression line plotted
through the graphed storage data allows the inclusion of
storage time as one of the factors affecting overall
precision. The SEE is similar to the standard deviation
except it is a measure of dispersion of data about a
regression line instead of about a mean. It is determined
with the following equation:
| SEE = |
[ |
S(Yobs -
Yest)2 |
] |
1/2 |
|
|
| n -
k |
|
| where |
| n |
= |
total no. of data
points |
| k |
= |
2 for a linear
regression |
| k |
= |
3 for a quadratic
regression |
| Yobs |
= |
observed % recovery at a
given time |
| Yest |
= |
estimated % recovery from
the regression line at the same given
time |
An
additional ±5% for pump error is added to the SEE by the
addition of variances. The precision at the 95% confidence
level is obtained by multiplying the SEE (with pump error
included) by 1.96 (the z-statistic from the
standard normal distribution at the 95% confidence level).
The 95% confidence intervals are drawn about their
respective regression lines in the storage graphs as shown
in Figure 4.5.1.
The data for Figure 4.5.1.
was used to determine the SEE of ±7.41% for MEK peroxide.
4.8. Reproducibility data
Six samples, liquid spiked with MEK peroxide, were
given to a chemist unassociated with this study. The samples
were analyzed after being stored for 7 days at about -25°C.
No sample result had a percent deviation greater than the
precision of the overall procedure, which was ±14.5%.
Table 4.8.
Reproducibility Data |
|
| µg spiked |
µg
recovered |
%
recovered |
%
deviation |
|
| 31.44 |
28.58 |
90.0 |
-10.0 |
| 31.44 |
27.87 |
88.6 |
-11.4 |
| 26.20 |
23.66 |
90.3 |
-9.7 |
| 26.20 |
24.11 |
92.0 |
-8.0 |
| 20.96 |
18.51 |
88.3 |
-11.7 |
| 20.96 |
19.74 |
94.2 |
-5.7 |
|
4.9. Sampler capacity
Aerosol
generation was found to be unsuitable for preparing a
standard atmosphere of MEK peroxide because it drastically
changes the latter's composition. The sampler capacity was
tested by the following method.
A plug of glass wool
was placed upstream of an XAD-4 tube. The back section of
the tube was removed. Another XAD-4 tube was placed
downstream. Humid air (80% RH at 22°C) was pulled through
the train at 1 L/min. At 15-min intervals, 229 µg of MEK
peroxide was spiked onto the glass wool and the downstream
XAD-4 tube was replaced with a new one. This simulates an
atmosphere of 15.3 mg/m3 MEK peroxide which is
about 10 times the target concentration. The test was
conducted for a total of 120 min, 8 times the recommended
sampling time. At the end of the test, all 8 downstream
tubes as well as the front tube were analyzed. Essentially
all of the MEK peroxide was collected on the front tube. The
sampler capacity is at least 8 times the recommended
sampling volume at 10 times the target concentration.
Table 4.9.
Breakthrough Data at 10×
Target Concentration |
|
| air
volume |
breakthrough |
| (L) |
(%) |
|
| 15 |
0.00 |
| 30 |
0.00 |
| 45 |
0.00 |
| 60 |
0.00 |
| 75 |
0.00 |
| 90 |
0.02 |
| 105 |
0.02 |
| 120 |
0.06 |
|
4.10. Desorption efficiency and stability of
desorbed samples
4.10.1. Desorption efficiency
The
desorption efficiency (DE) of MEK peroxide was determined
by liquid-spiking 80-mg portions of XAD-4 with MEK
peroxide at 0.5, 1 and 2 times the target concentration.
These samples were stored in a refrigerator overnight and
then desorbed and analyzed. The average desorption
efficiency over the studied range was 99.4%.
Table 4.10.1.
Desorption Efficiency of
MEK Peroxide |
|
| × target
conc. |
0.5× |
1× |
2× |
| µg/sample |
10.86 |
21.72 |
43.43 |
|
| DE, % |
98.3 |
98.8 |
98.6 |
|
100.9 |
100.2 |
99.7 |
|
96.5 |
99.6 |
99.9 |
|
96.8 |
98.0 |
101.5 |
|
97.5 |
98.2 |
101.5 |
|
97.9 |
102.3 |
102.9 |
|
 |
98.0 |
99.5 |
100.7 |
|
4.10.2. Stability of desorbed samples
The stability of desorbed samples was investigated
by reanalyzing the 1 times the target concentration
desorption samples about 24 h after the original analysis.
The samples had been recapped and stored at room
temperature.
They were reanalyzed with fresh
standards. The average of the reanalyzed samples relative
to the average of the original analysis was 101.7%.
Table 4.10.2.
Stability of Desorbed
Samples |
|
| original |
reanalyzed |
reanalyzed
relative |
| result
(%) |
result
(%) |
to
original (%) |
|
| 97.7 |
101.3 |
103.7 |
| 98.8 |
98.0 |
99.2 |
| 98.8 |
96.9 |
98.1 |
| 98.8 |
103.1 |
104.4 |
| 99.6 |
102.5 |
102.9 |
| 98.8 |
100.5 |
101.7 |
| 4.11. Chromatograms
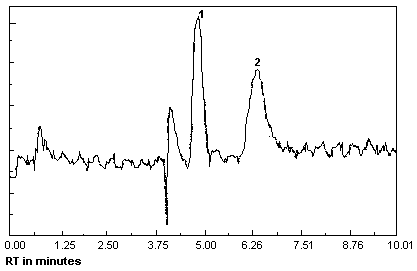
A chromatogram at the detection limit of the
analytical procedure is shown in Figure 4.1.
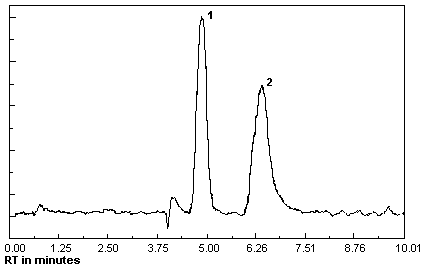
and a chromatogram at the target concentration is shown in
Figure 4.11.1.
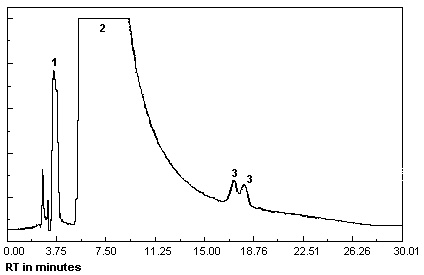
A chromatogram at target concentration under the alternate
analytical conditions is shown in Figure 4.11.2.
4.12. Standardization of MEK
peroxide solution
4.12.1. Apparatus
Round-bottomed
flask
Rotary evaporator
Separatory funnel
Vacuum
oven
Sintered-glass filtering funnel
4.12.2.
Reagents
Hydrogen peroxide, 30% aqueous
solution
Methyl ethyl ketone
Sulfuric acid
Methyl
t-butyl ether
Ammonium sulfate
Magnesium sulfate,
anhydrous
0.01 N Sodium thiosulfate
Potassium
dichromate, primary standard
Hexane
Liquid nitrogen
4.12.3. Synthesis of MEK peroxide monomer
Place methyl ethyl ketone (3.6 g, 0.05 mole) and
30% hydrogen peroxide (11.3 g, 0.1 mole) in a 20-mL
scintillation vial. Cap the vial and shake to mix well.
Let stand at room temperature overnight. Heat the vial in
a 40°C water bath for 1 h. Dilute the mixture in 500 mL of
methyl t-butyl ether and wash twice with a saturated
aqueous solution of ammonium sulfate and twice with water.
Dry over anhydrous magnesium sulfate and filter. Evaporate
the filtrate under vacuum. The yield of the residue is
approximately 2 g. Add approximately an equal volume of
dimethyl phthalate to the residue and store in a freezer.
Determine the monomer concentration by iodometric
titration.
4.12.4. Synthesis of MEK peroxide dimer
Assemble a 500-mL round-bottom flask equipped with
a stirring bar and a thermometer in an ice bath. Place
methyl ethyl ketone (14.4 g, 0.2 mole) and 30% hydrogen
peroxide (22.67 g, 0.2 mole) in the flask. Add sulfuric
acid (0.49 g, 0.005 mole) slowly with stirring so that the
temperature does not rise above 15°C. Continue stirring at
room temperature overnight. Take up the mixture in 500 mL
of hexane and wash twice with saturated aqueous solution
of ammonium sulfate and three times with 300 mL of water.
Dry over anhydrous magnesium sulfate and filter. Chill the
filtrate in an 2-propanol/liquid nitrogen bath. Collect
the precipitated white crystals. Recrystallize from
hexane, mp 43-49°C. The yield is approximately 5 g.
Determine the dimer purity by iodometric titration. It
should be near 100%. Store in a freezer.
4.12.5.
Iodometric titration of MEK peroxides (Ref. 5.16.)
Standardize the 0.01 N sodium thiosulfate solution
with a potassium dichromate primary standard. Prepare
stock solutions of monomer and dimer in 2-propanol. The
concentration should be approximately 5 mg/mL. Place 20 mL
of 2-propanol, 2 mL of glacial acetic acid, and 0.5 g of
potassium iodide in a 500-mL Erlenmeyer flask. Add 1.0 mL
of the MEK peroxide stock solution. Allow the flask to
stand in the dark for 15 min. Add 10 mL of water and
titrate the solution with 0.01 N sodium thiosulfate
solution. Perform blank corrections. Calculate the purity
of the monomer and dimer as follows.
| purity
(%) = |
(N) (V)
(EW)
(C) (D) |
×
100 |
| where |
N |
= |
normality of
thiosulfate |
|
V |
= |
volume (mL) of
thiosulfate solution |
|
EW |
= |
equivalent weight,
35.04 for dimer, 30.53 for monomer |
|
C |
= |
concentration (mg/mL)
of the dimer or monomer stock solution |
|
D |
= |
volume (mL) of the
stock solution |
|
| Figure 3.1.3.
Schematic of an HPLC postcolumn
system. |
|
Figure 4.1. Detection
limit of the analytical procedure.
1 = MEK
peroxide dimer, 2 = MEK peroxide
monomer. |
|
| Figure 4.4.1.
Calibration curve for MEK peroxide
dimer. |
|
| Figure 4.4.2.
Calibration curve for MEK peroxide
monomer. |
|
| Figure 4.5.1.
Storage test at reduced
temperature. |
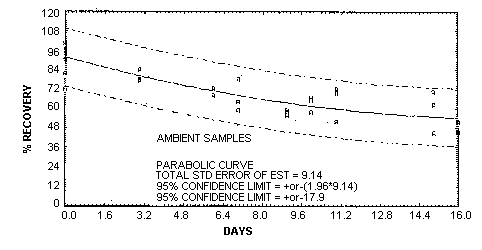
|
| Figure 4.5.2.
Storage test at ambient
temperature. |
|
Figure 4.11.1.
Chromatogram at target concentration.
1 = MEK
peroxide dimer, 2 = MEK peroxide
monomer. |
|
Figure 4.11.2.
Chromatogram at target concentration under alternate
analytical conditions.
1 = MEK peroxide monomer,
2 = oxygen, 3 = MEK peroxide
dimer. |
5. References
5.1. Elskamp, C.J.,
"Determination of Airborne Methyl Ethyl Ketone Peroxide",
Wisconsin State Laboratory of Hygiene, Madison, WI, 1975.
unpublished.
5.2. Purnell, C.J.,
Martin, G.L., and Wolfson, H., "Determination of Airborne
Methyl Ethyl Ketone Peroxide in the Glass Reinforced
Plastics Industry", Ann. Occup. Hyg., 1979,
Vol. 22, No. 4, pp. 383-387.
5.3. Method No. P&CAM 331 in "NIOSH
Manual of Analytical Methods", 2nd edition, U.S. Department
of Health, Education, and Welfare, Public Health Services,
Center for Disease Control, NIOSH, DHEW(NIOSH) Publication
No. 77-157-A.
5.4. Method No. 3508
in "NIOSH Manual of Analytical Methods", 3rd edition, 1985
Supplement, U.S. Department of Health and Human Services,
Center for Disease Control, NIOSH; Cincinnati, OH, 1986.
5.5. "Documentation of the
Threshold Limit Values and Biological Exposure Indices",
American Conference of Governmental Industrial Hygienists
Inc.: Cincinnati, OH, 5th ed. 1986.
5.6. Thomas, A., Jacyszyn, O., Schmitt, W.
and Kolczynski, J., "Methyl Ethyl Ketone Peroxides,
Relationship of Reactivity to Chemical Structure", 32nd
Annual Technical Conference, 1977, Reinforced
Plastics/Composites Institute, The Society of the Plastics
Industry, Inc.
5.7. Cassoni, J.P.,
Harpell, G.A., Wang, P.C. and Zupa, A.H., "Use of Ketone
Peroxides for Room Temperature Cure of Thermoset Resins",
32nd Annual Technical Conference, 1977, Reinforced
Plastics/Composites Institute, The Society of the Plastics
Industry, Inc.
5.8. Milas, N.A.
and Golubovic, A., "Studies in Organic Peroxides. XXV.
Preparation, Separation and Identification of Peroxides
Derived from Methyl Ethyl Ketone and Hydrogen Peroxide",
J. Am. Chem. Soc., 1959, 81, 5824-26.
5.9. Denney, D.B., Goodyear, W.F.
and Goldstein, B., "Concerning the Mechanism of the
Reduction of Hydroperoxides by Trisubstituted Phosphines and
Trisubstituted Phosphites", J. Am. Chem. Soc.,
1960, 82, 1393-195.
5.10. Shulsky, M., "Evaluation of Sampling
and Analytical Methods Specific for Methyl Ethyl Ketone
Peroxide", OSHA Analytical Laboratory, 1982. Unpublished.
5.11. Funk, M.O., Keller, M.B.
and Levison, B., "Determination of Peroxides by High
Performance Liquid Chromatography with Amperometric
Detection", Anal. Chem., 1980, 52,
771-773.
5.12. Cummins, K.,
"Analysis of Methyl Ethyl Ketone Peroxide by HPLC and
Detection by Electrochemical Reduction Using a Mercury-Gold
Electrode", OSHA Analytical Laboratory, 1988. Unpublished.
5.13. Deelder, R.S., Kroll,
M.G.F. and van den Berg, J.H.M., " Determination of Trace
Amounts of Hydroperoxides by Column Liquid Chromatography
and Colorimetric Detection", J. Chromatogr.,
1976, 125, 307-314.
5.14. Sittig, M., "Handbook of Toxic and
Hazardous Chemicals", Noyes Publications, Park Ridge, New
Jersey, 1981.
5.15. Sweet, D.V.,
ed., "Registry of Toxic Effects of Chemical Substances",
1985-86 edition, U.S. Department of Health and Human
Services, Government Printing Office, DHHS(NIOSH)
Publication No. 87-114.
5.16.
Johnson, R.M. and Siddiqi, I.W., The Determination of
Organic Peroxides, Pergamon Press, Oxford, 1970.
|
| |
| |

