ALDICARB (TEMIK)
| Method no.: | 74 |
| Matrix: | Air |
| Target concentration: | 0.1 mg/m3 |
| Procedure: | Samples are collected by drawing known volumes of air through
OSHA Versatile Samplers containing a glass fiber filter and two
sections of |
| Recommended air volume and sampling rate: |
480 L at 1.0 L/min |
| Reliable quantitation limit: | 0.25 µg/m3 |
| Standard error of estimate at the target concentration: (Section 4.7.) |
6.7% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: September 1988 | Chemist: Donald Burright |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
1.1. Background
1.1.1. History
This evaluation was undertaken to determine the effectiveness of
the OSHA Versatile Sampler containing
In the past, aldicarb was collected on
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The LD50 value for technical aldicarb was found to be 0.9 mg/kg for male rats when applied orally. Temik 10G has an LD50 400 mg/kg when wetted with saline and applied to the skin of rats. (Ref. 5.6.)
Aldicarb is a carbamate insecticide which causes cholinesterase inhibition at very low doses. It has muscarinic effects at exocrine, excretory, cardiac and bronchial sites which are exhibited overtly by salivation, lacrimation, defecation, urination, slowing of the heart and trouble with breathing. Aldicarb's chief metabolites, aldicarb sulfoxide and aldicarb sulfone, are also potent cholinesterase inhibitors. (Ref. 5.6.)
1.1.3. Workplace exposure
Occupational exposure can occur during the formulation,
distribution and application of aldicarb. Aldicarb is approved for
soil application around cotton, sugar beets, potatoes, peanuts,
sugarcane, lily bulbs, greenhouse plants, ornamentals selected,
citrus trees, pecans, sorghum, and soybeans. (Ref. 5.7.) An
estimated
1.1.4. Physical properties and other descriptive information (Ref. 5.7.)
| CAS no.: | 116-06-3 |
| molecular weight: | 190.25 |
| odor: | slightly sulfurous |
| melting point: | 99-100°C |
| vapor pressure: | 0.013 Pa (1 × 10-4 mm Hg) at 25°C |
| description: | white, crystalline solid |
| specific gravity: | 1.195 at 25/20°C |
| flash point: | between 23-60°C |
| solubility: | practically insoluble in hexane, but soluble in most other organic solvents, slightly soluble in water |
| synonyms: | |
| structure: | CH3-S-C(CH3) 2-CH=N-O-CO-NH-CH3 |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters.
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 60.3 pg per injection. This is the amount of analyte which gave an aldicarb peak whose area is about 5 times the area of an interference peak visible in a blank sample. (Section 4.1.)
1.2.2. Detection limit of the overall procedure is 120.5 ng per sample (0.25 µg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 120.5 ng per sample (0.25 µg/m3). This is the smallest amount of analyte spiked on the sampling device which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of the analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of aldicarb from samples (liquid spiked) used in a 17-day storage test remained above 103.1% when the samples were stored in a closed drawer at about 22°C. (Section 4.5.) The recovery of an analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical procedure only)
The pooled coefficient of variation obtained from replicate injections of analytical standards at 0.5, 1 and 2 times the target concentration is 0.014. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 17-day storage test is ±13.2%. (Section 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples, spiked by liquid injection with aldicarb, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after one day of storage at about 22°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantage
This sampling device can collect a variety of pesticides as aerosols or vapors.
1.4. Disadvantage
Currently the
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device attached.
2.1.2. Samples are collected with glass
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Attach the small end of the sampling device to the
sampling pump with flexible, plastic tubing such that the large
front section of the sampling tube is exposed directlyto the
atmosphere. Do not place any tubing in front of the sampler. Attach
the sampler vertically (large end down) in the worker's breathing
zone in such a manner that it does not impede work performance.
2.3.2. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps.
2.3.3. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.4. With each set of samples submit at least one blank sample. Handle the blank sample in the same manner as the other samples except draw no air through it.
2.4. Retention efficiency
To test the sampler's ability to retain aldicarb, two times the
target concentration of aldicarb (98.4 µg) was
2.5. Extraction and desorption efficiencies (Section 4.10.)
- 2.5.1. The combined extraction/desorption efficiency for
aldicarb from the glass fiber filter and the large
2.5.2. The extraction efficiency for aldicarb from glass fiber filters at the target concentration was 97.5%.
2.5.3. The average desorption efficiency for aldicarb over the
range of 0.5 to 2 times the target concentration from the lot of
cleaned
2.5.4. Extracted/desorbed samples remain stable for at least 48 h.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 480 L.
2.6.2. The recommended air sampling rate is 1.0 L/min.
2.6.3. When short-term air samples are required, the reliable
quantitation limit is 8.0 µg/m3 for a
2.7. Interferences (sampling)
Suspected interference should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. The sampling equipment should be attached to the worker
in such a manner that it will not interfere with work performance or
safety.
2.8.2. All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a nitrogen/phosphorus detector. For
this evaluation a
3.1.2. A GC column capable of separating the aldicarb peak from
potential interferences. A
3.1.3. An electronic integrator or other suitable means of
measuring detector response. A
3.1.4. Two- and four-milliliter vials with PTFE-lined caps were used for sample extraction/desorption and standard preparation.
3.2. Reagents
- 3.2.1. Aldicarb. Aldicarb (99.0% pure) used in this evaluation
was purchased from Chem Services Inc., West Chester, PA.
3.2.2. Acetone. American Burdick & Jackson "High Purity Solvent" brand acetone was used in this evaluation.
3.2.3. Triethylphosphate (TEP). TEP was purchased from ICN. This was used as the internal standard in the extracting/desorbing solution (25 microliters of TEP per liter of acetone).
3.3. Standard preparation
- 3.3.1. Prepare stock standards by adding acetone to preweighed
amounts of aldicarb. Include the percent purity in the calculation.
3.3.2. Prepare analytical standards by injecting microliter
amounts of diluted aldicarb stock standards into
3.4. Sample preparation
- 3.4.1. Transfer the glass fiber filter and the 270-mg section of
the sampling tube to a
Discard the rear foam plug. Do not discard the glass sampling tube as it can be reused after cleaning with surfactant or suitable solvent.
3.4.2. Add 2.0 mL of extracting/desorbing solution to each vial.
3.4.3. Seal the vials with PTFE-lined caps and allow them to extract/desorb for 1 h. Shake the vials vigorously by hand several times during the extraction/desorption time.
3.4.4. If necessary, transfer some of the solution from each of the 4-mL vials to smaller glass vials suitable for an autosampler.
3.5. Analysis
- 3.5.1. Analytical conditions
GC conditions
| temperature: | 50°C (column) 200°C (injector) 220°C (detector) |
| temp program: | hold initial temp 1.0 min, increase temp at 25°C/min to 110°C, hold temp for 3.5 min, increase temp at 25°C/min to 135°C, hold temp for 5.0 min |
| column gas flow: | 1.7 mL/min (helium) |
| septum purge: | 3.8 mL/min (helium) |
| injection size: | 1.0 µL (splitless mode) |
| column: | SPB-1, 30-m × 0.32-mm i.d. fused silica
|
| retention time: | 6.44 min (aldicarb) 11.23 min (TEP) |
| NPD conditions | |
| make-up gas flow: | 36 mL/min (helium) |
| hydrogen flow: | 3.5 mL/min |
| air flow: | 100 mL/min |
| chromatogram: | Figure 3.5.1. |
3.5.2. Measure detector response using a suitable method such as electronic integration.
3.5.3. Use an external or internal standard procedure to prepare a calibration curve using several standards over a range of concentrations. Prepare the calibration curve daily. Bracket the samples with analytical standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound having a similar retention time as the
analyte is a potential interference. Generally, chromatographic
conditions can be altered to separate an interference from the
analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. Prepare calibration curves from analytical standards by
plotting detector response for aldicarb versus the analytical
standard concentrations (in terms of micrograms aldicarb per
milliliter). Determine, the
3.7.2. Determine the concentration, in micrograms of aldicarb per milliliter, of a sample by comparing its detector response to the calibration curve. Perform blank corrections for each section before adding the results together. Add the amount of aldicarb on the backup section to the amount found on the front section.
3.7.3. The air concentration of aldicarb can be expressed in mg/m3 by using the following equation:
mg/m3 = (A)(B) / (C)(D)
| where | A = | µg/mL of aldicarb from Section 3.7.2. |
| B = | extraction/desorption volume in milliliters | |
| C = | liters of air sampled | |
| D = | combined extraction/desorption efficiency (decimal) |
The combined extraction/desorption efficiency should be determined for the particular batch of resin and lot of filter used for the sample.
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood.
3.8.3. Wear safety glasses in all laboratory areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure, 60.3 pg per
injection, is based on a
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 120.5 ng per sample
(0.25 µg/m3). The injection size recommended
in the analytical procedure (1.0 µL) was used in the determination of
the detection limit of the overall procedure. Six samples containing a
glass fiber filter and 270 mg of
|
| ||
| sample number |
theoretical amount (ng) |
amount recovered (ng) |
|
| ||
| 1 | 120.5 | 103.5 |
| 2 | 120.5 | 95.5 |
| 3 | 120.5 | 92.5 |
| 4 | 120.5 | 93.9 |
| 5 | 120.5 | 100.0 |
| 6 | 120.5 | 100.3 |
|
| ||
4.3. Reliable quantitation limit data
The reliable quantitation limit is 120.5 ng per sample (0.25
µg/m3). The injection size recommended in
the analytical procedure (1.0 µL) was used in the determination of the
reliable quantitation limit. Six samples containing "a glass fiber
filter and 270 mg of
|
| |||
| sample number |
percent recovered |
statistics | |
|
| |||
| 1 | 85.9 | ||
| 2 | 79.2 | 81.0 | |
| 3 | 76.8 | SD = | 3.5 |
| 4 | 77.9 | Precision = | ±(1.96)(3.5) |
| 5 | 83.0 | = | ±6.9 |
| 6 | 83.2 | ||
|
| |||
4.4. Instrument response to aldicarb
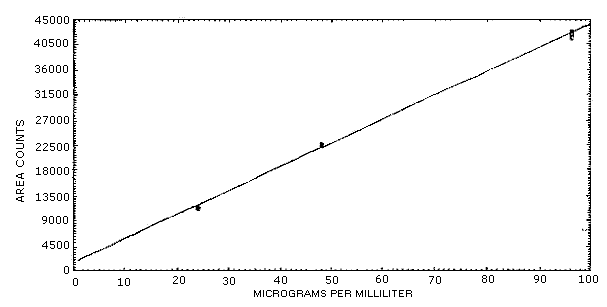
The instrument response to aldicarb over the range of 0.5 to 2 times the target concentration is linear with a slope of 424 area counts per microgram per milliliter. The response to aldicarb was determined by multiple injections of aldicarb standards. The data listed below is presented graphically in Figure 4.4.
|
| |||
| × target conc. µg/sample |
0.5× 24.1 |
1× 48.4 |
2× 96.8 |
|
| |||
| area counts | 11753 | 22861 | 42825 |
| 11651 | 22868 | 42613 | |
| 11523 | 22707 | 42579 | |
| 11276 | 22603 | 42241 | |
| 11178 | 22484 | 41806 | |
| 11246 | 22640 | 41737 | |
| 11438 | 22694 | 42300 | |
|
| |||
4.5. Storage data
Storage samples were generated by liquid-spiking 36 sampling tubes
with 48.2 µg of aldicarb and then pulling 120 L of humid air (about
80% relative humidity) through them.
|
| ||||||||
| storage time | % recovery | |||||||
| (days) | (ambient) | (refrigerated) | ||||||
|
| ||||||||
| 0 | 99.8 | 100.9 | 101.6 | 99.8 | 100.9 | 101.6 | ||
| 101.7 | 111.7 | 106.9 | 101.7 | 111.7 | 106.9 | |||
| 4 | 103.7 | 99.2 | 110.7 | 101.4 | 108.2 | 100.7 | ||
| 7 | 96.8 | 99.7 | 106.7 | 110.0 | 96.2 | 100.4 | ||
| 11 | 100.0 | 101.4 | 99.4 | 96.2 | 102.7 | 106.7 | ||
| 14 | 109.1 | 104.5 | 104.7 | 103.6 | 105.5 | 99.2 | ||
| 17 | 111.4 | 102.7 | 100.3 | 106.6 | 101.4 | 100.6 | ||
|
| ||||||||
4.6. Precision (analytical method only)
The precision of the analytical procedure is defined as the pooled
coefficient of variation determined from replicate injections of
aldicarb standards at 0.5, 1 and 2 times the PEL. The coefficients of
variation (CV) for the three levels and the pooled coefficient of
variation ![]() )
)
|
| |||
| × target conc. µg/sample |
0.5× 24.1 |
1× 48.2 |
2× 96.4 |
|
| |||
| SD (area counts) | 238 | 151 | 451 |
| CV | 0.0207 | 0.0067 | 0.0107 |
|
| |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with, the following equation:
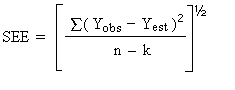
| where | |
| n = k = k = |
total no. of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed % recovery at a given time |
| Yest = | estimated % recovery form the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the, SEE (with pump error included) by 1.96 (the
4.8. Reproducibility data
Six samples, liquid-spiked with aldicarb, were given to a chemist unassociated with this study. The samples were analyzed after being stored for one day at 22°C. The results were corrected for extraction/desorption efficiency and are listed below. No sample result had a percent deviation greater than the precision of the overall procedure, which is ±13.2%.
|
| ||||
| sample | µg spiked | µg recovered | % recovered | % deviation |
|
| ||||
| 1 | 48.5 | 44.3 | 91.3 | -8.7 |
| 2 | 48.5 | 48.9 | 100.8 | +0.8 |
| 3 | 48.5 | 50.0 | 103.1 | +3.1 |
| 4 | 48.5 | 49.6 | 102.3 | +2.3 |
| 5 | 48.5 | 44.0 | 90.7 | -9.3 |
| 6 | 48.5 | 48.8 | 100.6 | +0.6 |
|
| ||||
4.9. Retention efficiency
To test the ability of the sampling device to retain the analyte,
12 sampling devices were
|
| ||||
| air volume (L) |
"A" section (µg) |
"B" section (µg) |
total (µg) |
breakthrough (%) |
|
| ||||
| 264 | 102.2 | 0 | 102.2 | 0 |
| 381 | 99.3 | 0.6 | 99.9 | 0.6 |
| 424 | 89.8 | 1.6 | 91.4 | 1.7 |
| 480 | 97.7 | 2.9 | 100.6 | 2.9 |
| 539 | 98.9 | 1.0 | 99.9 | 1.0 |
| 572 | 97.2 | 3.1 | 100.3 | 3.1 |
| 656 | 93.0 | 1.6 | 94.6 | 1.7 |
| 689 | 93.0 | 2.8 | 95.8 | 2.9 |
| 749 | 93.0 | 1.7 | 94.7 | 1.8 |
| 805 | 99.4 | 1.7 | 101.1 | 1.7 |
| 907 | 102.4 | 3.5 | 105.9 | 3.3 |
| 965 | 105.0 | 4.5 | 109.5 | 4.1 |
|
| ||||
4.10. Extraction and desorption efficiencies
- 4.10.1. Extraction from glass fiber filter
The extraction efficiency of aldicarb was determined by
|
| ||
| sample number |
µg recovered |
percent recovered |
|
| ||
| 1 | 47.3 | 97.7 |
| 2 | 47.9 | 99.0 |
| 3 | 46.1 | 95.2 |
| 4 | 47.9 | 99.0 |
| 5 | 46.7 | 96.5 |
| 6 | 47.1 | 97.3 |
| |
97.6 | |
|
| ||
4.10.2. Desorption from
The desorption efficiency (DE) of aldicarb was determined by
|
| |||
| × target conc. µg/sample |
0.5× 24.2 |
1× 48.4 |
2× 96.8 |
|
| |||
| DE, % | 87.5 | 106.2 | 107.4 |
| 96.5 | 108.3 | 104.6 | |
| 102.3 | 110.7 | 103.1 | |
| 103.3 | 109.8 | 101.7 | |
| 104.7 | 100.4 | 100.4 | |
| 92.8 | 104.8 | 95.0 | |
| 97.8 | 106.7 | 102.0 | |
|
| |||
4.10.3. Combined extraction/desorption efficiency
The combined extraction/desorption efficiency of aldicarb was
determined by
|
| ||
| original | 24 h later | |
|
| ||
| % | 107.0 | 110.7 |
| 109.5 | 109.5 | |
| 107.9 | 108.1 | |
| 107.8 | 108.3 | |
| 103.9 | 102.3 | |
| 104.4 | 103.0 | |
| 106.8 | 107.0 | |
| % of original | 100.2 | |
|
| ||
4.11. Preparing the OVS tube
It is anticipated that this glass sampling tube can be used to
collect a broad range of airborne contaminants when packed with
various adsorbents. Therefore, the suffix will reflect the type of
adsorbent contained in the sampler. For example, a sampler containing
Tenax will be designated
- 4.11.1. Apparatus
- 4.11.1.1. Soxhlet extractor
4.11.1.2. Rotary evaporator
4.11.1.3. Miscellaneous glassware: vacuum flask, 2-L
round-bottom flask, Erlenmeyer flask,
4.11.1.4. Urethane foam plugs, 3/8-in. × 1/2-in. diameter and
4.11.1.5. Glass fiber filters, 1/2-in. or 13-mm diameter.
4.11.1.6. PTFE retainer. The retainer is made by removing a 50°
arc from a piece of PTFE tubing,
4.11.1.7. Glass sampling tube. The sampling tube is constructed
from two pieces of borosilicate glass tubing that have been joined
together by a glass blower. One of the pieces is 50 mm ×
4.11.1.8. Plastic cap, 7/8 in. × 1/2-in. i.d. (Alliance Plastics, Inc., Erie, PA).
4.11.1.9. Plastic cap, 3/4 in. × 7/32-in. i.d. (SKC, Inc.,
4.11.2. Reagents
- 4.11.2.1. Toluene, HPLC grade.
4.11.2.2. Methanol, HPLC grade.
4.11.2.3. Acetonitrile, HPLC grade.
4.11.2.4. Amberlite
4.11.3. Cleaning the
Add 500 g of crude
4.11.4. Assembly of the
Place a large foam plug in the bottom of the large end of the
glass tube. Add 140 mg of cleaned
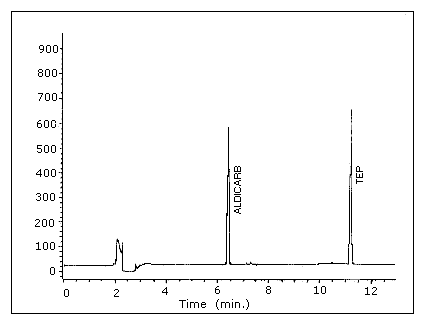
Figure 3.5.1. Chromatogram
of aldicarb at the target concentration.
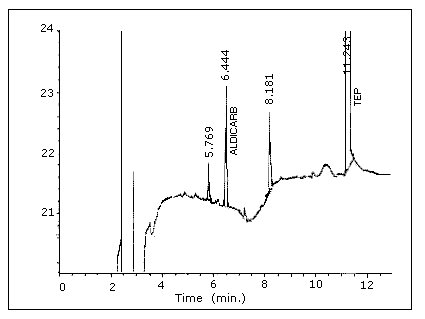
Figure 4.1.1. Chromatogram
of aldicarb at the detection limit (60 pg per injection).
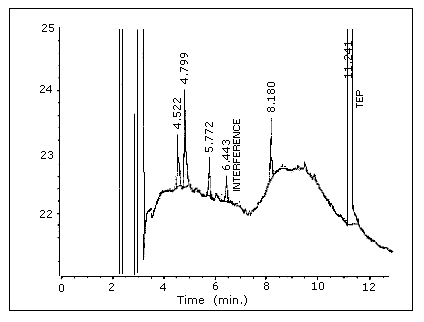
Figure 4.1.2. Chromatogram
of a blank "A" section.
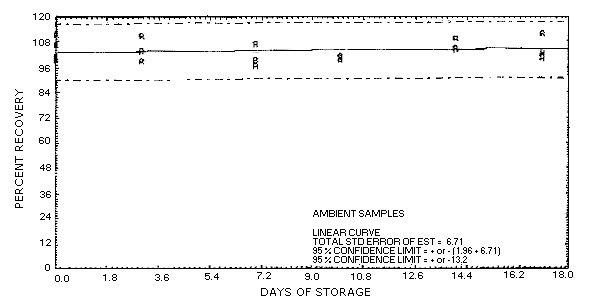
Figure 4.5.1. Ambient
storage test for aldicarb, liquid-spiked.
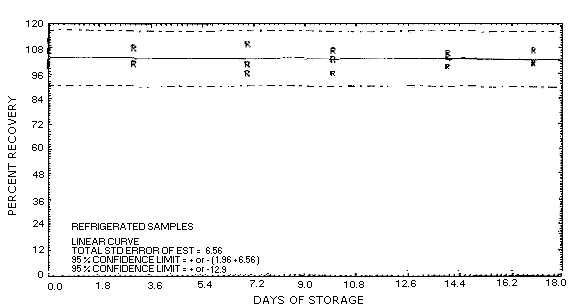
Figure 4.5.2. Refrigerated
storage test for aldicarb, liquid-spiked.
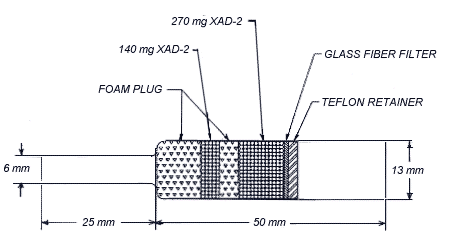
Figure 4.11.4. A drawing of an
5. References
- 5.1. Burright, D. Method #62, "Chlorpyrifos, DDVP, Diazinon,
Malathion, and Parathion", U.S. Department of Labor, OSHA Analytical
Laboratory, Salt Lake City, unpublished, 1986.
5.2. Burright, D. Method #63, "Carbaryl (Sevin)", U.S. Department of Labor, OSHA Analytical Laboratory, Salt Lake City, unpublished, 1987.
5.3. Burright, D. Method #67, "Chlordane", U.S. Department of Labor, OSHA Analytical Laboratory, Salt Lake City, unpublished, 1987.
5.4. Burright, D. Method #70, "Pyrethrum", U.S. Department of Labor, OSHA Analytical Laboratory, Salt Lake City, unpublished, 1988.
5.5. "Chemical Information Manual", U.S. Department of Labor, Occupational Safety and Health Administration, Directorate of Technical Support, Washington, D.C.; October 20, 1987.
5.6. "Initial Scientific and Minieconomic Review of Aldicarb", U.S.
Environmental Protection Agency, Office of Pesticide Programs,
Criteria and Evaluation Division; Washington, D.C.; 1975;
5.7. NIOSH Registry of Toxic Effects, RTECS Online Database available through National Library of Medicine, Bethesda, MD.