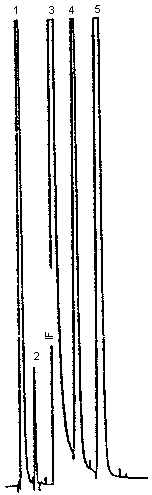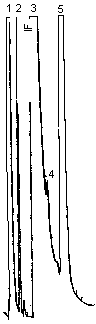| Method no.: | 72 |
| Matrix: | Air |
| Target concentration: | 5 ppm (20 mg/m3) (OSHA PEL) (skin) |
| Procedure: | Air samples are collected by drawing known volumes of air through sampling tubes containing petroleum-based charcoal. Each sampling tube contains a 100-mg sampling section and a 50-mg backup section. The samples are desorbed with carbon disulfide containing 1% dimethylformamide. Magnesium sulfate is added to remove any collected water. The desorbed samples are analyzed by gas chromatography using a flame ionization detector. |
| Recommended air volume and sampling rate: |
180 L and 1 L/min |
| Reliable quantitation limit: | 42 ppb (166 µg/m3) |
| Standard error of estimate at the target concentration: (Section 4.7.) |
5.4% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: June 1988 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
NIOSH has published two methods to monitor occupational exposure to furfural. One of the methods, NIOSH Method S-17, requires sample collection and derivatization using a bubbler containing Girard T reagent (Ref. 5.1.). The other method, NIOSH Method 2529, specifies that air samples be collected and derivatized using a sampling tube containing XAD-2 adsorbent which has been coated with 2-(hydroxymethyl) piperidine (Ref. 5.2.). Direct collection of furfural on charcoal has been reported in the literature (Ref. 5.3.). It was decided to evaluate direct collection because of its relative simplicity.
The direct sampling and analytical method for furfural collected on charcoal (Ref. 5.3.) was reported to have a low and non-linear desorption efficiency. The desorption efficiency was found to vary from about 61 to 72% when 0.1 to 2.4 mg of furfural was desorbed from 150 mg of charcoal with 1 mL of methylene chloride. Several other solvents, including carbon disulfide, methanol, acetone, and benzene, were evaluated but methylene chloride provided the best desorption efficiency results.
Work performed at the OSHA Analytical Laboratory has shown that the desorption efficiency of 3.6 mg of furfural from 100 mg of charcoal was 80% when 1 mL of carbon disulfide containing 1% dimethylformamide (DMF) was used as the desorbing solvent. The desorption efficiency increased to 92% when the volume of desorbing solvent was increased to 5 mL. The desorption efficiency remained constant when samples containing 1.8, 3.6 and 7.2 mg of furfural were desorbed with 5 mL of carbon disulfide containing 1% DMF.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Furfural vapor is an irritant of the skin, eyes and respiratory tract. It may also cause unconsciousness. Long-term exposure to furfural may cause sensitization of the skin, loss of the sense of taste, numbness of the tongue, and breathing problems. Furfural has an odor similar to almonds. The odor threshold for furfural is reported to be between 0.25 and 5 ppm. (Ref. 5.4.)
The OSHA PEL for furfural is 5 ppm. The American Conference of Governmental Industrial Hygienists lowered the TLV-TWA for furfural from 5 to 2 ppm because significant irritation of the eyes and respiratory passages was found to occur at 5 ppm (Ref. 5.5.).
Furfural is currently undergoing a carcinogenesis bioassay by the National Toxicology Program (NTP). Preliminary results are expected in 1988.
1.1.3. Workplace exposure
In 1978, the non-Communist world production of furfural was 159,000 metric tons. Furfural is produced from renewable agricultural sources such as corncobs, oat hulls, rice hulls and wood wastes. Furfural is used as a selective solvent in petroleum refining, as an extraction agent in the distillation of butadiene, as a solvent for the production of light-colored wood rosins and as a solvent and processing aid in the production of anthracene. It is used as a solvent and wetting agent in the manufacture of abrasive wheels and brake linings. Furfural is used in several furfuryl alcohol resins and in phenol-aldehyde resins. Furfural is used to manufacture furfuryl alcohol, tetrahydrofurfuryl alcohol, furan, tetrahydrofuran, poly-(oxytetramethylene) glycol and a variety of synthetic resins. (Ref. 5.6.) Furfural has also been used as a weed killer, fungicide, and flavoring agent (Ref. 5.5.).
1.1.4. Physical properties (Refs. 5.4. and 5.5.)
| CAS no.: | 98-01-1 |
| molecular weight: | 96.08 |
| appearance: | colorless, oily liquid that turns reddish-brown when exposed to air and light |
| melting point: | -36.5°C |
| boiling point at 1 atm: | 161.7°C |
| vapor pressure at 20°C: | 266.6 Pa (2 mm Hg) |
| density at 20°C: | 1.16 g/mL |
| solubility: | 8% soluble in water; miscible with alcohol, ether and benzene |
| flash point (closed cup): | 60°C |
| structural formula: |  |
| autoignition temperature: | 316°C |
| flammable limits: | lower 2.1% |
| (% by volume in air): | upper 19.3% |
| synonyms: |
The furfural air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm and ppb are referenced to 25°C and 760 mm Hg.
- 1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 4.5 ng per injection. This is the amount of analyte which will give a peak whose height is about 5 times the height of a nearby contaminant peak. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 29.9 µg per sample (166 µg/m3 or 42 ppb). This is the amount of furfural spiked on the sampling device which allows recovery of an amount of analyte approximately equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 29.9 µg per sample (166 µg/m3 or 42 ppb). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of the analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of furfural from samples used in an 18-day storage test remained above 94.5% when the samples were stored at about 23°C. (Section 4.5.) The recovery of the analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.016. (Section 4.6.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 18-day ambient temperature storage test is ±10.7%. (Section 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples, spiked by liquid injection, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 10 days of storage at 5°C. No individual sample deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
1.3. Advantage
This method provides a simple, convenient, and precise means to monitor occupational exposure to furfural.
1.4. Disadvantage
Furfuryl alcohol is a sampling interference.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device attached.
2.1.2. Samples are collected with 7-cm × 4-mm i.d. × 6-mm o.d. glass tubes which are packed with a 50-mg backup section and a 100-mg sampling section of petroleum-based charcoal. The two sections of charcoal are separated and retained with small plugs of foam and glass-wool. The ends of the sampling tube are flame-sealed. Petroleum-based charcoal sampling tubes, Lot 104, were obtained from SKC, Inc. for use in this evaluation.
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Break open both ends of the sampling tube so that the
holes in the tube ends are at least one-half the i.d. of the tube.
Attach the sampling tube to the sampling pump with flexible, plastic
tubing such that the large, front section of the sampling tube is
exposed directly to the atmosphere. Do not place any tubing in front
of the sampler. Attach the sampler vertically in the worker's
breathing zone in such a manner that it does not impede work
performance or safety. Be certain that the sharp end of the sampling
tube does not injure the worker.
2.3.2. Remove the sampler after sampling for the appropriate time and then seal the tube with plastic end caps. Wrap the tube lengthwise with an official OSHA seal (Form 21).
2.3.3. Submit at least one blank sample with each set of samples. Handle the blank the same as the other samples with the exception of drawing air through it.
2.3.4. List any potential interferences on the sample data sheet.
2.4. Sampler capacity
Five-percent breakthrough occurred after sampling a controlled test atmosphere containing 46.2 mg/m3 furfural (2.3 times the OSHA PEL) for 266 min at 1.0 L/min. At the end of this time 266 L of air had been sampled and 12.3 mg of furfural had been collected. Breakthrough data were obtained by sampling the test atmosphere for increasing periods of time with several of the recommended two-section charcoal tubes. Five-percent breakthrough was defined as the point at which 5% of the total amount of furfural collected on the entire tube was found on the backup section. (Section 4.9.)
2.5. Desorption efficiency
- 2.5.1 The average desorption efficiency for furfural from 100 mg
of petroleum-based charcoal (SKI, Inc. Lot 104) over the range of
0.5 to 2 times the target concentration was 92.3%. (Section 4.10.)
2.5.2. Desorbed samples remain stable for at least 24 h. (Section 4.10.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 180 L and the recommended
sampling rate is 1.0 L/min.
2.6.2. When short-term air samples are required, the recommended sampling rate is 1.0 L/min. A 15-min sample at the reliable quantitation limit is equivalent to 0.5 ppm furfural.
2.7. Interferences (sampling)
- 2.7.1. Furfuryl alcohol is a sampling interference. Several SKI,
Inc. Lot 104 petroleum-based charcoal tubes were liquid spiked with
11.3 mg of furfuryl alcohol. An air volume of 180 L at 68% relative
humidity and 26°C was drawn through each of the spiked samples which
were then stored either at ambient temperature or at -20°C for 14
days. Upon analysis, it was found that 410 µg of furfural had formed
on the charcoal tubes stored at ambient temperature. Sample results
showed that 135 µg of furfural was formed on charcoal tubes stored
at -20°C. The formation of furfural was probably due to the
oxidation of furfuryl alcohol. The oxidation of furfuryl alcohol to
furfural was retarded by storing samples at -20°C. Furfural samples
collected in locations where furfuryl alcohol is in use should be
refrigerated to help prevent the oxidation of furfuryl alcohol to
furfural.
2.7.2. The presence of other substances in the sampled air can reduce the capacity of the sampler for furfural.
2.7.3. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. The sampling equipment should be attached to the worker
in such a manner that it will not interfere with work performance or
safety.
2.8.2. All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph (GC) equipped with a flame ionization
detector (FID). A Hewlett-Packard 5840A GC fitted with an FID was
used in this evaluation. Injections were performed using a
Hewlett-Packard 7671A automatic sampler.
3.1.2. A GC column capable of resolving furfural from potential interferences. A 6-ft × 1/4-in. o.d. (2-mm i.d.) glass GC column containing 20% SP-2100 with 0.1% Carbowax 1500 on 100/120 mesh Supelcoport was used in this evaluation. The GC packing material was obtained from Supelco, Inc. Injections were performed on-column. The separation can also be performed on a 60-m × 0.32-mm i.d. fused silica capillary column, containing DB-5 (1-µm film thickness) which is obtained from J & W Scientific.
3.1.3. Vials,
3.1.4. Volumetric flasks, pipets and syringes for preparing standards, making dilutions and performing injections.
3.2. Reagents
- 3.2.1. Carbon disulfide, imethylformamide (DMF) and ethyl
benzene, reagent grade or better. Carbon disulfide was obtained from
EM Science, DMF from American Burdick and Jackson and ethyl benzene
from Eastman Kodak.
3.2.2. Nitrogen, hydrogen, and air, GC grade.
3.2.3. Furfural, of known high purity. Aldrich Chemical Co. furfural (99%) was used, as received, for this evaluation. Aldrich states that the dark coloration which occurs upon storage does not affect the furfural purity and that the reagent should be redistilled if a lighter color is desired.
3.2.4. Desorbing solution. Carbon disulfide containing 1% DMF (v/v). Ethyl benzene (1 µL/mL of desorbing solution) was used as an internal standard in this evaluation. The use of the internal standard is optional.
3.2.5. Magnesium sulfate, anhydrous powder. J.T. Baker "Baker Analyzed" Reagent grade magnesium sulfate was used in this evaluation.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by diluting a known amount of 99%
furfural with desorbing solution. Store the stock standards in a
freezer. The furfural and desorbing solution may separate during
freezer storage. Shake the mixture after it has been warmed to room
temperature. Prepare fresh stock standards every week.
3.3.2. Prepare analytical standards by diluting stock standards with desorbing solution.
3.3.3. Prepare a sufficient number of standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4. Sample preparation
- 3.4.1. Transfer the 100-mg front section of the sampling tube
and the front glass-wool plug to a
3.4.2. Add approximately 100 mg of anhydrous magnesium sulfate to each sample vial. This reagent is added to remove any collected water which can cause low results because of furfural partitioning in the water phase.
3.4.3. Add 5.0 mL of desorbing solution to each vial.
3.4.4. Seal the vials with Teflon-lined caps and allow them to desorbed for 1 h. Mix the contents of the vials vigorously by hand several times during the desorption time.
3.4.5. Transfer some of the desorbed sample to autosampler vials if an autosampler is used.
3.4.6. Prepare additional standards to determine the detector response when analyzed samples are not in the concentration range of the standards. Samples containing high amounts of furfural can be diluted with desorbing reagent.
3.5. Analysis
- 3.5.1.
| GC Conditions (packed column) | |||
| column temperature: | 90°C | ||
| injector temperature: | 180°C | ||
| nitrogen flow rate: | 30 mL/min (carrier gas) | ||
| injection volume: | 0.90 µL | ||
| GC column: | 6-ft × 1/4-in. o.d. (2-mm i.d.) glass CC column containing 20% SP-2100 with 0.1% Carbowax 1500 on 100/120 mesh Supelcoport | ||
| FID conditions | |||
| hydrogen flow rate: | 30 mL/min | ||
| air flow rate: | 250 mL/min | ||
| detector temperature: | 275°C | ||
| retention time: | 5.1 min | ||
| chromatogram: | Figure 3.5.1. | ||
| |||
3.5.2.
| GC Conditions (capillary column) | |
| column temperature: | 50°C for 5 min, then temperature program to 150°C at 5°C/min |
| injector temperature: | 170°C |
| nitrogen flow rate: | 6 mL/min (carrier gas) |
| injection volume: | 1.0 µL (1 to 8 split ratio) |
| GC column: | 60-m × 0.32-mm i.d., fused silica capillary column, DB-5, 1-µm film thickness |
| FID conditions | |
| nitrogen flow rate: | 30 mL/min (detector make-up gas) |
| hydrogen flow rate: | 40 mL/min |
| air flow rate: | 300 mL/min |
| detector temperature: | 200°C |
| retention time: | 16.0 min |
| chromatogram: | Figure 3.5.2. |
3.5.3. Use a suitable method, such as electronic integration, to measure detector response. Prepare an internal standard procedure on the integrator if the internal standard option is employed.
3.5.4. Analyze several standard solutions of different concentration to generate the calibration curve. Prepare the calibration curve daily.
3.5.5. Bracket sample concentrations with standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound having a similar retention time as furfural
or the internal standard is a potential interference. Interferences
which were identified by the person submitting the samples must be
carefully considered before the samples are analyzed.
3.6.2. GC parameters (temperature, column, etc.) may be changed to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity.
3.6.4. GC/MS is a useful means of structure determination. This means should be used to confirm samples whenever possible.
3.7. Calculations
- 3.7.1. Prepare a calibration curve from analytical standards by
plotting the detector response against concentration (µg/sample) for
each standard. Determine the best line through the data points by
curve fitting.
3.7.2. Determine the actual concentration, in µg/sample, for a particular sample by comparing its detector response to the calibration curve. If furfural is found on the backup section, add it to the amount found on the front section. Perform blank corrections before adding the results for the front and backup sections together.
3.7.3. Express furfural air concentration using the following equation:
| where | A B C |
= = = |
µg/sample from Section 3.7.2. liters of air sampled desorption efficiency (decimal form) |
3.7.4. Convert furfural results in mg/m3 to ppm using the following equation:
| where | mg/m3 24.46 96.08 |
= = = |
result from Section 3.7.3. molar volume at 760 mm Hg and 25°C molecular weight of furfural |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood.
3.8.3. Wear safety glasses and a lab coat in laboratory areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The injection size recommended in the analytical procedure (0.90 µL) was used in the determination of the detection limit of the analytical procedure. The detection limit of the analytical procedure is 4.5 ng per injection. This is the amount of analyte which will give a peak whose height is about 5 times the height of a nearby contaminant peak. This detection limit was determined by the analysis of a standard containing 5.0 µg/mL furfural. Figure 4.1. is a chromatogram of the detection limit of the analytical procedure.
4.2. Detection limit of the overall procedure
The detection limit of the overail procedure is 29.9 µg per sample (166 µg/m3 or 42 ppb). This is the amount of furfural spiked on the sampler which allows recovery of an amount of analyte approximately equivalent to the detection limit of the analytical procedure. This detection limit was determined by analyzing six 100-mg portions of SKC, Inc. Lot 104 petroleum-based charcoal which had been spiked with 29.9 µg of furfural. The samples were stored at ambient temperature overnight before analysis. The injection size recommended in the analytical procedure (0.90 µL) was used in the determination of the detection limit of the overall procedure. The results of this study are presented In Table 4.2.
Detection Limit of the Overall
procedure
|
| |||||
| sample | theoretical | amount | sample | theoretical | amount |
| amount (µg) | found (µg) | amount (µg) | found (µg) | ||
|
| |||||
| 1 | 29.9 | 23.9 | 4 | 29.9 | 23.1 |
| 2 | 29.9 | 23.9 | 5 | 29.9 | 23.6 |
| 3 | 29.9 | 23.6 | 6 | 29.9 | 21.6 |
|
| |||||
4.3. Reliable quantitation limit
The reliable quantitation limit is 29.9 µg per sample (166 µg/m3 or 42 ppb). This is the smallest amount of furfural which can be spiked on the front section of a sampling tube and result in a recovery of at least 75% and a precision of ±25% or better. The injection size recommended in the analytical procedure (0.90 µL) was used in the determination of the reliable quantitation limit. The data presented in Table 4.3. were calculated from the data in Table 4.2.
Reliable Quantitation Limit
|
| ||||
| sample | percent | |||
| number | recovered | statistics | ||
|
| ||||
| 1 2 3 4 5 6 |
79.9 79.9 78.9 77.2 78.9 72.2 |
SD precision |
= = = = |
77.8% 2.9% ±(1.96)(2.9%) ±5.7% |
|
| ||||
4.4. Instrument response to the analyte
The instrument response to furfural was evaluated by performing multiple injections of analytical standards. The instrument response to furfural over the range of 0.5 to 2 times the target concentration was linear with a slope of 359 area counts per µg/mL.
Instrument Response to Furfural
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 1795.3 | 3590.6 | 7181.3 |
| ppm | 2.5 | 5.1 | 10.2 |
|
| |||
| area | 121300 | 252200 | 509900 |
| counts | 122100 | 257500 | 530000 |
| 122000 | 258400 | 519000 | |
| 126000 | 259300 | 509700 | |
| 126000 | 254500 | 529300 | |
| 124800 | 250800 | 512300 | |
| 123700 | 255450 | 518367 | |
|
| |||
4.5. Storage data
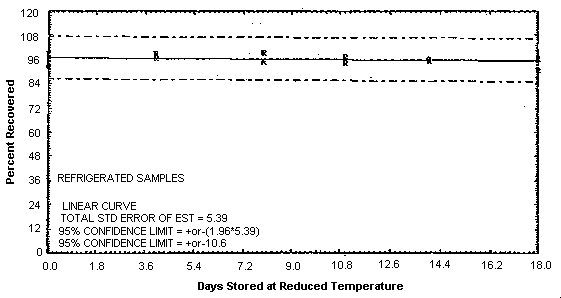
Storage samples were generated by collecting samples at 1.0 L/min for 1 h from a controlled test atmosphere containing 14.3 ppm furfural. The results of the storage test are presented in Table 4.5. and are shown graphically in Figures 4.5.1. and 4.5.2.
|
| ||||||
| storage time | % recovery | |||||
| (days) | (refrigerated) | (ambient) | ||||
|
| ||||||
| 0 | 93.1 | 99.2 | 96.2 | 93.1 | 99.2 | 96.2 |
| 0 | 96.1 | 95.1 | 97.3 | 96.1 | 95.1 | 97.3 |
| 4 | 98.4 | 99.1 | 96.8 | 97.6 | 93.3 | 96.8 |
| 8 | 99.5 | 95.5 | 95.0 | 99.5 | 96.8 | 97.0 |
| 11 | 96.7 | 94.5 | 97.7 | 97.4 | 94.5 | 94.3 |
| 14 | 96.3 | 96.6 | 95.2 | 96.8 | 93.7 | 92.2 |
| 18 | 90.8 | 97.0 | 96.6 | 97.6 | 92.1 | 92.8 |
|
| ||||||
4.6. Precision (analytical method only)
The precision of the analytical method was evaluated from the data in Table 4.4.
Precision of the Analytical Method
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 1795.3 | 3590.6 | 7181.3 |
| ppm | 2.5 | 5.1 | 10.2 |
|
| |||
| SD (area counts) | 2145 | 3488 | 9369 |
| CV | 0.0173 | 0.0137 | 0.0181 |
|
| |||
4.7. Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
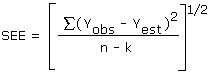
| where | ||
| n | = | total no. of data points |
| k | = | 2 for a linear regression |
| k | = | 3 for a quadratic regression |
| Yobs | = | observed % recovery at a given time |
| Yest | = | estimated % recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility
Reproducibility samples prepared by liquid spiking SKC Lot 104 petroleum-based charcoal with furfural. The samples and a draft copy of the method were given to a chemist who was not associated with this evaluation. The samples were analyzed after 10 days of storage at 5°C. No sample deviated from its theoretical value by more than the precision of the overall procedure which was ±10.7.
Reproducibility
|
| ||||
| sample | theoretical | determined | determined | deviation |
| number | amount (µg) | amount (µg) | amount (%) | (%) |
|
| ||||
| 1 | 3660.6 | 3700.5 | 101.1 | +1.1 |
| 2 | 3660.6 | 3828.2 | 104.6 | +4.6 |
| 3 | 3660.6 | 3683.7 | 100.6 | +0.6 |
| 4 | 3660.6 | 3818.5 | 104.3 | +4.3 |
| 5 | 3660.6 | 3744.3 | 102.3 | +2.3 |
| 6 | 3660.6 | 3772.1 | 103.0 | +3.0 |
|
| ||||
4.9. Sampler capacity
The breakthrough study was performed using several of the recommended two-section collection devices to sample a controlled test atmosphere containing furfural in air for increasing periods of time. The furfural concentration was 46.2 mg/m3 (2.3 times the OSHA PEL) and the relative humidity was 80% at 29°C. The sampling rate was 1.0 L/min. Breakthrough was defined as the amount of furfural found on the backup section divided by the total amount of furfural collected on the entire sampling tube. Five-percent breakthrough occurred after sampling for 266 min. At the end of this time, 266 L of air had been sampled and 12.3 mg of furfural had been collected. The results of the study are presented in Table 4.9. and Figure 4.9.
Furfural Breakthrough Data
|
| |
| air volume | breakthrough |
| (L) | (%) |
|
| |
| 221 | 1.3 |
| 244 | 1.6 |
| 297 | 6.7 |
| 323 | 11.3 |
| 348 | 16.5 |
| 354 | 14.2 |
|
| |
4.10. Desorption efficiency and stability of desorbed samples
- 4.10.1. Desorption efficiency
The desorption efficiency of furfural from SKC, Inc. Lot 104 petroleum-based charcoal was determined by liquid spiking 100-mg portions of the adsorbent with furfural. The samples were allowed to stand overnight at room temperature before analysis. A significant increase in desorption efficiency was observed when the volume of desorbing solvent was increased from 1 to 5 mL. The results of the desorption efficiency studies are presented in Tables 4.10.1.1. and 4.10.1.2. The target concentration (3591 µg/sample) was the only level studied when samples were desorbed with 1 mL of solvent.
Desorption Efficiency of
Samples
Desorbed with 1 mL of Solvent
|
| |
| sample | desorption |
| number | efficiency (%) |
|
| |
| 1 | 79.8 |
| 2 | 80.2 |
| 3 | 79.7 |
| 4 | 81.1 |
| 5 | 81.7 |
| 6 | 80.4 |
|
| |
Desorption Efficiency of
Samples
Desorbed with 5 mL of Solvent
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 1795 | 3591 | 7181 |
|
| |||
| desorption | 90.3 | 92.4 | 95.5 |
| efficiency, % | 91.3 | 91.6 | 94.0 |
| 90.5 | 92.9 | 94.2 | |
| 90.5 | 92.1 | 93.5 | |
| 91.8 | 92.0 | 92.9 | |
| 90.6 | 92.3 | 92.6 | |
| 90.8 | 92.2 | 93.8 | |
| average desorption efficiency = 92.3 | |||
|
| |||
4.10.2. Stability of desorbed samples
The stability of desorbed samples was investigated by reanalyzing desorbed target concentration samples following 24 h storage at room temperature. Fresh standards were used and the sample vials were resealed immediately after the first analysis. The average recovery, relative to the original analysis, was 101.0%. The results of this study are presented in Table 4.10.2.
Stability of Desorbed Samples
|
| ||
| original | reanalyzed | percent |
| result (%) | result (%) | of original |
|
| ||
| 92.4 | 93.2 | 100.9 |
| 91.6 | 92.5 | 101.0 |
| 92.9 | 93.8 | 101.0 |
| 92.1 | 93.1 | 101.1 |
| 92.0 | 93.1 | 101.2 |
| 92.3 | 93.2 | 101.0 |
|
| ||
Chromatographic notes: note the forced tangent skim integrator function command (IF) on the leading edge of the DMF peak in Figures 3.5.1. and 4.1. This special integrator function is used to prevent overestimating the area of the furfural peak. The technique is not necessary when the capillary column (Figure 3.5.2.) is used because better peak resolution is attained. Peak identification was as follows: 1, carbon disulfide; 2, benzene; 3, DMF; 4, furfural; 5, ethyl benzene. The benzene was a contaminant of the carbon disulfide.
5. References
- 5.1. "NIOSH Manual of Analytical Methods, 2nd.
ed.; U.S. Department of Health and Human Services, Center for
Disease Control, NIOSH: Cincinnati, OH, Aug 1979; Vol. 5, Method
S-17, Publ. No. DHEW (NIOSH) 79-141.
5.2. "NIOSH Manual of Analytical Methods", 3rd. ed.; U.S. Department of Health and Human Services, Center for Disease Control, NIOSH: Cincinnati, OH, Aug 1987; 2nd. supplement to Vol. 2, Method 2529, Publ. No. DHEW (NIOSH) 84-100.
5.3. Greinke, R.A.; Am. Ind. Hyg. Assoc. J., 1974, 35, 809-814.
5.4. "NIOSH/OSHA Occupational Health Guidelines for Chemical Hazards, U.S. Dept. of Health and Human Services, Public Health Services, Center for Disease Control, NIOSH and U.S. Dept. of Labor, OSHA: U.S. Government Printing Office Washington, DC, Jan 1981, Furfural, DHHS (NIOSH) Publ. No. 81-123.
5.5. "Documentation of the Threshold Limit Values and Biological Exposure Indices", 5th ed.; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, 1986; p. 280.
5.6. McKillip, W.J. and Sherman, E. in "Kirk-Othmer Encyclopedia of Chemical Technology"; Grayson, M., Ed.; John Wiley & Sons, New York, 1980, pp. 499-510.