s-DIANISIDINE
4,4'
METHYLENEBIS(2-CHLOROANILINE) (MOCA)
s-TOLIDINE
| Method no.: | 71 | ||
| Matrix: | Air | ||
| Procedure: | Samples are collected closed-face by drawing known
volumes of air through sampling devices consisting of
| ||
| Recommended air volume and sampling rate: |
100 L at 1 L/min | ||
|
| |||
| s-Dianisidine | MOCA | s-Tolidine | |
|
| |||
| Target conc.: ppb (µg/m3) | 1 (10) | 20 (218) | 1 (8.7) |
| Reliable quantitation limits: ppt (ng/m3) |
1.2 (12) | 40 (440) | 1.3 (11) |
| Standard errors of estimate at the target concentration: (Section 4.7.) |
7.8% | 5.8% | 8.0% |
|
| |||
| Special requirements: | Samples for s-dianisidine must be shipped and stored at 0°C or colder to minimize loss of analyte. These samples should be analyzed as soon as possible. | ||
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | ||
| Date: April 1988 Updated: July 1989 |
Chemist: Carl J.
Elskamp | ||
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The previous OSHA-recommended procedures to determine airborne
concentrations of
Methodology exists which has previously been validated for
benzidine,
The derivatives are analyzed by capillary gas chromatography
using an electron capture detector. This sampling and analytical
scheme was used in the validation of the following method for
Note: As a consequence of later evaluation tests done for
toluidine, this method has been updated. The sampling device now
consists of two acid-treated glass fiber filters assembled in a
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
s-DIANISIDINE: s-Dianisidine has been shown to be carcinogenic
to rats and hamsters. There are no conclusive epidemiological
studies to show that
MOCA: MOCA has been shown to be carcinogenic to rats and mice. There are no conclusive epidemiological studies to show that MOCA is carcinogenic to humans. (Ref. 5.5.) Exposure to MOCA results in the same general toxic effects that are characteristic of aromatic amines. These effects include cyanosis (a bluish or purplish discoloration due to deficient oxygenation of the blood) and methemoglobinemia (the presence of methemoglobin in the blood). ACGIH has designated MOCA as a suspect human carcinogen and has assigned it a TLV of 0.02 ppm with a "skin" notation. (Ref. 5.6.)
s-TOLIDINE: Rats that had been
administered
1.1.3. Potential workplace exposure
s-DIANISIDINE: The principal use
for s-dianisidine is as a chemical
intermediate in the production of dyes. It has been reported that 89
dyes are produced with
MOCA: MOCA is primarily used as a curing agent for
isocyanate containing polymers. It is also widely used for curing
s-TOLIDINE: The most important
use for
1.1.4. Physical properties and other descriptive information
| s-DIANISIDINE (Ref. 5.4.) | ||
| CAS no.: | 119-90-4 | |
| molecular weight: | 244.3 | |
| melting point: | 137-138°C | |
| description: | Colorless crystals which turn violet on standing | |
| solubility: | Almost insoluble in water, soluble in ethanol, ether, acetone, benzene and chloroform; probably soluble in most organic solvents and lipids | |
| chemical reactivity: | A weak base; has the general characteristics of primary aromatic amines | |
| synonyms: | 3,3'-Dimethoxybenzidine; bianisidine;
| |
| structural formula: |  | |
| MOCA (Ref. 5.5.) | ||
| CAS no.: | 101-14-4 | |
| molecular weight: | 267.2 | |
| melting point: | 110°C | |
| description: | Colorless crystals | |
| solubility: | Almost insoluble in water; soluble in alcohol and ether and probably in most organic solvents and lipids | |
| synonyms: | ||
| trade names: | Curalin M; Curene 442; DACPM; MOCA; Cyanaset | |
| structural formula: | 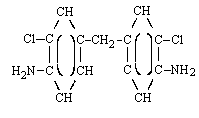 | |
| s-TOLIDINE (Ref. 5.7.) | ||
| CAS no.: | 119-93-7 | |
| molecular weight: | 212.3 | |
| melting point: | 129-131°C | |
| description: | White to reddish crystals or powder | |
| solubility: | Slightly soluble in water, soluble in alcohol, ether or dilute acids | |
| synonyms: | 3,3'-dimethylbenzidine | |
| structural formula: | 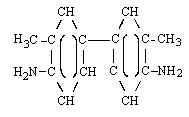 | |
| The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppb and ppt are referenced to 25°C and 760 mm Hg. Although the derivatives of the amines are analyzed, the equivalent masses of the amines are listed throughout the method. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limits of the analytical procedure are 6.0, 5.5,
and 5.4 fg per injection for
1.2.2. Detection limit of the overall procedure
The detection limits of the overall procedure are 1.2, 44, and
1.1 ng per sample for
1.2.3. Reliable quantitation limit
The reliable quantitation limits are 1.2, 44, and 1.1 ng per
sample for
| The reliable quantitation limits and detection limits reported in this method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters. |
1.2.4. Instrument response to the analyte
The instrument response over concentration ranges representing
1.2.5. Recovery
The recoveries of
1.2.6. Precision (analytical method only)
The pooled coefficients of variation obtained from replicate
injections of analytical standards at 0.5, 1, and 2 times the target
concentrations are 0.020, 0.034, and 0.025 for
1.2.7. Precision (overall procedure)
The precisions at the 95% confidence level for the 15-day storage
tests are ±15.4, ±11.3, and ±15.7% for
1.2.8. Reproducibility
Six samples for each analyte, spiked by liquid injection, and a
draft copy of this procedure were given to a chemist unassociated
with this evaluation. The
1.3. Advantages
- 1.3.1. The
1.3.2. The analysis is rapid, sensitive, and precise.
1.4. Disadvantages
- 1.4.1. Sample filters must be transferred to vials containing
water before being submitted to the laboratory for analysis.
1.4.2. Samples for
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated within ±5% of the recommended flow rate with
the sampling filter in line.
2.1.2. Samples are collected closed-face using a sampling device
consisting of two
2.1.3. Small sealable vials capable of holding at least 7 mL of liquid are needed for sample shipment and storage. Glass scintillation vials with caps containing Teflon liners are recommended.
2.2. Reagents
Deionized water is needed for addition to the vials described in Section 2.1.3.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, remove the plastic plugs
from the filter cassettes.
2.3.2. Attach the cassette to the sampling pump with flexible tubing and place the cassette in the employee's breathing zone.
2.3.3. After sampling, seal the cassettes with plastic plugs until the filters are transferred to the vials containing deionized water.
2.3.4. At some convenient time within 10 h of sampling, carefully remove the filters from the cassettes and individually transfer them to separate vials. Add approximately 2 mL of deionized water to each vial. This can be done before or after the filters are transferred.
2.3.5. Seal the vials lengthwise with OSHA Form 21.
2.3.6. Ship and store samples for
2.3.7. Submit at least one blank filter with each sample set. Handle the blank filters in the same manner as the air samples, but draw no air through them.
2.3.8. Record air volumes (in liters) for each sample, along with any potential interferences.
2.4. Retention efficiency
A retention efficiency study was performed by drawing 100 L of air
(76% relative humidity) at 1 L/min through six sample filters that had
been spiked with 1.00 µg of
2.5. Extraction efficiency
- 2.5.1. The average extraction efficiencies from six filters for
each amine spiked at the target concentrations were 97.2, 95.7, and
99.2% for
2.5.2. The stability of extracted and derivatized samples was
verified by reanalyzing the above samples 24 h later using fresh
standards. The average extraction efficiencies for the reanalyzed
samples were 98.9, 93.5, and 100.0% for
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 100 L.
2.6.2. The recommended sampling rate is 1 L/min.
2.6.3. If a smaller air volume is desired, the reliable
quantitation limits will be larger. For example, the reliable
quantitation limit for
2.7. Interferences (sampling)
- 2.7.1. Any compound in the sampled air that will react with the
sulfuric acid on the treated filters or with the collected analyte
is a potential sampling interference.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the employees so that it
will not interfere with work performance or safety.
2.8.2. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with an electron capture detector. For this
evaluation, a
3.1.2. A GC column capable of separating the amine derivatives
from the solvent and interferences. A
3.1.3. An electronic integrator or some other suitable means of
measuring peak areas or heights. A
3.1.4. Small resealable vials with Teflon-lined caps capable of holding 4 mL.
3.1.5. A dispenser or pipet for toluene capable of delivering 2.0 mL.
3.1.6. Pipets (or repetitive pipets with plastic or Teflon tips) capable of delivering 1 mL, for dispensing the sodium hydroxide and buffer solutions.
3.1.7. Repetitive pipets, one to deliver 25 µL of HFAA and one to
transfer
3.1.8. Disposable pipets to transfer the toluene layers after the samples are extracted.
3.2. Reagents
- 3.2.1. Saturated and 0.5 N NaOH solutions, prepared from reagent
grade Na0H.
3.2.2. Toluene. American Burdick and Jackson "High Purity Solvent" brand toluene was used.
3.2.3. Heptafluorobutyric acid anhydride (HFAA). HFAA from Pierce Chemical Company was used.
3.2.4. Phosphate buffer, prepared from 136 g of reagent grade potassium dihydrogen phosphate and 1 L deionized water. The pH is adjusted to 7.0 with saturated sodium hydroxide solution.
3.2.5. s-Dianisidine, MOCA,
3.3. Standard preparation
- 3.3.1. CAUTION. THESE AROMATIC AMINES ARE OR SHOULD BE
CONSIDERED CARCINOGENIC TO HUMANS. Restrict use of pure compounds
and concentrated standards to regulated areas. Prepare concentrated
stock standards by diluting the pure amines with toluene. Prepare
analytical standards by injecting microliter amounts of diluted
stock standards into vials that contain 2.0 mL of toluene. In order
to keep the response of MOCA standards which are at or around the
target concentration (20 ppb for a
3.3.2. Add 25 µL of HFAA to each vial. Recap and shake the vials for 10 s.
3.3.3. After allowing 10 min for the derivatives to form, add 1 mL of buffer to each vial to destroy the excess HFAA and to extract the heptafluorobutyric acid that is formed.
3.3.4. Recap and shake the vials for 10 s.
3.3.5. After allowing the two layers to separate, analyze the toluene (upper) layer of each standard by GC.
3.3.6. Bracket sample concentrations with analytical standard concentrations. If sample concentrations are higher than the upper range of prepared standards, prepare additional standards to ascertain detector response or derivatize a smaller aliquot of the toluene extract of the high samples using toluene as the diluent.
3.4. Sample preparation
- 3.4.1. The sample filters are received in vials containing
deionized water.
3.4.2. Add 1 mL of 0.5 N Na0H and 2.0 mL of toluene to each vial.
3.4.3. Recap and shake the vials for 10 min.
3.4.4. If the samples are to be analyzed for
3.4.5. Add 25 µL of HFAA to each vial. Recap and shake the vials for 10 s.
3.4.6. After allowing 10 min for the derivatives to form, add 1 mL of buffer to each vial to destroy the excess HFAA and to extract the heptafluorobutyric acid that is formed.
3.4.7. Recap and shake the vials for 10 s.
3.4.8. After allowing the two layers to separate, analyze the toluene (upper) layer of each sample by GC.
3.5. Analysis
- 3.5.1. GC conditions and information
| zone temperatures: | column, 250°C injector, 225°C detector, 300°C | |
| gas flows: | column | 2.3 mL/min hydrogen (35 kPa head pressure) |
| make up | 45 mL/min nitrogen | |
| injection volume: | 1.0 µL | |
| split ratio: | 100:1 | |
| column: | SPB-5, 1.0-µm film, 15-m × 0.32-mm i.d. fused silica (Supelco, Inc.) | |
| retention times of derivatives: |
s-Dianisidine, 5.5 min MOCA, 4.2 min s-tolidine, 3.9 min | |
| chromatograms: | Section 4.10. | |
3.5.2. Measure peak areas or heights by use of an integrator or by other suitable means.
3.5.3. Construct a calibration curve by plotting response (peak areas or heights) of standard injections versus micrograms of analyte per sample. Bracket sample concentrations with standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that elutes in the same general time as the
HFAA derivative of the amine of interest is a potential
interference. Suspected interferences reported to the laboratory
with submitted samples by the industrial hygienist must be
considered before samples are derivatized.
3.6.2. GC parameters may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Analyte identity should be confirmed by GC/MS if possible.
3.7. Calculations
The analyte concentration for samples is obtained from the calibration curve in micrograms of analyte per sample. If any analyte is found on the blank, that amount is subtracted from the sample amounts. The air concentrations are calculated using the following formulae.
| µg/m3 = | (micrograms of analyte per
sample) (1000)
(liters of air sampled) (extraction efficiency) |
| where extraction efficiencies are: | 97.2% ( 95.7% (MOCA) 99.2% ( |
| ppb = | (µg/m3) (24.46)
(molecular weight of analyte) |
| where | 24.46 is the molar volume (liters) at 25°C and 760
mm Hg molecular weights are: 244.3 ( 267.2 (MOCA), 212.3 ( | |
3.8. Safety precautions (analytical)
- 3.8.1. CAUTION. THESE AROMATIC AMINES ARE OR SHOULD BE
CONSIDERED CARCINOGENIC TO HUMANS. Restrict use of pure compounds
and concentrated standards to regulated areas.
3.8.2. Avoid skin contact and inhalation of all chemicals. Restrict the use of chemicals to a fume hood if possible. Wear safety glasses and a lab coat while in the lab area.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The injection volume listed in Section 3.5.1., 1.0 µL with a 1 to
100 split, was used in the determination of the detection limits of
the analytical procedure. The detection limits of 6.0 fg of
4.2. Detection limit of the overall procedure
The detection limits of the overall procedure were determined by
analyzing filters spiked with loadings equivalent to the detection
limits of the analytical procedure. Samples were prepared by injecting
1.20 ng of
|
| ||
| sample no. | ng spiked | ng recovered |
|
| ||
| 1 2 3 4 5 6 |
1.20 1.20 1.20 1.20 1.20 1.20 |
1.07 1.27 1.13 1.01 1.25 1.31 |
|
| ||
|
| ||
| sample no. | ng spiked | ng recovered |
|
| ||
| 1 2 3 4 5 6 |
43.8 43.8 43.8 43.8 43.8 43.8 |
37.9 46.3 39.9 47.8 42.7 41.1 |
|
| ||
|
| ||
| sample no. | ng spiked | ng recovered |
|
| ||
| 1 2 3 4 5 6 |
1.09 1.09 1.09 1.09 1.09 1.09 |
1.02 1.15 1.01 0.97 1.07 1.06 |
|
| ||
4.3. Reliable quantitation limit
The reliable quantitation limits were determined by analyzing
filters spiked with loadings equivalent to the detection limits of the
analytical procedure. Samples were prepared by injecting 1.20 ng of
|
| |||
| sample | % recovered | statistics | |
|
| |||
| 1 2 3 4 5 6 |
89.2 105.8 94.2 84.2 104.2 109.2 |
SD = Precision = = |
97.8% 10.1% ±(1.96)(10.1%) ±19.8 |
|
| |||
|
| |||
| sample | % recovered | statistics | |
|
| |||
| 1 2 3 4 5 6 |
86.5 105.7 91.1 109.1 97.5 93.8 |
SD = Precision = = |
97.3% 8.69% ±(1.96)(8.69%) ±17.0 |
|
| |||
|
| |||
| sample | % recovered | statistics | |
|
| |||
| 1 2 3 4 5 6 |
96.6 105.5 92.7 89.0 98.2 97.2 |
SD = Precision = = |
96.0% 5.70% ±(1.96)(5.70%) ±11.2 |
|
| |||
4.4. Instrument response to the analyte
The instrument response to the analytes over the range of 0.5 to 2
times the target concentrations was determined from multiple
injections of analytical standards. These data are given in Tables
|
| |||
| × target conc. µg/sample ppb |
0.5× 0.50 0.50 |
1× 1.00 1.00 |
2× 2.00 2.00 |
|
| |||
| area counts |
106412 103872 100769 106147 105799 103801 104467 |
195200 191643 189055 195327 188406 186838 191078 |
333377 337847 340122 335264 321881 325757 332375 |
|
| |||
|
| |||
| × target conc. µg/sample ppb |
0.5× 10.9 10.0 |
1× 21.8 20.0 |
2× 43.6 39.9 |
|
| |||
| area counts |
74749 75422 79894 75636 77790 75830 76554 |
127250 122572 133929 137936 132986 132634 131218 |
244357 255069 248678 232331 235188 239419 242507 |
|
| |||
|
| |||
| × target conc. µg/sample ppb |
0.5× 0.434 0.50 |
1× 0.868 1.00 |
2× 1.736 2.00 |
|
| |||
| area counts |
115996 112884 110286 116518 115563 110950 113700 |
214903 209958 205280 212341 204580 201801 208144 |
367038 373575 371890 365314 349354 354703 363646 |
|
| |||
4.5. Storage test
Test atmospheres containing these potentially carcinogenic amines
could not be safely generated in our laboratory. Storage samples were
generated by spiking
|
| ||||||||
| days of | % recovery | |||||||
| storage | refrigerated | ambient | ||||||
|
| ||||||||
| 0 0 3 5 8 12 15 |
94.0 102.8 87.5 77.0 86.4 73.2 85.6 |
97.6 91.2 92.4 83.3 88.6 72.8 89.5 |
90.6 89.4 92.5 84.2 85.8 77.0 84.6 |
94.0 102.8 74.5 81.2 62.6 69.4 53.7 |
97.6 91.2 70.2 49.9 68.8 34.4 70.0 |
90.6 89.4 72.0 63.9 54.6 45.9 67.8 | ||
|
| ||||||||
|
| ||||||||
| days of | % recovery | |||||||
| storage | refrigerated | ambient | ||||||
|
| ||||||||
| 0 0 3 5 8 12 15 |
98.2 104.9 98.6 101.4 101.8 102.0 101.3 |
95.0 97.0 103.4 108.4 100.6 99.0 102.3 |
95.4 100.4 104.7 99.7 102.3 100.9 104.6 |
98.2 104.9 102.5 98.4 102.6 99.7 103.2 |
95.0 97.0 100.8 102.0 104.8 97.2 99.2 |
95.4 100.4 101.9 99.4 104.3 98.1 100.6 | ||
|
| ||||||||
|
| ||||||||
| days of | % recovery | |||||||
| storage | refrigerated | ambient | ||||||
|
| ||||||||
| 0 0 3 5 8 12 15 |
98.4 109.2 94.8 98.1 103.2 93.6 100.6 |
102.7 96.8 101.1 102.6 101.2 98.2 101.0 |
99.0 97.2 99.3 101.3 101.5 95.6 96.5 |
98.4 109.2 96.0 96.2 96.6 86.6 92.2 |
102.7
96.8 87.4 92.0 98.2 76.2 97.2 |
99.0 97.2 93.6 91.4 99.0 83.6 96.8 | ||
|
| ||||||||
4.6. Precision (analytical method only)
The precision of the analytical method for each analyte is the
pooled coefficient of variation determined from replicate injections
of standards. The standards analyzed for these determinations are
described in Section 4.4. The precision of the analytical method for
each analyte is given in Tables
|
| |||
| × target conc. µg/sample ppb |
0.5× 0.50 0.50 |
1× 1.00 1.00 |
2× 2.00 2.00 |
|
| |||
| SD (area counts) | 2138 | 3594 | 7117 |
| CV | 0.020 | 0.019 | 0.021 |
|
| |||
|
| |||
| × target conc. µg/sample ppb |
0.5× 10.9 10.0 |
1× 21.8 20.0 |
2× 43.6 39.9 |
|
| |||
| SD (area counts) | 1928 | 5443 | 8555 |
| CV | 0.025 | 0.041 | 0.035 |
|
| |||
|
| |||
| × target conc. µg/sample ppb |
0.5× 0.434 0.50 |
1× 0.868 1.00 |
2× 1.736 2.00 |
|
| |||
| SD (area counts) | 2704 | 5054 | 9644 |
| CV | 0.024 | 0.024 | 0.027 |
|
| |||
4.7.Precision (overall procedure)
The precision of the overall procedure is determined from the storage data. The determination of the standard error of estimate (SEE) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEE is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:
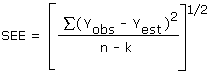
| where | |
| n = k = k = |
total no. of data points 2 for linear regression 3 for quadratic regression |
| Yobs = | observed % recovery at a given time |
| Yest = | estimated % recovery from the regression line at the same given time |
An additional 5% for pump error is added to the SEE by the addition
of variances. The precision at the 95% confidence level is obtained by
multiplying the SEE (with pump error included) by 1.96 (the
4.8. Reproducibility
Six samples for each analyte were prepared by injecting microliter
quantities of standards onto
|
| ||||
| sample no. | µg found | µg expected | % found | % deviation |
|
| ||||
| 1 2 3 4 5 6 |
0.927 0.940 0.963 0.959 0.988 0.960 |
0.960 0.960 0.960 0.960 0.960 0.960 |
96.6 97.9 100.3 99.9 102.9 100.0 |
-3.4 -2.1 +0.3 -0.1 +2.9 0.0 |
|
| ||||
|
| ||||
| sample no. | µg found | µg expected | % found | % deviation |
|
| ||||
| 1 2 3 4 5 6 |
20.80 20.05 20.42 19.82 21.90 21.59 |
22.07 22.07 22.07 22.07 22.07 22.07 |
94.2 90.8 92.5 89.8 99.2 97.8 |
-5.8 -9.2 -7.5 -10.2 -0.8 -2.2 |
|
| ||||
|
| ||||
| sample no. | µg found | µg expected | % found | % deviation |
|
| ||||
| 1 2 3 4 5 6 |
0.859 0.861 0.886 0.868 0.871 0.859 |
0.872 0.872 0.872 0.872 0.872 0.872 |
98.5 98.7 101.6 99.5 99.9 98.5 |
-1.5 -1.3 +1.6 -0.5 -0.1 -1.5 |
|
| ||||
4.9. Extraction efficiency data
Six sample filters for each amine were spiked with the target
concentration amounts by liquid injection (1.00 µg of
|
| ||
| sample no. | % extracted | reanalyzed after 24 h |
|
| ||
| 1 2 3 4 5 6 |
99.2 95.7 94.0 98.2 97.0 99.4 97.2 |
106.2
97.3 96.2 96.8 96.5 100.3 98.9 |
|
| ||
|
| ||
| sample no. | % extracted | reanalyzed after 24 h |
|
| ||
| 1 2 3 4 5 6 |
95.4 92.7 99.5 94.0 92.2 100.5 95.7 |
94.4 94.1 96.6 92.9 90.3 92.8 93.5 |
|
| ||
|
| ||
| sample no. | % extracted | reanalyzed after 24 h |
|
| ||
| 1 2 3 4 5 6 |
101.7 97.1 96.5 100.5 97.7 101.8 99.2 |
107.5
95.9 98.9 98.2 95.8 103.7 100.0 |
|
| ||
4.10. Chromatogram
Chromatograms at the target concentrations are shown in Figures 4.10.1.
and 4.10.2.
The chromatograms are from

Figure 4.1.1.
Detection limit chromatogram for

Figure 4.1.2. Detection
limit chromatogram for MOCA.

Figure 4.4.1. Instrument
response to
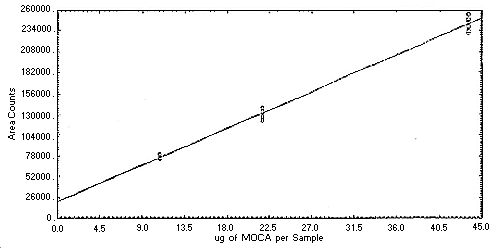
Figure 4.4.2. Instrument
response to MOCA.

Figure 4.4.3. Instrument
response to
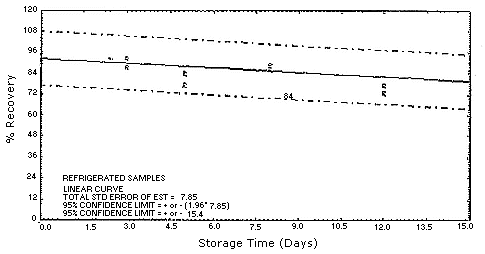
Figure 4.5.1.1.

Figure 4.5.1.2.
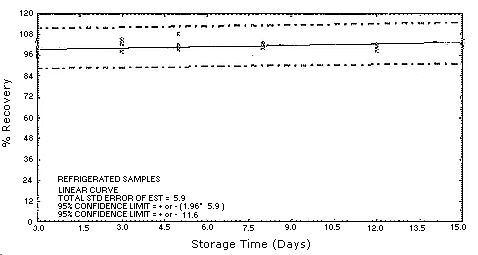
Figure 4.5.2.1. MOCA
refrigerated storage samples.

Figure 4.5.2.2. MOCA
refrigerated storage samples.
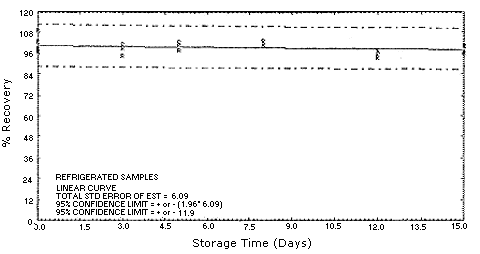
Figure 4.5.3.1.
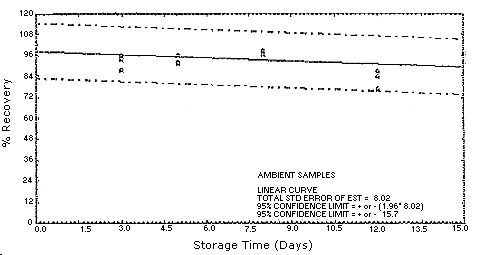
Figure 4.5.3.2.

Figure 4.10.1.
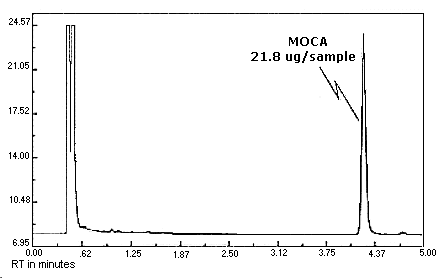
Figure 4.10.2. MOCA
chromatogram.
5. References
- 5.1. "Chemical Information Manual", U.S. 1 Department of Labor,
Occupational Safety and Health Administration, OSHA Instruction CPL
5.2. Elskamp, Carl J. "OSHA Method No. 65; Benzidine,
5.3. Elskamp, Carl J. "OSHA Method No. 57;
5.4. "IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man: Some aromatic amines, hydrazine and related
substances,
5.5. "IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man: Some aromatic amines, hydrazine and related
substances,
5.6. "Documentation of the Threshold Limits Values and Biological Exposure Indices", 5th ed.; American Conference of Governmental Industrial Hygienists Inc.: Cincinnati, OH, 1986; p 392.4(86).
5.7. "Documentation of the Threshold Limits Values and Biological Exposure Indices", 5th ed.; American Conference of Governmental Industrial Hygienists Inc.: Cincinnati, OH, 1986; p 577.