|
| Method no.: |
68 |
| |
|
| Matrix: |
Air |
| |
|
| Target concentration: |
200 ppm (360 mg/m3)
(OSHA PEL) |
| |
|
| Target Concentration: |
Insert Info |
| |
|
| Procedure: |
Air samples are collected by
drawing known volumes of air through 8-mm o.d.
sampling tubes containing XAD-2 adsorbent which
has been coated with 2-(hydroxymethyl)
piperidine. Each sampling tube contains a 450-mg sampling
section and a 225-mg backup section. The samples
are desorbed with toluene and the derivatized acetaldehyde is
analyzed by gas chromatography using a nitrogen selective
detector. |
| |
|
| Recommended air volume and
sampling rate studied: |
3 L at 0.05 L/min |
| |
|
| Reliable quantitation limit: |
580 ppb (1050
µg/m3) |
| |
|
Standard error of estimate
at
the target concentration:
(Section
4.7.) |
6.1% |
| |
|
| Status of method: |
Evaluated method. This method
has been subjected to the established evaluation procedures of
the Organic Methods Evaluation Branch. |
| |
|
| Date: |
January 1988 |
| |
|
| Chemist: |
Warren Hendricks |
| |
|
Organic Methods Evaluation Branch
OSHA
Analytical Laboratory
Sandy, Utah
|
1.
General Discussion
1.1 Background
1.1.1. History
This work was performed
be
cause the existing NIOSH sampling and analytical
method for acetaldehyde is too impractical for use by
OSHA. The NIOSH method (Ref.
5.1.) requires air sample collection using a bubbler
containing Girard T reagent. Analysis of the resulting
derivative is performed by HPLC with UV detection. Girard
T reagent must be used within 2 weeks of preparation and
specially treated glassware must be used to store the
reagent and to collect the samples.
XAD-2
adsorbent coated with 2-(hydroxymethyl)piperidine (2-HMP)
is used to collect phosgene (Ref.
5.2.), acrolein, and formaldehyde (Ref.
5.3.). Acetaldehyde was also found to react with 2-HMP
when the aldehyde was spiked on the coated adsorbent. Gas
chromatographic analysis revealed the presence of two
derivative peaks. The ratio of the two derivative peaks is
reasonably constant and is independent of the technique
(liquid spike or collection from a controlled test
atmosphere) used to prepare the derivatives on coated
XAD-2 adsorbent. Mass spectrometric analysis
showed that the two derivative peaks have the same
molecular formula. The two acetaldehyde derivatives are
probably due to the relative positions of the methyl group
and the hydrogen atom which are bonded to the carbon atom
indicated by the asterisk in the proposed derivatization
reaction shown below. The proposed names of the
derivatives are syn- and
anti-9-methyl-l-aza-8-oxabicyclo[4.3.0.]nonane.
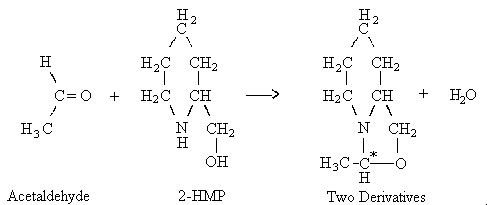
A
larger tube containing more coated adsorbent must be used
for acetaldehyde than for phosgene, formaldehyde, and
acrolein because a greater amount of acetaldehyde is
collected. Attempts to use the OSHA Versatile Sampler
(OVS) tube containing the coated adsorbent were
unsuccessful because of excessive migration of the
derivatives to the backup section during storage. This
effect was not observed with the sampling tube recommended
in this method because of its longer adsorbent bed length.
The length of the 450-mg bed in an OVS tube
is 1.3 cm while that in the recommended tube is 4 cm.
1.1.2. Toxic effects (This section is for
information only and should not be taken as the basis of
OSHA policy.)
Acetaldehyde vapor is an irritant of
the eyes, nose, and throat. Inhalation of high
concentrations may cause drowsiness, dizziness, and
unconsciousness. Acetaldehyde has a penetrating fruity
odor. The odor threshold for acetaldehyde is reported to
be between 0.031 and 2.3 ppm. Ingestion of acetaldehyde
may cause drowsiness, dizziness, unconsciousness, kidney
damage, and respiratory problems. The onset of respiratory
symptoms may be delayed. Eye contact with acetaldehyde may
cause a burning sensation, lacrimation, and blurred
vision. Skin contact with acetaldehyde may cause erythema
and burns. Repeated skin contact may result in dermatitis
which can be caused either by primary irritation or by
sensitization. (Ref.
5.4.)
The International Agency for Research on
Cancer (IARC) reports that acetaldehyde is an animal
teratogen, mutagen, and carcinogen. IARC also reports that
there is inadequate evidence of the carcinogenicity of
acetaldehyde to humans. IARC states that in the absence of
adequate human data, it is reasonable to treat chemicals
for which sufficient evidence of animal carcinogenicity
exists as if they were carcinogenic to humans. (Ref.
5.5.)
1.1.3. Workplace exposure
In
1982, the U.S. production of acetaldehyde was 281,000
metric tons. Most of this amount was produced by the
liquid-phase oxidation of ethylene using a
catalytic solution of palladium and copper chlorides. In
that year, 61% of the acetaldehyde production was used to
produce acetic acid, 9% to produce pyridine and pyridine
bases, 8% to produce peracetic acid, 7% to produce
pentaerythritol, 2% to produce 1,3-butylene
glycol, 1% to produce chloral, and 12% for other
applications which include food additives and exports. (Ref.
5.5.)
NIOSH estimated in 1974 that 1700
workers were potentially exposed to acetaldehyde.
Occupational exposure to acetaldehyde occurs primarily in
the manufacture of organic chemicals from acetaldehyde.
Examples of such chemical manufacturing operations
include: production of acetic acid, acetic anhydride,
aldol compounds, synthetic resins, pesticides,
pharmaceuticals, and rubber processing chemicals. Exposure
may also occur in industrial operations where acetaldehyde
is used. Uses for acetaldehyde include: coating of
mirrors, hardening agent in photography, preservative for
food and leather products and manufacture of gelatin,
glue, and casein products. (Ref.
5.4.)
1.1.4. Physical properties (Ref.
5.5. unless otherwise noted)
| CAS no.: |
|
75-07-0 |
| molecular weight: |
44.1 |
| appearance: |
colorless liquid |
| melting point: |
-123.5° |
| boiling point at 1 atm: |
20.16°C |
| vapor pressure at 20°C: |
100.6 kPa (755 mm Hg) |
| density at 20°C: |
0.7780 g/mL |
| solubility: |
miscible with water and most
organic solvents |
| flash point (closed cup): |
-38°C |
| structural formula: |
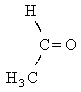 |
| autoignition temperature: |
175°C (Ref.
5.4.) |
flammable limits:
(% by volume
in air) |
lower 4 (Ref.
5.4.)
upper 60 |
| synonyms: |
acetic aldehyde; "aldehyde";
ethanal; ethyl aldehyde; ethylaldehyde;
NCIC56326 | 1.2. Limit defining parameters (The analyte
air concentrations listed throughout this method are based
on an air volume of 3 L and a solvent desorption volume of
5.0 mL. Air concentrations listed in ppm are referenced to
25°C and 760 mm Hg. The analyte concentrations are listed as
acetaldehyde even though the derivatives are the actual
species analyzed.)
1.2.1. Detection limit of the analytical
procedure
The ratio of the two derivative peaks is
about 14 to 1 and the smaller derivative peak is just
visible at the detection limit. The detection limit of the
analytical procedure is based on the peak height of the
larger derivative peak. The detection limit of the
analytical procedure is 565 pg per injection. This is the
amount of analyte which will give a peak whose height is
about 5 times the height of a nearby contaminant peak. (Section
4.1.)
1.2.2. Detection limit of the overall
procedure
The detection limit of the overall
procedure is 3.14 µg per sample (580 ppb or 1050
µg/m3). This is the amount of acetaldehyde
spiked on the sampling device which allows recovery of an
amount of analyte equivalent to the detection limit of the
analytical procedure. (Section 4.2.)
1.2.3.
Reliable quantitation limit
The reliable
quantitation limit is 3.14 µg per sample (580 ppb or 1050
µg/m3). This is the smallest amount of analyte
which can be quantitated within the requirements of a
recovery of at least 75% and a precision (±1.96 SD) of
±25% or better. (Section
4.2.)
The reliable quantitation limit and detection limits
reported in the method are based upon optimization of the
instrument for the smallest possible amount of the
analyte. When the target concentration of the analyte is
exceptionally higher than these limits, they may not be
attainable at the routine operating parameters.
1.2.4. Instrument response to the analyte
The
instrument response over the concentration range of 0.5 to
2 times the target concentration was not linear. (Section
4.4.)
1.2.5. Recovery
The recovery of
acetaldehyde from samples used in a 23-day storage test
remained above 92.8% when the samples were stored at about
23°C. (Section
4.7.) The recovery of the analyte from the collection
medium during storage must be 75% or greater.
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from
replicate determinations of analytical standards at 0.5,
1, and 2 times the target concentration is 0.0065. (Section
4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the
23-day ambient temperature storage test is ±11.9%. (Section
4.7.) This includes an additional ±5% for sampling
error. The overall procedure must provide results at the
target concentration that are ±25% or better at the 95%
confidence level.
1.2.8. Reproducibility
Six samples, spiked by liquid injection, and a
draft copy of this procedure were given to a chemist
unassociated with this evaluation. The samples were
analyzed after 41 days of storage at 10°C. No individual
sample deviated from its theoretical value by more than
the precision reported in Section 1.2.7. (Section
4.8.) 1.3. Advantage
This sampling
and analytical procedure provides a simple, convenient and
precise means to monitor occupational exposure to
acetaldehyde.
1.4. Disadvantage
The sampling
tubes are not currently commercially available.
2. Sampling Procedure
2.1. Apparatus
2.1.1. Samples are collected by use of a
personal sampling pump that can be calibrated to within
±5% of the recommended flow rate with the sampling device
attached.
2.1.2. Samples are collected with 11-cm
× 6-mm i.d. × 8-mm o.d. silane-treated glass
tubes which are packed with a 225-mg backup
section and a 450-mg sampling section of pretreated
XAD-2 adsorbent coated with
2-HMP. The two sections of coated adsorbent
are separated and retained with small plugs of silanized
glass-wool. Instructions for the pretreatment and the
coating of XAD-2 adsorbent are given in
Section 4.10. of this method. 2.2. Reagents
No sampling reagents are required.
2.3.
Sampling technique
2.3.1. Break open both ends of the sampling
tube so that the holes in the tube ends are at least
one-half the i.d. of the tube. Attach the
sampling tube to the sampling pump with flexible, plastic
tubing such that the large, front section of the sampling
tube is exposed directly to the atmosphere. Do not place
any tubing in front of the sampler. Attach the sampler
vertically in the worker's breathing zone in such a manner
that it does not impede work performance or safety. Be
certain that the sharp end of the sampling tube does not
injure the worker.
2.3.2. Remove the sampling
device after sampling for the appropriate time and seal
the tube with plastic end caps. Wrap the tube lengthwise
with an official OSHA seal (Form 21).
2.3.3.
Submit at least one blank sample with each set of samples.
Handle the blank the same as the other samples with the
exception of drawing air through it.
2.3.4. List
any potential interferences on the sample data sheet.
2.4. Sampler capacity
When controlled
test atmospheres containing 840 mg/m3 of
acetaldehyde (2.3 times the OSHA PEL) in air at 78% relative
humidity and 28°C were sampled using the recommended
sampling method, 5% breakthrough occurred after sampling for
304 min at 0.05 L/min. At the end of this time 15.2 L of air
had been sampled and 12.8 mg of acetaldehyde had been
collected.
Five-percent breakthrough was defined as
the point at which 5% of the total amount of acetaldehyde
collected on the entire tube was found on the backup
section. (Section 4.5.)
At the 5% breakthrough
point, 74% of the theoretically available 2-HMP
on the front section of the sampling tube had been consumed.
Other chemicals with carbonyl groups may also deplete
available 2-HMP. Clearly, a safety margin is
necessary and this is the basis of the recommended
3-L air volume. After sampling an atmosphere
containing 2 times the OSHA PEL of acetaldehyde for 3 L,
only 15% of the theoretically available 2-HMP
will be consumed.
An additional sampler capacity
experiment was performed to determine if the amount of water
in the sampled air could affect the ability of the sampler
to collect acetaldehyde. When a controlled test atmosphere
containing 823 mg/m3 acetaldehyde (2.3 times the
OSHA PEL) in air at 19% relative humidity and 27°C was
sampled using the recommended sampling method, 14%
breakthrough occurred after sampling for 3 L. Even though
the breakthrough was excessive, the amount of acetaldehyde
recovered was 99.7% of theoretical. The high breakthrough
observed when sampling essentially dry air further supports
the recommended 3-L air volume. (Section 4.5.)
2.5 Desorption efficiency
2.5.1 No desorption efficiency corrections are
necessary to compute results because analytical standards
are prepared using coated adsorbent. However, desorption
efficiencies were determined to investigate the recovery
of acetaldehyde from the sampling device. The average
desorption efficiency for acetaldehyde from
XAD-2 adsorbent coated with
2-HMP over the range of 0.3 to 2 times the
target concentration was essentially 100%. (Section 4.6.)
2.5.2. Desorbed samples are stable for at least 72
h. (Section 4.6.) 2.6. Recommended air volume
and sampling rate
2.6.1. The recommended air volume is 3 L and
the recommended sampling rate is 0.05 L/min.
2.6.2. When short-term air samples are required,
the recommended sampling rate is 0.05 L/min. A
15-min sample at the reliable quantitation
limit is equivalent to 2.3 ppm acetaldehyde.
2.7. Interferences (sampling)
2.7.1. Any collected substance that is capable
of reacting with 2-HMP is a potential
interference. Mineral acids may neutralize
2-HMP and chemicals which contain a carbonyl
group (such as acetone) may be capable of reacting with
2-HMP.
2.7.2. Suspected interferences
should be reported to the laboratory with submitted
samples. 2.8. Safety precautions
2.8.1. The sampling equipment should be
attached to the worker in such a manner that it will not
interfere with work performance or safety.
2.8.2.
All safety practices that apply to the work area being
sampled should be followed.
3. Analytical Procedure
3.1. Apparatus
3.1.1 A gas chromatograph (GC) equipped with a
nitrogen selective detector. A
Hewlett-Packard 5840A GC fitted with a
nitrogen-phosphorus detector (NPD) was used
in this evaluation. Injections were performed using a
Hewlett-Packard 7671A automatic sampler.
3.1.2. A GC column capable of resolving the
acetaldehyde derivative from potential interferences. A
6-ft × 1/4-in. o.d.
(2-mm i.d.) glass GC column containing 10%
UCON 50-HB-5100 with 2% KOH on 80/100 mesh
Chromosorb W-AW was used in this evaluation. Injections
were performed on-column.
3.1.3.
Vials, 7-mL and 2-mL glass with Teflon-lined caps.
3.1.4. Volumetric flasks, pipets, and syringes for
preparing standards, making dilutions, and performing
injections.
3.1.5. Vortex mixer, a S/P Deluxe
Mixer, obtained from American Scientific Products, was
used in this evaluation. 3.2. Reagents
3.2.1. Toluene and dimethylformamide, reagent
grade or better. American Burdick and Jackson solvents
were used in this evaluation.
3.2.2. Helium,
hydrogen, and air, GC grade.
3.2.3. Acetaldehyde,
of known high purity. Aldrich Chemical Co. acetaldehyde
(99%) was used in this evaluation. Store the acetaldehyde
in a freezer at approximately -20°C. CAUTION!
ACETALDEHYDE IS AN ANIMAL CARCINOGEN.
3.2.4. Amberlite XAD-2 adsorbent
coated with 10%, by weight,
2-(hydroxymethyl)piperidine. (Section 4.10.)
3.2.5. Desorbing solution. This solution is
prepared by adding 20 µL of dimethylformamide which is
used as an internal standard to 100 mL of toluene. This is
the same solution used to desorb phosgene, formaldehyde,
and acrolein air samples. 3.3. Standard
preparation
3.3.1. Prepare stock standards by diluting a
known amount of 99% acetaldehyde with toluene. CAUTION!
ACETALDEHYDE IS AN ANIMAL CARCINOGEN. Use of the
following technique to prepare stock standards is strongly
recommended: Determine the weight of a sealed
25-mL volumetric flask containing about 15 mL
of toluene. Place the sealed volumetric flask and a
1-mL pipet in the same freezer as used to
store the 99% acetaldehyde. After waiting 30 min, remove
the sealed volumetric flask, 1-mL pipet, and
99% acetaldehyde from the freezer. Immediately pipet 1 mL
of 99% acetaldehyde into the cold flask, reseal the flask,
and then allow the flask to warm to room temperature.
Reweigh the flask and subtract the tare weight from the
weight of the reweighed flask to determine the weight of
the added acetaldehyde. Dilute the contents of the
volumetric flask to the mark with toluene. A stock
standard containing 31.20 mg/mL acetaldehyde was prepared
using this technique. Store the stock standards in a
freezer. Prepare fresh stock standards every 10 days.
3.3.2. Prepare analytical standards about 16 h
before the air samples are to be analyzed in order to
ensure that the reaction between acetaldehyde and
2-HMP is complete.
3.3.3. Place
450-mg portions of coated XAD-2 adsorbent,
from the same lot number as used to collect the air
samples, into each of several 7-mL glass
vials. Seal each vial with a Teflon-lined
cap. Standards are prepared with coated adsorbent to
ensure that analytical standards are similar to air
samples.
3.3.4. Prepare standards by injecting
appropriate volumes of acetaldehyde onto the coated
adsorbent contained in the sealed 7-mL vials.
A standard containing 1092 µg (target concentration) of
acetaldehyde was prepared by injecting 35.0 µL of a
standard containing 31.20 mg/mL acetaldehyde into a vial
containing 450 mg of coated adsorbent.
3.3.5.
Prepare a sufficient number of standards to generate a
calibration curve. Analytical standard concentrations must
bracket sample concentrations.
3.3.6. Desorb the
standards in the same manner as the samples following the
16-h reaction time. 3.4. Sample
preparation
3.4.1. Transfer the 450-mg section of the
sampling tube and the front glass-wool plug
to a 7-mL glass vial. Place the
225-mg section and the center glass-wool plug
in a separate vial. Discard the rear
glass-wool plug.
3.4.2. Add 5.0 mL of
desorbing solution to each vial.
3.4.3. Seal the
vials with Teflon-lined caps and allow them to desorb for
1 h. Mix the contents of the vials using a vortex mixer
several times during the desorption time.
3.4.4.
Dilute samples containing high amounts of acetaldehyde
with desorbing reagent. When samples are not in the
concentration range of the prepared standards, additional
standards must be prepared to determine detector response.
3.5. Analysis
3.5.1. GC Conditions
| column temperature: |
|
bi-level temperature
program first level: 100 to 140°C at
4°C/min upon injection
second level:
140 to 180°C at 20°C/min following completion
of the first level
isothermal period: hold
column at 180°C until the recorder pen returns to
baseline (usually about 25 min after
injection) |
| injector temperature: |
180°C |
| helium flow rate: |
30 mL/min (detector response will
be reduced if nitrogen is substituted for helium
carrier gas) |
| injection volume: |
0.90 µL |
| GC column: |
6-ft × 1/4-in. o.d. (2-mm i.d.)
glass GC column containing 10% UCON
50-HB-5100 + 2% KOH on
80/100 Chromosorb W-AW |
NPD conditions
|
| hydrogen flow rate: |
3 mL/min |
| air flow rate: |
50 mL/min |
| detector temperature: |
275°C |
3.5.2. Retention times: 5.8 and 7.7 min (two
peaks)
3.5.3. Chromatogram: Figure
3.5.3.
3.5.4. Use a suitable method, such as
electronic integration, to measure detector response.
3.5.5. Analyze a standard at the target
concentration. Determine the relative concentration
(µg/sample) for each of the two acetaldehyde peaks by
multiplying the factor, which is obtained by dividing the
area of each peak by the total area for both peaks, times
the concentration (µg/sample) of the standard. For
example: The area of the 5.8-min peak
was 186700 and the area of the 7.7-min peak
was 12110. The total area was 198810 and the standard
concentration was 1092 µg/sample. The relative
concentration of the 5.8-min peak is
[(186700/198810)](1092) = 1025.48 µg/sample
and for the 7.7-min peak,
[(12110/198810)](1092) = 66.52 µg/sample.
Calibrate the integrator using the appropriate amounts and
an internal standard procedure. An internal standard is
used to compensate for minor variations in injection
volume and detector response.
3.5.6. Analyze
several standard solutions of different concentration to
generate the calibration curve. A calibration curve must
be used to compensate for possible nonlinear detector
response. Prepare the calibration curve daily.
3.5.7. Bracket sample concentrations with
standards. 3.6.
Interferences
3.6.1. Any compound having a similar retention
time as the acetaldehyde derivatives is a potential
interference.
3.6.2. GC parameters (temperature,
column, etc.) may be changed to circumvent Interferences.
3.6.3. Retention time on a single column is not
proof of chemical identity.
3.6.4. GC/MS is a
useful means of structure determination. It is recommended
that this procedure be used to confirm samples whenever
possible. 3.7. Calculations
3.7.1. Add the integrator results for both
peaks to obtain total µg/sample. A calibration curve must
be applied to these results to compensate for possible
nonlinear detector response.
3.7.2. Prepare a
calibration curve by plotting total µg/sample against the
actual concentration for each standard. Determine the best
line through the data points by curve fitting.
3.7.3. Determine the actual concentration, in
µg/sample, for a particular sample by comparing its summed
integrator results to the calibration curve. If
acetaldehyde is found on the backup section, add it to the
amount found on the front section. Perform blank
corrections before adding the results for the front and
backup sections together.
3.7.4. Express
acetaldehyde air concentration using the following
equation:
mg/m3 =
(A)/(B)
| where |
A = µg/sample from
Section 3.7.3. |
|
B = liters of air
sampled |
No desorption efficiency correction is
required because analytical standards are prepared using
coated adsorbent.
3.7.5. Convert acetaldehyde
results in mg/m3 to ppm using the following
equation:
ppm =
(mg/m3)(24.46)/(44.1)
| where |
mg/m3 |
= result from
Section 3.7.4. |
|
24.46 |
= molar volume at
760 mm Hg and 25°C |
|
44.1 |
= molecular weight
of acetaldehyde | 3.8. Safety precautions
3.8.1. Acetaldehyde is an animal carcinogen.
Treat it as if it were a human carcinogen.
3.8.2.
Avoid skin contact and inhalation of all chemicals.
3.8.3. Restrict the use of all chemicals to a fume
hood.
3.8.4. Wear safety glasses and a lab coat in
laboratory areas. 4. Backup Data
4.1. Detection limit of the
analytical procedure
The injection size recommended
in the analytical procedure (0.90 µL) was used in the
determination of the detection limit of the analytical
procedure. The ratio of the two derivative peaks is about 14
to 1 and the smaller derivative peak is just visible at the
detection limit. Therefore, the detection limit of the
analytical procedure is based on the peak height of the
larger derivative peak. The detection limit of the
analytical procedure was 565 pg per injection. This is the
amount of analyte which will give a peak whose height is
about five times the height of a nearby contaminant peak.
This detection limit was determined by the analysis of a
standard containing 0.628 µg/mL acetaldehyde. Figure
4.1.1. is a chromatogram of a blank sample and Figure
4.1.2. is a chromatogram of the detection limit of the
analytical procedure. A small acetaldehyde peak is present
in the blank sample. The acetaldehyde elution times for the
blank sample are indicated with arrows. The formaldehyde
derivative, which is a contaminant of the coated adsorbent,
is present in both chromatograms.
4.2. Detection limit of the overall procedure
and reliable quantitation limit data
The injection
size recommended in the analytical procedure (0.90 µL) was
used in the determination of the detection limit of the
overall procedure and in the determination of the reliable
quantitation limit. Samples were prepared by the liquid
injection of 10 µL of a solution containing 314 µg/mL
acetaldehyde in toluene onto 450-mg portions of
coated XAD-2 adsorbent. Standards were prepared
by injecting acetaldehyde into several 7-mL
glass vials, each containing 5 mL of a solution containing 9
mg/mL of 2-HMP in toluene. The samples and
standards were stored for about 16 h at room temperature
before analysis. Since the recoveries of acetaldehyde from
the samples were high and approximately equivalent to the
detection limit of the analytical procedure, the detection
limit of the overall procedure and the reliable quantitation
limit were 3.14 µg/sample (580 ppb or 1050
µg/m3).
Table
4.2.
Data for the Detection Limit of the
Overall
Procedure and the Reliable Quantitation Limit |
|
sample
number |
theoretical
amount
(µg) |
amount
recovered
(µg) |
|
percent
recovered |
|
| 1 |
3.14 |
3.28 |
104 |
| 2 |
3.14 |
2.89 |
92.0 |
| 3 |
3.14 |
3.42 |
109 |
| 4 |
3.14 |
3.28 |
104 |
| 5 |
3.14 |
3.04 |
96.8 |
| 6 |
3.14 |
3.00 |
95.5 |
|
| X
= |
100.2 |
| SD
= |
6.4 |
| 1.96 SD
= |
12.5 |
|
4.3. Precision (analytical method only)
The precision of the analytical method was evaluated
by performing multiple injections of analytical standards.
The standards were prepared by injecting appropriate amounts
of acetaldehyde into sealed 7-mL glass vials
containing 450 mg of coated adsorbent.
The standards
were allowed to stand 16 h prior to desorption and analysis.
The results of this study are presented in Tables 4.3.1. to
4.3.3. Tables 4.3.1 and 4.3.3. contain peak area data which
have not been corrected by the internal standard. Tables
4.3.2. and 4.3.3. contain peak concentration data which has
been corrected by the internal standard. The peak area data
is presented for use in Figure
4.4. and the precision of the analytical method was
evaluated using the combined peak concentration data.
Table
4.3.1.
Acetaldehyde Individual Peak Area Data |
|
| × target conc. |
0.5× |
1× |
2× |
| µg/sample |
542.5 |
1085 |
2170 |
| ppm |
100
|
201
|
401
|
| peak |
1 |
2 |
1 |
2 |
1 |
2 |
|
| area |
574600 |
34250 |
*1051000 |
69120 |
1739000 |
136200 |
| counts |
550900 |
33170 |
1023000 |
66760 |
1866000 |
147200 |
|
536600 |
31990 |
1044000 |
68800 |
1840000 |
144600 |
|
560100 |
33090 |
1066000 |
70140 |
1803000 |
139700 |
|
555900 |
33040 |
1042000 |
68540 |
1760000 |
135000 |
|
540700 |
31800 |
1036000 |
68380 |
1738000 |
133200 |
|
| * Areas from this
standard were used to calibrate the
integrator. |
Table
4.3.2.
Acetaldehyde Individual Peak Concentration
Data |
|
| × target conc. |
0.5× |
1× |
2× |
| µg/sample |
542.5 |
1085 |
2170 |
| ppm |
100
|
201
|
401
|
| peak |
1 |
2 |
1 |
2 |
1 |
2 |
|
| µg/sample |
542.0 |
32.3 |
1018.0 |
67.0 |
1766.8 |
137.9 |
| found |
539.5 |
32.5 |
1012.0 |
66.0 |
1748.3 |
137.9 |
|
541.6 |
32.3 |
1013.1 |
66.8 |
1761.5 |
138.4 |
| 540.7 |
32.0 |
1014.5 |
66.8 |
1797.6 |
139.3 |
| 541.6 |
32.2 |
1013.9 |
66.7 |
1791.8 |
137.4 |
| 541.3 |
31.8 |
1014.9 |
67.0 |
1796.2 |
137.7 |
|
Table
4.3.3.
Acetaldehyde Combined Peak Area
and
Combined Peak Concentration Data |
|
| 0.5× TC |
1×
TC |
2×
TC |
| 542.5
µg/sample |
1085
µg/sample |
2170
µg/sample |
100 ppm
|
200 ppm
|
400 ppm
|
| area |
µg/sample |
area |
µg/sample |
area |
µg/sample |
|
| 608850 |
574.3 |
1120120 |
1085.0 |
1875200 |
1904.7 |
| 584070 |
572.0 |
1089760 |
1078.0 |
2013200 |
1886.2 |
| 568590 |
573.9 |
1112800 |
1079.9 |
1984600 |
1899.9 |
| 593190 |
572.7 |
1136140 |
1081.3 |
1942700 |
1936.9 |
| 588940 |
573.8 |
1110540 |
1080.6 |
1895000 |
1929.2 |
| 572500 |
573.1 |
1104380 |
1081.9 |
1871200 |
1933.9 |
|
 |
573.3 |
|
1081.1 |
|
1915.1 |
| SD |
0.860 |
2.33 |
20.98 |
| CV |
0.00150 |
0.00216 |
0.0110 |
|
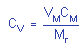 =
0.0065 =
0.0065 |
|
| TC =
target
concentration | 4.4. Instrument response to
the analyte
The combined area data in Table 4.3.3.
are presented graphically in Figure
4.4. This figure shows instrument response over the
concentration range of 0.5 to 2 times the target
concentration. The instrument response was not linear over
this range.
4.5. Breakthrough data
4.5.1. Breakthrough studies were performed
using several of the recommended two-section
collection devices to sample controlled test atmospheres
containing acetaldehyde in air for increasing periods of
time. The average acetaldehyde concentration was 840
mg/m3 (2.3 times the OSHA PEL) and the average
relative humidity was 78% at 28°C. The sampling rate was
0.05 L/min. Breakthrough was defined as the amount of
acetaldehyde found on the backup section divided by the
total amount of acetaldehyde collected on the entire
sampling tube. Five-percent breakthrough
occurred after sampling for 304 min. At the end of this
time, 15.2 L of air had been sampled and 12.8 mg of
acetaldehyde had been collected. The results of these
studies are presented in Table 4.5.1 and Figure
4.5.
Table
4.5.1.
Acetaldehyde Breakthrough Data |
|
volume
(L) |
breakthrough
(%) |
air
volume
(L) |
breakthrough
(%) |
|
| 8.6 |
0.17 |
16.7 |
3.6 |
| 15.0 |
0.80 |
20.2 |
23.1 |
| 15.0 |
1.2 |
20.8 |
32.0 |
| 16.3 |
4.8 |
23.9 |
42.8 |
| 16.4 |
3.5 |
|
4.5.2. An additional sampler capacity
experiment was performed as in Section 4.5.1. to determine
if the amount of water in the sampled air could affect the
ability of the sampler to collect acetaldehyde. A
controlled test atmosphere containing 823 mg/m3
acetaldehyde (2.3 times the OSHA PEL) in air at 19%
relative humidity and 27°C was sampled using the
recommended sampling method. The dilution air used in this
experiment was not humidified. The humidity was due to the
water with which the acetaldehyde was diluted. Even though
the observed breakthrough was excessive, the amount of
acetaldehyde recovered was high in all cases.
Table
4.5.2.
Acetaldehyde Breakthrough Data |
|
air
volume
(L) |
breakthrough
(%) |
amount
recovered
(%) |
|
| 2.7 |
13.3 |
104 |
| 3.0 |
14.0 |
99.7 |
| 4.7 |
15.1 |
91.0 |
| 5.1 |
16.1 |
92.5 |
| 6.4 |
16.0 |
91.3 |
| 6.5 |
14.6 |
91.2 |
| 4.6. Desorption efficiency and stability of
desorbed samples
4.6.1. Desorption efficiency
No
desorption efficiency corrections are required to
calculate air sample results because standards are
prepared using coated adsorbent. However, the desorption
efficiency of acetaldehyde from XAD-2
adsorbent coated with 2-HMP was investigated
by liquid spiking acetaldehyde into sealed
7-mL glass vials containing
450-mg portions of coated XAD-2
adsorbent. Analytical standards were prepared by injecting
equivalent amounts of acetaldehyde into 5-mL
aliquots of toluene containing 9 mg/mL of
2-HMP. The samples and standards were stored
at room temperature overnight before analysis.
Table
4.6.1.
The Desorption Efficiency
of
Acetaldehyde From XAD-2 Coated with 10% 2-HMP |
|
amount
spiked (µg) |
%
of
PEL |
amount
recovered (µg) |
desorption
efficiency (%) |
|
| 310.0 |
29.0 |
316.2 |
102 |
| 620.0 |
57.4 |
652.0 |
105 |
| 930.0 |
86.1 |
952.3 |
102 |
| 1240 |
115 |
1277 |
103 |
| 1550 |
144 |
1604 |
103 |
| 1860 |
172 |
1971 |
106 |
| 2170 |
201 |
2282 |
105 |
|
|
 =
104 =
104 |
|
4.6.2. Stability of desorbed samples
The stability of desorbed samples was investigated
by reanalyzing desorbed air samples following 72 to 120 h
storage at room temperature. Fresh standards were used and
the sample vials were resealed immediately after the first
analysis. The average recovery, relative to the original
analysis, was 103%.
Table
4.6.2.
The Stability of Desorbed Samples |
|
original
analysis (µg) |
storage
time
(h) |
reanalysis
(µg) |
% of
original
analysis |
|
| 1166 |
120 |
1185 |
102 |
| 1226 |
120 |
1270 |
104 |
| 1134 |
120 |
1156 |
102 |
| 1184 |
120 |
1198 |
101 |
| 1177 |
72 |
1211 |
103 |
| 1130 |
72 |
1173 |
104 |
|
|
 =
103 =
103 |
| 4.7. Storage data
Samples stored at approximately 23°C were collected
from a controlled test atmosphere containing 231 ppm
acetaldehyde in air at 80% relative humidity and 28°C.
Samples stored at -20°C were collected from a
controlled test atmosphere containing 211 ppm acetaldehyde
in air at 81% relative humidity and 27°C. All of the storage
samples were generated by sampling the controlled test
atmosphere for 60 min at 0.05 L/min. The results of the
storage tests are shown graphically in Figures
4.7.1. and 4.7.2.
Table
4.7.1.
Ambient Storage Data |
|
storage
time
(days) |
|
recovery
(%) |
|
| 0 |
95.0 |
91.8 |
97.4 |
| 5 |
91.7 |
92.9 |
88.1 |
| 8 |
92.4 |
96.6 |
94.7 |
| 12 |
94.6 |
98.2 |
100
|
| 15 |
89.8 |
91.1 |
90.1 |
| 23 |
99.3 |
96.6 |
98.8 |
|
Table
4.7.2.
Refrigerated Storage Data |
|
storage
time
(days) |
|
recovery
(%) |
|
| 0 |
95.3 |
91.0 |
94.5 |
| 2 |
94.0 |
95.9 |
93.9 |
| 6 |
87.3 |
95.4 |
99.0 |
| 9 |
101 |
96.1 |
95.7 |
| 13 |
101 |
98.7 |
99.7 |
| 16 |
95.4 |
92.4 |
98.4 |
|
4.8. Reproducibility data
Reproducibility samples were prepared by the liquid
injection of acetaldehyde on the sampling sections of coated
XAD-2 tubes. The samples and a draft copy of
this evaluation were given to a chemist unassociated with
this evaluation. The samples were analyzed after 41 days of
storage at about 10°C. No individual sample deviated from
its theoretical value by more than the precision (±11.9%) at
the 95% confidence level for the 23-day storage
test (Section
4.7.)
Table
4.8.
Reproducibility Results |
|
sample
no. |
theoretical
amount
(µg) |
analytical
result(µg) |
recovery
(%) |
|
| 1 |
1022 |
1048 |
102 |
| 2 |
1022 |
1044 |
102 |
| 3 |
1022 |
1077 |
105 |
| 4 |
1022 |
1026 |
100 |
| 5 |
1022 |
997.2 |
97.6 |
| 6 |
1022 |
1083 |
106 |
|
4.9.
Generation of controlled test atmospheres
The
controlled test atmospheres which were used in this
evaluation were generated by pumping an acetaldehyde/water
solution into a heated glass manifold with a Sage
Instruments Model 355 Syringe Pump. The acetaldehyde/water
solution was volatilized and then diluted with heated air.
The dilution air was metered into the heated glass manifold
using a calibrated precision rotameter. The air was
humidified, if desired, by passing it through a water
bubbler prior to its entering the heated glass manifold. The
water bubbler was contained in a
temperature-controlled water bath. The relative
humidity of the dilution air could be varied by changing the
temperature of the water bath. If dry dilution air was
required, the water bubbler was not used. The relative
humidity of the test atmosphere was monitored, after mixing,
with a YSI Model 91 Dew Point Hygrometer. The test
atmosphere passed through a manifold from which samples
could be collected.
The acetaldehyde concentration
of the test atmosphere was adjusted to the desired level by
varying the aldehyde concentration of the acetaldehyde/water
solution.
The theoretical acetaldehyde
concentrations of the test atmospheres were calculated using
the concentration of the acetaldehyde/water solution, the
flowrate of the syringe pump, and the volume of the dilution
air. Air sample results agreed with the theoretical
concentrations. The theoretical concentrations were used
throughout this evaluation.
4.10. Procedure to coat
XAD-2 adsorbent with 2-HMP
4.10.1. Apparatus
4.10.1.1. Soxhlet extractor
4.10.1.2. Rotary evaporator
4.10.1.3.
Miscellaneous glassware: One-liter vacuum flask,
1-L round-bottomed evaporative
flask, 1-L Erlenmeyer flask,
250-mL Buchner funnel with a coarse fritted
disc, etc. 4.10.2. Reagents
4.10.2.1. Methanol, isooctane, and toluene,
reagent grade or better. American Burdick and Jackson
solvents were used in this evaluation.
4.10.2.2.
2-(hydroxymethyl)piperidine. Technical grade
2-HMP, obtained from Aldrich Chemical
Company, was recrystallized from isooctane for use in
this evaluation.
4.10.2.3. Amberlite
XAD-2 non-ionic polymeric
adsorbent, 20 to 60 mesh. Aldrich Chemical
XAD-2 adsorbent was used in this
evaluation. 4.10.3. Procedure
This
procedure is similar to the one described in Section 4.10.
of OSHA Method 61: Phosgene (Ref.
5.2.) and in Section 4.8. of OSHA Method 52: Acrolein
and/or Formaldehyde (Ref.
5.3.). Coated adsorbent prepared by the procedure
described below can be used for Method 61 and for Method
52 if the formaldehyde blank level is determined to be
acceptably low.
Weigh 125 g of crude
XAD-2 adsorbent into a 1-L Erlenmeyer flask.
Add about 200 mL of water to the flask and then swirl the
mixture to wash the adsorbent. Discard any adsorbent that
floats to the top of the water and then filter the mixture
using a fritted-Buchner funnel. Transfer the
adsorbent back to the Erlenmeyer flask and repeat the
water wash and the filtration. Air dry the adsorbent for
about 2 min. Transfer the adsorbent back to the Erlenmeyer
flask and then add about 200 mL of methanol to the flask.
Swirl and then filter the mixture as before. Transfer the
washed adsorbent to a 1-L evaporative flask
and remove the methanol using the rotary evaporation
apparatus. Cool the flask to room temperature and then add
13 g of 2-HMP and 200 mL of toluene to the
flask. Swirl the mixture and then allow it to stand for 1
h. Remove the toluene using rotary evaporation. Seal the
evaporative flask and allow the coated adsorbent to stand
overnight at ambient temperature.
Transfer the
coated adsorbent to a Soxhlet extractor and then extract
the material with toluene for about 24 h. Replace the
contaminated toluene with fresh toluene and continue the
extraction for an additional 24 h. Replace the second
aliquot of contaminated toluene with methanol and continue
the Soxhlet extraction for 4 h. Transfer the adsorbent to
a weighed 1-L round-bottomed
evaporative flask and remove the methanol using the rotary
evaporation apparatus. Determine the weight of the
adsorbent and then add an amount of 2-HMP,
which is 10%, by weight, of the adsorbent. Add 200 mL of
toluene and then swirl the mixture. Allow the flask to
stand for 1 h. Remove the toluene using rotary
evaporation. If the last traces of toluene are difficult
to remove, add about 100 mL of methanol to the flask,
swirl the mixture, and then remove the solvents using
rotary evaporation.
XAD-2 adsorbent
treated in this manner will often contain residual
formaldehyde derivative levels of about 0.5 µg/150 mg of
adsorbent. The formaldehyde blank level and potential
acrolein and acetaldehyde chromatographic interferences
should be determined at this time. If the formaldehyde
blank and/or any interference is determined to be
excessive the batch should be returned to the Soxhlet
extractor, extracted with toluene again and then recoated
with 2-HMP. This process can be repeated
until an acceptable blank and/or level of chromatographic
interferences are attained.
The coated adsorbent
is now ready to be packed into sampling tubes. The
sampling tubes should be stored in the dark and segregated
by lot number. A sufficient amount of each lot of coated
adsorbent should be retained to prepare analytical
standards for use with air samples from that lot
number.
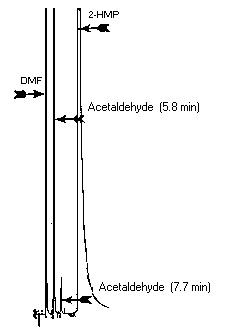
Figure 3.5.3. Acetaldehyde
chromatogram.

Figure 4.1.1. Blank sample
chromatogram.

Figure 4.1.2. Detection limit of the
analytical procedure chromatogram for
acetaldehyde.

Figure 4.4. Instrument response to
acetaldehyde.

Figure 4.5. Sampler capacity for
acetaldehyde.

Figure 4.7.1. Ambient temperature storage
study for acetaldehyde.
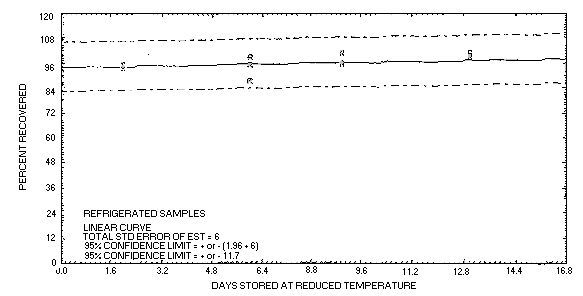
Figure 4.7.2. Refrigerated temperature
storage study for acetaldehyde.
5. References
5.1. NIOSH Manual of
Analytical Methods, 2nd ed.; U.S. Dept. of Health and
Human Services, Center for Disease Control, NIOSH:
Cincinnati, Aug 1979, Vol. 5, Method S345, Publ. No.
79-141.
5.2. Hendricks, W. Phosgene Method #61, OSHA Analytical
Laboratory, Salt Lake City, UT, unpublished, 1986.
5.3. OSHA Analytical Methods
Manual, U.S. Department of Labor, Occupational Safety
and Health Administration, OSHA Analytical Laboratory: Salt
Lake City, UT, Method 52, American Conference of
Governmental Hygienists (ACGIH): Cincinnati, 1985, ISBN:
0-936712-66-X.
5.4. NIOSH/OSHA Occupational Health Guidelines for
Chemical Hazards, U.S. Dept. of Health and Human
Services, Public Health Services, Center for Disease
Control, NIOSH and U.S. Dept. of Labor, OSHA: U.S.
Government Printing Office Washington, DC, Jan 1981,
Acetaldehyde, DHHS (NIOSH) Publ. No. 81-123.
5.5. IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans:
Allyl Compounds, Aldehydes, Epoxides and Peroxides,
International Agency for Research on Cancer: Lyon, 1984,
Vol. 36, 101-132. |
| |
| |

