| Method no.: | 67 |
| Matrix: | Air |
| Target concentration: | 0.5 mg/m3 (OSHA PEL) |
| Procedure: | Samples are collected by drawing known volumes of air through
OSHA Versatile Sampler tubes containing a glass fiber filter and two
sections of |
| Recommended air volume and sampling rate: |
480 L at 1.0 L/min |
| Reliable quantitation limit: | 0.064 µg/m3 |
| Standard error of estimate at the target concentration: (Section 4.6.) |
7.7% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: December 1987 | Chemist: Donald Burright |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
This evaluation was undertaken to determine the effectiveness of
the OSHA Versatile Sampler containing
In the past, technical grade chlordane was collected on a 37-mm
mixed cellulose ester membrane filter mounted in a cassette and
backed up by a Chromosorb 102 sorbent tube. The analytical procedure
developed by NIOSH (Method 5278) required that the sample be
desorbed with toluene and analyzed by GC with an electron capture
detector (ECD). (Ref. 5.3.) The NIOSH approach was to base the
analysis on a- and
Upon initial investigation, it was unclear if the OSHA PEL
pertained to technical grade chlordane or to a- and
1.1.2. Toxic effects (This section is for information only and should not be taken as a basis for OSHA policy.)
Technical grade chlordane is toxic to humans by ingestion, skin absorption and inhalation. It has been estimated that the fatal oral dose for an adult lies between 6 and 60 g, with onset of symptoms within 45 min to several hours after ingestion. (Ref. 5.8.)
Technical grade chlordane is a stimulant to the central nervous system but its exact mode of action is unknown. The general symptoms are convulsions and tremors followed by depression. Cycles of excitement and depression may be repeated several times. Other symptoms are liver damage, anorexia and weight loss. (Ref. 5.8.)
Chronic poisoning in animals produces loss of appetite and degenerative lesions in the liver and renal tubules. Like most halogenated hydrocarbon insecticides, technical grade chlordane is very slowly metabolized and excreted primarily in the feces. It alsois stored in body fat and is recognized as an inducer of hepatic microsomal enzyme activity. (Ref. 5.8.)
International Agency for Research on Cancer (IARC) reports on the carcinogenicity of chlordane (Ref. 5.9.): "There is sufficient evidence that technical grade chlordane is carcinogenic in mice. A report of a number of cases of cancer in humans was also available, but these data do not allow an evaluation of the carcinogenicity of technical grade chlordane to humans to be made."
1.1.3. Workplace exposure
The major use of technical grade chlordane is for termite control, in which treatment of the site with a 1% emulsion is enough to give residual control for at least 5 years. The size of the work force potentially exposed is not available, but would include workers involved in the manufacture, formulation, application and transportation of technical grade chlordane. (Ref. 5.10.)
1.1.4. Physical properties (Ref. 5.9. unless otherwise indicated)
Capillary GC and GC-MS investigations show that technical grade
chlordane contains at least 50 compounds, which in the majority of
cases belong to the tetrahydromethanoindene or
tetrahydromethanoindane system. The typical principle constituents
are heptachlor,
| CAS no.: | 57-74-9 |
| vapor pressure: | 0.0013 Pa at 25°C (1×10-5 mm Hg) |
| appearance: | viscous, amber-colored liquid |
| density: | 1.59-1.63 g/mL at 25°C |
| viscosity: | 0.75-1.20 stokes at 55°C |
| refractive index: | 1.56-1.57 at 25°C |
| solubility: | insoluble in water, soluble in most organic solvents |
| trade names: | 1068; Aspon; Belt; CD 68; Chlordan; Chlor Kil; Chlorindan;
Chlorodane; Corodane; |
| structural formula of a- and | |
 | |
1.2. Limit defining parameters (The analyte air concentrations
listed throughout this method are based on an air volume of 480 L and
a solvent extraction/desorption volume of 4 mL. All of the amounts
listed below are for technical grade chlordane but the actual peaks
analyzed were a- and
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 7.6 pg per
injection. This is the amount of analyte which gave a- and
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 30.5 ng per sample (0.064 µg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 30.5 ng per sample (0.064 µg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.2.)
|
|
| The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of the analyte. When the target concentration of the analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters. |
|
|
1.2.4. Instrument response to the analyte
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
The recovery of technical grade chlordane from samples used in a
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.028. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 15-day ambient temperature storage test is ±15.1%. (Section 4.6.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples, spiked by liquid injection with technical grade
chlordane and a draft copy of this procedure were given to a chemist
unassociated with this evaluation. The samples were analyzed after
65 days of storage at about
1.3. Advantage
This sampling device has the capability to collect both vapor and aerosols of technical grade chlordane without having to use two separate samplers in series.
1.4. Disadvantage
Currently the
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device attached.
2.1.2. Samples are collected with
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Attach the small end of the sampling tube to the sampling
pump with flexible, plastic tubing such that the large front section
of the sampling tube is exposed directly to the atmosphere. Do not
place any tubing in front of the sampler. The sampler should be
attached vertically (large end down) in the worker's breathing zone
in such a manner that it does not impede work performance.
2.3.2. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps.
2.3.3. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.4. With each set of samples submit at least one blank. The blank should be handled the same as the other samples except that no air is drawn through it.
2.4. Retention efficiency and sampler capacity
- 2.4.1. To test the sampler's ability to retain technical grade
chlordane, twice the target concentration of technical grade
chlordane, 488 µg, was
2.4.2. An aerosol of technical grade chlordane was generated and
the atmosphere was sampled for 65 min with an
2.5. Extraction and desorption efficiencies (Section 4.5.)
- 2.5.1. The combined extraction/desorption efficiency for
technical grade chlordane from the the glass fiber filter and the
large
2.5.2. The extraction efficiency for technical grade chlordane from glass fiber filters at the target concentration was essentially 100%.
2.5.3. The average desorption efficiency for technical grade
chlordane from the lot of cleaned
2.5.4. Extracted/desorbed samples remain stable for at least 48 h.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 480 L.
2.6.2. The recommended air sampling rate is 1.0 L/min.
2.6.3. When short-term air samples are required, the recommended
sampling rate is 1.0 L/min. The reliable quantitation limit for a
2.7. Interferences (sampling)
Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. The sampling equipment should be attached to the worker
in such a manner that it will not interfere with work performance or
safety.
2.8.2. All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with an electron capture detector. For this
evaluation a
3.1.2. A GC column capable of separating the peaks of a- and
3.1.3. An electronic integrator or other suitable means of
measuring detector response. A
3.1.4. Two- and four-milliliter vials with PTFE-lined caps were used for sample extraction/desorption and standard preparation.
3.2. Reagents
- 3.2.1. Chlordane (technical grade) 45%, (Dexol Industries).
3.2.2. Toluene, (American Burdick & Jackson).
3.2.3. Hexachlorobenzene (HCB) 97%, (Aldrich Chemical Co.). This may be used as the internal standard in the extracting/desorbing solution. The solution is prepared by adding 2 mg of HCB to 1 L of toluene.
3.3. Standard preparation
- 3.3.1. Prepare stock standards by adding toluene to preweighed
amounts of technical grade chlordane, be careful to include the
percentage of purity in the calculation. If
3.3.2. Prepare analytical standards by injecting microliter amounts of diluted technical grade chlordane stock standards into vials containing 4.0 mL of extracting/desorbing solution.
3.4. Sample preparation
- 3.4.1. Transfer the glass fiber filter and the 270-mg section of
the sampling tube to a
3.4.2. Add 4.0 mL of extracting/desorbing solution to each vial.
3.4.3. Seal the vials with PTFE-lined caps and allow them to extractdesorb for 1 h. Shake the vials vigorously by hand several times during the extraction/desorption time.
3.4.4. Transfer some of the solution from each of the 4-mL vials to smaller glass vials suitable for an autosampler if necessary.
3.5. Analysis
- 3.5.1. Analytical conditions
| temperatures: | 210°C (column) 250°C (injector) 300°C (detector) |
| column gas flow: | 9 mL/min (95% argon/5% methane) |
| make-up gas flow: | 24 mL/min (95% argon/5% methane) |
| injection size: | 1.0 µL |
| column: | DB-1, 1.5 µm thick, 30 m × 0.53-mm i.d. fused silica (J & W Scientific) |
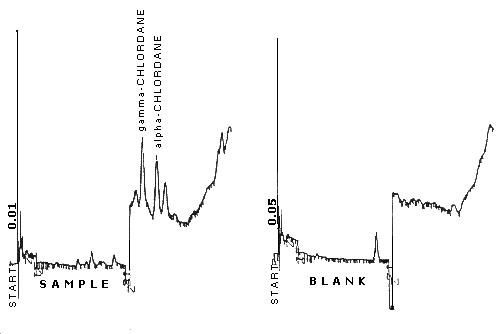
| retention time: | 7.7 min for 8.5 min for |
| chromatogram: | Figure 3.5.1. |
3.5.2. Measure detector response using a suitable method such as
electronic integration. Since there is only one source of
manufactured technical grade chlordane (Velsicol Chemical Corp.),
the pattern of peaks in the technical grade chlordane standards
should look like the pattern in the samples. When the patterns are
different use the bulk material from the sampling site as an
analytical standard if the bulk material is readily available or
analyze the samples for the major constituents of technical grade
chlordane: heptachlor, a- and
3.5.3. Use an internal standard procedure to prepare a calibration curve using several solutions over a range of concentrations. Prepare the calibration curve daily. Bracket the samples with analytical standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound having a similar retention time as the
analyte is a potential interference. Generally, chromatographic
conditions can be altered to separate an interference from the
analyte.
3.6.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. Prepare calibration curves from analytical standards by
plotting detector response for technical grade chlordane (the summed
response for the
3.7.2. Determine the concentration, in micrograms of technical
grade chlordane per milliliter, for a particular sample by comparing
its detector response (the summed response for the
3.7.3. The air concentration of technical grade chlordane can be expressed in mg/m3 by using the following equation:
| where | A = | concentration of technical grade chlordane from Section 3.7.2. |
| B = | extraction/desorption volume in milliliters | |
| C = | liters of air sampled | |
| D = | combined extrac./desorp. efficiency (decimal) |
The combined extraction/desorption efficiency should be determined for the particular batch of resin and lot of filter used for the sample.
3.7.4. If the requested information is for the concentration of
3.8. Safety precaution (analytical)
- 3.8.1. Because technical grade chlordane is an animal
carcinogen, handle it as if it were a human carcinogen. Observe a
similar precaution with the pure isomers of
3.8.2. Avoid skin contact and inhalation of all chemicals.
3.8.3. Restrict the use of all chemicals to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 7.63 pg per
injection of technical grade chlordane. This amount produced peaks of
4.2. Detection limit of the overall procedure and reliable quantitation limit
The detection limit of the overall procedure and the reliable
quantitation limit are 30.5 ng per sample (0.064
µg/m3). The injection size recommended in
the analytical procedure (1.0 µL) was used in the determination of the
detection limit of the overall procedure and in the determination of
the reliable quantitation limit. Six samples were
|
| |||
| sample | % recovered | statistics | |
|
| |||
| 1 | 108.8 | ||
| 2 | 107.5 | 106.0 | |
| 3 | 107.5 | SD = | 3.8 |
| 4 | 98.9 | 1.96 SD = | 7.4 |
| 5 | 108.8 | ||
| 6 | 104.5 | ||
|
| |||
4.3. Precision (analytical method only)
The precision of the analytical method was determined by multiple
injections of technical grade chlordane standards and summing the area
counts for the peaks of
|
| |||
| × target conc. µg/sample |
0.5× 122 |
1× 244 |
2× 488 |
|
| |||
| area | 503818 | 932305 | 1844530 |
| counts | 536341 | 956413 | 1768390 |
| 530464 | 991515 | 1837810 | |
| 536679 | 1027010 | 1788040 | |
| 540964 | 951229 | 1822170 | |
| 546248 | 949299 | 1885210 | |
| 538045 | 950531 | 1808700 | |
| 540952 | 959986 | 1867040 | |
| 561979 | 939683 | 1873950 | |
| 509477 | 989442 | 1824240 | |
| 534497 | 964741 | 1832008 | |
| SD | 16935 | 29058 | 37547 |
| CV | 0.0317 | .0301 | 0.0205 |
|
| |||
4.4. Instrument response to the analytes
The data in Table 4.3. is presented graphically in Figure 4.4. This figure is a calibration curve over the concentration range of 0.5 to 2 times the target concentration. The instrument response is linear over this range. The slope of the line (14200 area counts per µg/mL) is a measure of the response of the instrument to the analyte.
4.5. Extraction and desorption efficiencies
- 4.5.1. Extraction from glass fiber filter
The extraction efficiency of technical grade chlordane was
determined by
|
| |
| extraction efficiency, % | |
|
| |
| 98.7 | 102.3 |
| 102.9 | 102.3 |
| 102.7 | 100.5 |
|
| |
4.5.2. Desorption from
The desorption efficiency of technical grade chlordane was
determined by
|
| |||
| × target conc. µg/sample |
0.5× 122 |
1× 244 |
2× 488 |
|
| |||
| desorption | 99.7 | 100.7 | 100.9 |
| efficiency, | 98.7 | 101.1 | 100.3 |
| % | 99.2 | 100.3 | 99.2 |
| 98.4 | 101.1 | 100.9 | |
| 99.0 | 100.4 | 100.3 | |
| 98.8 | 100.5 | 99.5 | |
| 99.0 | 100.7 | 100.2 | |
|
| |||
The average desorption efficiency over the studied range was 100.0%.
4.5.3. Combined extraction/desorption efficiency
The combined extraction/desorption efficiency of technical grade
chlordane was determined by
|
| ||
| original | 48 h later | |
|
| ||
| extraction/ | 102.7 | 99.1 |
| desorption | 99.5 | 99.6 |
| efficiency, | 100.3 | 99.0 |
| % | 101.4 | 99.6 |
| 100.1 | 98.6 | |
| 99.4 | 98.2 | |
| 100.6 | 99.0 | |
| % of original = 98.4 | ||
|
| ||
4.6. Storage data
- 4.6.1. Storage samples were generated by liquid-spiking 36
sampling tubes with technical grade chlordane, 244.2 µg, and then
pulling 20 L of humid air through them (about 74% relative
humidity). One-half of the tubes was stored in a freezer
|
| ||||||||
| storage time (days) |
ambient recovery (percent) |
refrigerated
recovery (percent) | ||||||
|
| ||||||||
| 0 | 97.7 | 97.4 | 97.9 | 97.7 | 97.4 | 97.9 | ||
| 97.1 | 97.8 | 97.5 | 97.1 | 97.8 | 97.5 | |||
| 3 | 98.8 | -- | 98.3 | 97.8 | 97.4 | 97.5 | ||
| 6 | 95.5 | 96.5 | 95.2 | 98.4 | 98.6 | 97.4 | ||
| 9 | 96.1 | 96.4 | 96.0 | 96.6 | 95.8 | 96.6 | ||
| 13 | 98.0 | 98.1 | 98.0 | 98.2 | 99.1 | 97.9 | ||
| 15 | 98.7 | -- | 94.8 | 93.2 | 90.7 | 92.6 | ||
|
| ||||||||
4.6.2. Another storage test was performed by sampling a
dynamically generated aerosol test atmosphere (9.6
mg/m3 and about 80% relative humidity) of
technical grade chlordane. The average value of the Day 0 samples
was 202.2 µg/sample.
|
| ||||||||
| storage time (days) |
ambient recovery (percent) |
refrigerated
recovery (percent) | ||||||
|
| ||||||||
| 0 | 100 | 105 | 100 | 96.9 | 103 | 94.5 | ||
| 96.9 | 103 | 94.5 | 100 | 105 | 100 | |||
| 3 | 87.3 | 102 | 91.4 | 79.9 | 86.1 | 97.2 | ||
| 6 | 93.7 | 103 | 87.9 | 97.8 | 99.3 | 96.9 | ||
| 9 | 99.0 | 100 | 97.9 | 95.2 | 90.4 | 101 | ||
| 13 | 99.9 | 109 | 94.1 | 92.9 | 103 | 107 | ||
| 15 | 91.7 | 92.6 | 90.6 | 95.5 | 99.3 | 97.9 | ||
|
| ||||||||
4.7. Reproducibility data
Six samples, liquid-spiked with technical grade chlordane, were
given to a chemist unassociated with this study. The samples were
analyzed after being stored for 65 days at
|
| |||
| sample | µg spiked | % recovered | % deviation |
|
| |||
| 1 | 366 | 98.4 | -1.6 |
| 2 | 244 | 91.3 | -8.7 |
| 3 | 366 | 92.4 | -7.6 |
| 4 | 244 | 94.2 | -5.8 |
| 5 | 244 | 92.8 | -7.2 |
| 6 | 244 | 92.1 | -7.9 |
|
| |||
4.8. Retention efficiency
To test the ability of the sampler to retain the analytes, eight
samplers were
|
| ||
| air volume (L) | µg recovered | % recovered |
|
| ||
| 15.2 | 480.1 | 96.0 |
| 30.3 | 484.3 | 96.7 |
| 45.5 | 480.2 | 96.1 |
| 60.6 | 482.6 | 96.5 |
| 90.9 | 448.1 | 90.7 |
| 120.0 | 475.7 | 95.3 |
| 181.8 | 476.3 | 95.4 |
| 255.0 | 477.1 | 95.5 |
|
| ||
4.9. Preparation of the
It is anticipated that this sampler containing different adsorbents
can be used to collect a broad range of airborne contaminants. For
these applications the suffix will reflect the type of resin contained
in the sampler. For example, a sampler containing Tenax will be
designated
- 4.9.1. Apparatus
- 4.9.1.1. Soxhlet extractor
4.9.1.2. Rotary evaporator
4.9.1.3. Miscellaneous glassware: vacuum flask, 2-L
round-bottom flask, Erlenmeyer flask,
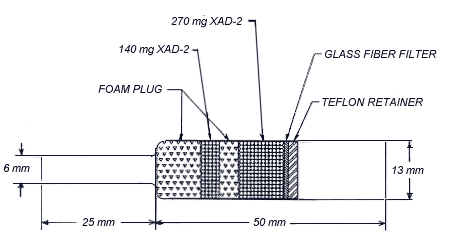
4.9.1.4. Urethane foam plugs, 3/8-in. × 1/2-in. diameter and
4.9.1.5. Glass fiber filters, 1/2-in. diameter or 13-mm diameter.
4.9.1.6. PTFE retainer. The retainer is made by removing a 50
arc from a piece of PTFE tubing,
4.9.1.7. Glass sampling tube. The sampling tube is constructed
of two pieces of borosilicate glass tubing that have been joined
together by a glass blower. One of the pieces is 50 mm ×
4.9.1.8. Plastic cap, 7/8 in. × 1/2-in. i.d. (Alliance Plastics, Inc., Erie PA).
4.9.1.9. Plastic cap, 3/4 in. × 7/32-in. i.d. (SKC, Inc,
4.9.2. Reagents
- 4.9.2.1. Acetonitrile, HPLC grade.
4.9.2.2. Toluene, HPLC grade.
4.9.2.3. Methanol, HPLC grade.
4.9.2.4. Amberlite
4.9.3. Cleaning of adsorbent
Add 500 g of crude
4.9.4. Assembly of the
Place a large foam plug in the bottom of the large end of the
glass tube. Add 140 mg of cleaned
4.10. Comparisons of commercially available technical grade chlordane
A study was done to compare several different sources of technical
grade chlordane to check the fingerprint pattern of the chromatogram.
An
To determine how well the labeled concentrations of the three
solutions compared to each other, two weighed aliquots of each
solution were diluted with toluene. From each of the six stock
solutions three dilute solutions were prepared which were
approximately 0.5, 1 and 2 times the target concentration. The six
dilute standards from each source (D1, BF, or D2) were then used to
determine the concentration of the remaining solutions from the other
two sources. The results of this
|
| |||
| sample | D1 | standard BF |
D2 |
|
| |||
| D1 | -- | 78.9% | 83.1% |
| BF | 41.6% | -- | 47.5% |
| D2 | 39.2% | 42.9% | -- |
|
| |||
The fingerprint patterns of the three solutions were similar to each other. After comparing the results, the two newer solutions were found to be close to their labeled percentages and that over the years the D1 solution had loss some of its solvent. The concentration of the D1 solution is now about 81% technical grade chlordane.
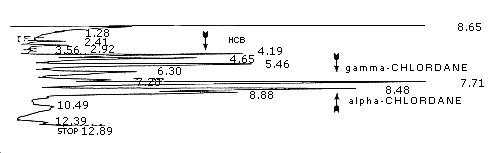
Figure
3.5.1.
Chromatogram of technical grade chlordane at the target
concentration.
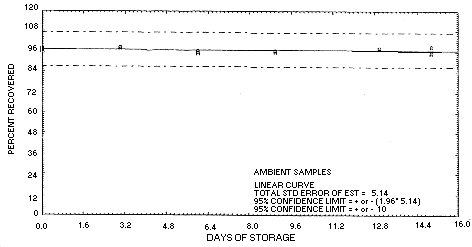
Figure 4.6.1.1.
Ambient
storage for technical grade chlordane, liquid spiked.

Figure
4.6.1.2.
Refrigerated storage test for technical grade chlordane,
liquid spiked.

Figure 4.6.2.1.
Ambient
storage test for technical grade chlordane, generated aerosol.
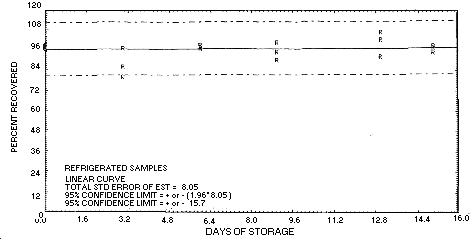
Figure
4.6.2.2.
Refrigerated storage test for technical grade chlordane,
generated aerosol.