| Method no.: | 61 |
| Matrix: | Air |
| Target concentration: | 100 ppb (0.4 mg/m3) (OSHA PEL) |
| Procedure: | Air samples are collected by drawing known volumes of air through sampling tubes containing XAD-2 adsorbent which has been coated with 2-(hydroxymethyl)piperidine. The samples are desorbed with toluene and then analyzed by gas chromatography using a nitrogen selective detector. |
| Recommended air volume and sampling rate: |
240 L at 1 L/min |
| Reliable quantitation limit: | 3.5 ppb (0.014 mg/m3) |
| Standard error of estimate at the target concentration: (Section 4.7.) |
6.7% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: August 1986 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The procedures that have been used by OSHA to monitor occupational exposure to phosgene include detector tubes, monitoring dosimeters and infrared gas analyzers. None of these procedures have proven to be completely adequate for use by OSHA. These methods lack either the desired precision and accuracy or they are awkward and inconvenient for field use.
This procedure was developed after it was found that phosgene
would react quantitatively with
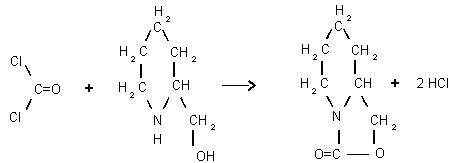
Phosgene 2-HMP Derivative
XAD-2 adsorbent coated with
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Phosgene gas is a severe eye and respiratory irritant. The lowest concentration that will cause immediate throat irritation is 3 ppm. A level of 4 ppm will cause immediate eye irritation, 4.8 ppm causes coughing and brief exposure to 50 ppm may be fatal. Symptoms of moderate exposure include a dryness or a burning sensation in the throat, vomiting, chest pain and difficulty in breathing. Inhalation of a sufficient quantity of phosgene causes pulmonary edema which may be characterized by a delay in the onset of symptoms of up to 72 h. The length of the delay in the onset of symptoms depends on the severity of the exposure. The pulmonary edema may progress to pneumonia which may result in cardiac failure. (Ref. 5.2.)
Splashes of liquid phosgene on the skin or in the eye may produce severe tissue damage (Ref. 5.2.).
The odor threshold for phosgene is about 0.5 to 1 ppm. This level will vary with the individual and is usually higher following exposure because of olfactory fatigue. The odor of phosgene at 0.5 ppm has been described as pleasant and similar to new-mown hay or cut-green corn. At higher levels, the odor may be strong, stifling and unpleasant. (Ref. 5.3.)
1.1.3. Workplace exposure
Most phosgene is used to manufacture other chemicals in the same plant in which it is produced. It is manufactured by the reaction of carbon monoxide and chlorine over activated charcoal. In 1981, the United States consumed almost a million metric tons. The primary user of phosgene is the polyurethane industry which consumes 85% of the total phosgene produced. The polycarbonate industry consumes about 6% and the remaining 9% is used in miscellaneous applications. Phosgene can be employed in a variety of metal recovery operations and in the production of chloroformates which are intermediates in the production of perfumes, herbicides, insecticides and pharmaceuticals. Phosgene also has numerous other minor uses and suggested uses. (Ref. 5.3.) In 1976, NIOSH estimated that 10,000 workers had potential occupational exposure to phosgene during its manufacture and use (Ref. 5.4.).
Phosgene can be unintentionally produced when chlorinated hydrocarbons are subjected to heat or light. An example of this is the production of phosgene in the vicinity of a welding operation in a facility in which perchloroethylene is used. (Ref. 5.4.)
1.1.4. Physical properties (Ref. 5.3.)
| CAS no.: | 75-44-5 |
| molecular weight: | 98.92 |
| appearance: | colorless gas |
| melting point: | -127.84°C |
| boiling point at 1 atm: | 7.48°C |
| vapor pressure at 20°C: | 1212 mm Hg |
| vapor density (air = 1.0): | 3.4 |
| molecular structure: |  |
| synonyms: | carbonyl chloride; carbon oxychloride; chloroformyl chloride |
- 1.2. Limit defining parameters (The analyte air concentrations
listed throughout this method are based on an air volume of 240 L and
a solvent desorption volume of 1.0 mL. Air concentrations listed in
ppm are referenced to 25°C and 760 mm Hg. The analyte concentrations
are listed as phosgene even though the derivative is the actual
species analyzed.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.7 ng per injection. This is the amount of analyte which will give a peak whose height is sufficiently large to permit its visual detection in a sample chromatogram when it is compared to a blank sample chromatogram. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 3.4 µg per sample (3.5 ppb or 0.014 mg/m3). This is the amount of phosgene spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 3.4 µg per sample (3.5 ppb or 0.014 mg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Instrument response to the analyte
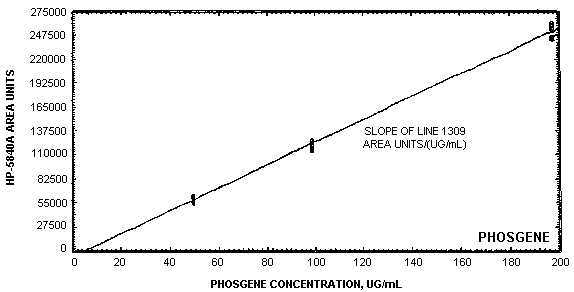
The instrument response over the concentration range of 0.5 to 2 times the target concentration is linear. (Section 4.4.)
1.2.5. Recovery
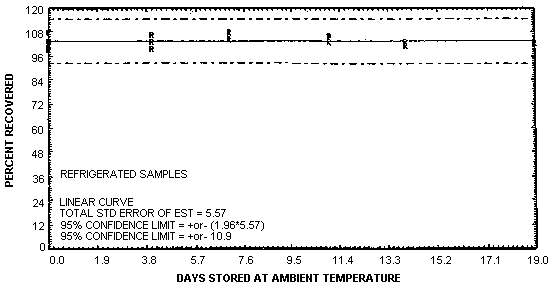
The recovery of phosgene from samples used in a 19-day storage test remained above 102% when the samples were stored at about 23°C. (Section 4.7.) The recovery of the analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical procedure)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.035. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 19-day ambient temperature storage test is ±13%. (Section 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples, collected from a controlled test atmosphere, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 1 day of storage at 23°C. No individual sample deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.8.)
- 1.3. Advantage
This sampling and analytical procedure provides a simple, convenient and precise means to monitor occupational exposure to phosgene.
1.4. Disadvantage
The sampling tubes currently must be obtained from the Salt Lake City Analytical Laboratory (SLCAL).
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated to within ±5% of the recommended flow rate
with the sampling device in line.
2.1.2. Samples are collected on 4-mm i.d. × 6-mm o.d. × 11-cm
long silane-treated glass tubes which are packed with a 75-mg backup
section and a 150-mg sampling section of pretreated XAD-2 adsorbent
which has been coated with
2.1.3. Commercially available sampling tubes, marketed by Supelco for the collection of acrolein, while similar to those in this method, are not recommended by SLCAL for the collection of phosgene. These sampling tubes provided consistently lower results than SLCAL tubes when the same test atmosphere was sampled with both tubes. (Section 4.5.2.) It should be noted that the Supelco tubes were not intended by the manufacturer to collect phosgene.
- 2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. Break open both ends of the sampling tube so that the
holes in the tube ends are at least one-half the i.d. of the tube.
Attach the sampling tube to the sampling pump with flexible, plastic
tubing such that the large, front section of the sampling tube is
exposed directly to the atmosphere. Do not place any tubing in front
of the sampler. The sampler should be attached vertically in the
worker's breathing zone in such a manner that it does not impede
work performance or safety. Be certain that the sharp end of the
sampling tube does not injure the worker.
2.3.2. After sampling for the appropriate time, remove the sampling device and then seal the tube with plastic end caps. Wrap the tube lengthwise with an official OSHA seal (Form 21).
2.3.3. With each set of samples, submit at least one blank. The blank should be handled the same as the other samples except that no air is drawn through it.
2.3.4. List any potential interferences on the sample data sheet.
- 2.4. Sampler capacity
When controlled test atmospheres, each containing 0.7 ppm phosgene (7 times the OSHA PEL) in air at 78% relative humidity and 23°C, were sampled using the recommended sampling method, the average 5% breakthrough occurred after sampling for 275 min at 1 L/min. At the end of this time 275 L of air had been sampled and 778 µg of phosgene had been collected. Five-percent breakthrough was defined as the point at which 5% of the total amount of phosgene collected on the entire tube was found on the backup section. (Section 4.5.)
The amount of water in the sampled air can affect the ability of
the sampler to collect phosgene. When no humidity was added to a test
atmosphere containing 0.3 ppm phosgene, considerable break through was
observed after sampling for 240 min at 1 L/min. Even though the
breakthrough from the sampling section of the tube was considerable,
the total amount of phosgene recovered from the entire tube was still
97% of the theoretical amount. The breakthrough was probably caused by
a reduction in the reaction rate between phosgene and
2.5. Desorption efficiency
- 2.5.1 No desorption efficiency corrections are necessary to
compute air sample results because analytical standards are prepared
using coated adsorbent. However, desorption efficiencies were
determined to investigate the recovery of phosgene from the sampling
device. The average desorption efficiency for phosgene from XAD-2
adsorbent coated with
2.5.2. Desorbed samples remain stable for at least 16 h. (Section 4.6.)
- 2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 240 L and the recommended
sampling rate is 1 L/min.
2.6.2. When the humidity of the sampled air is very low, the recommended air volume is 120 L and the recommended sampling rate is 0.5 L/min. (Section 4.5.)
2.6.3. When short-term air samples are required, the recommended sampling rate is 1 L/min. A 15-min sample at the reliable quantitation limit is equivalent to 60 ppb phosgene.
- 2.7. Interferences (sampling)
- 2.7.1. Any collected substance that is capable of reacting with
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
- 2.8. Safety precautions (sampling)
- 2.8.1. The sampling equipment should be attached to the worker
in such a manner that it will not interfere with work performance or
safety.
2.8.2. All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph (GC) equipped with a nitrogen
selective detector. A Hewlett-Packard 5840A GC fitted with a
nitrogen-phosphorus flame ionization detector (NPD) was used in this
evaluation. Injections were performed using a Hewlett-Packard 7671A
automatic sampler.
3.1.2. A GC column capable of resolving the phosgene derivative
from potential interferences. A 6-ft × 1/4-in. o.d. × 2-mm i.d.
glass GC column containing 10% UCON 50-HB-5100 with 2% KOH on 80/100
mesh Chromosorb W-AW was used in this evaluation. Injections were
performed
3.1.3. Vials, 2-mL glass with Teflon-lined caps.
3.1.4. Volumetric flasks, pipets and syringes for preparing standards, making dilutions and performing injections.
- 3.2. Reagents
- 3.2.1. Toluene and dimethylformamide. Burdick and Jackson
solvents were used in this evaluation.
3.2.2. Helium, hydrogen and air, GC grade.
3.2.3. Phosgene, of known high purity. Matheson Gas Products 99.0% minimum grade phosgene was used in this evaluation.
3.2.4. Amberlite XAD-2 adsorbent coated with 10%, by weight, 2-(hydroxymethyl)piperidine. (Section 4.10.)
3.2.5. Desorbing solution. This solution is prepared by adding 20 µL of dimethylformamide to 100 mL of toluene. This is the same solution used to desorb formaldehyde and acrolein air samples.
- 3.3. Standard preparation
- 3.3.1. It is recommended that standards be prepared about 16 h
before the air samples are to be analyzed in order to insure the
complete reaction between phosgene and
3.3.2. Place 150-mg portions of coated XAD-2 adsorbent, from the same lot number as used to collect the air samples, into each of several 2-mL glass vials. Seal each vial with a Teflon-lined cap.
3.3.3. Prepare standards by injecting appropriate volumes of phosgene gas into the headspace above the coated adsorbent contained in the sealed 2-mL vials. A standard containing 97.0 µg of phosgene was prepared by injecting 28 µL of 99.0% phosgene gas, at 653 mm Hg and 23°C, into a vial containing 150 mg of coated adsotbent.
Note: Phosgene is a corrosive gas. The syringes used to prepare standards must be cleaned after each use. The syringes must be checked for plugging before and after each use.
3.3.4. The mass of phosgene gas which was used to prepare standards can be determined by use of the following equations:
| where | MV | = | ambient molar volume |
| BP | = | ambient barometric pressure | |
| T | = | ambient temperature |
µg/µL = 98.92/MV
µg per standard = (µg/µL)(µL)(P)
| where | µg/µL | = | ambient density of phosgene gas |
| µL | = | µL of phosgene used to prepare the standard | |
| P | = | decimal form of the phosgene purity |
3.3.5. Prepare a sufficient number of standards to generate a calibration curve. Analytical standard concentrations should bracket sample concentrations. Thus, if samples are not in the concentration range of the prepared standards, additional standards must be prepared to determine detector response.
3.3.6. Desorb the standards in the same manner as the samples following the 16-h reaction time.
- 3.4. Sample preparation
- 3.4.1. Transfer the 150-mg section of the sampling tube to a
2-mL glass vial. Place the 75-mg section in a separate vial. If the
glass wool plugs contain a significant number of adsorbent beads,
place them with the appropriate sampling tube section. Discard the
glass wool plugs if they do not contain a significant number of
adsorbent beads.
3.4.2. Add 1.0 mL of desorbing solution to each vial.
3.4.3. Seal the vials with Teflon-lined caps and allow them to desorb for 1 h. Shake the vials by hand with vigorous force several times during the desorption time.
- 3.5. Analysis
- 3.5.1. GC conditions
| column temperature: | 180°C |
| injector temperature: | 180°C |
| helium flow rate: | 30 mL/min |
| injection volume: | 0.80 µL |
| GC column: | 6-ft × 1/4-in. o.d. (2-mm i.d.) glass GC column packed with 10% UCON 50-HB-5100 with 2% KOH on 80/100 mesh Chromosorb W-AW |
| NPD hydrogen flow rate: | 3 mL/min |
| NPD air flow rate: | 50 mL/min |
| NPD temperature: | 275°C |
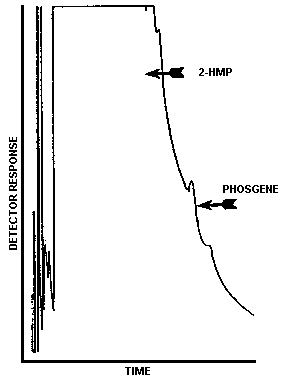
| Retention time: | 14.6 min (phosgene) |
| Chromatogram: | Figure 3.5.1. |
3.5.2. Use a suitable method, such as electronic integration, to measure detector response.
3.5.3. Use an external standard procedure to prepare a calibration curve with several standard solutions of different concentrations. Prepare the calibration curve daily. Program the integrator to report results in µg/mL.
3.5.4. Bracket sample concentrations with standards.
- 3.6. Interferences (analytical)
- 3.6.1. Any compound having a similar retention time as phosgene
is a potential interference.
3.6.2. GC parameters (temperature, column, etc.) may be changed to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity.
3.6.4. GC/MS is a useful means of structure determination. It is recommended that this procedure be used to confirm samples whenever possible. Phosgene was confirmed by GC/MS in a desorbed sample containing 3.7 µg of phosgene.
- 3.7. Calculations
- 3.7.1. Results are obtained by use of calibration curves.
Calibration curves are prepared by plotting detector response
against concentration for each standard. The best line through the
data points is determined by curve fitting.
3.7.2. The concentration, in µg/mL, for a particular sample is determined by comparing its detector response to the calibration curve. If phosgene is found on the backup section, it is added to the amount found on the front section. Blank corrections should be performed before adding the results together.
3.7.3. Phosgene air concentration can be expressed using the following equation:
| mg/m3 (A)(B)/(C) where | A = µg/mL from Section 3.7.2. B = desorption volume C = liters of air sampled |
No desorption efficiency correction is required because analytical standards are prepared using coated adsorbent.
3.7.4. The following equation can be used to convert phosgene results in mg/m3 to ppm:
ppm = (mg/m3)(24.46)/(98.92)
| where | mg/m3 | = | result from Section 3.7.3. |
| 24.46 | = | molar volume of an ideal gas at 760 mm Hg and 25°C | |
| 98.92 | = | molecular weight of phosgene |
- 3.8. Safety precautions
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood.
3.8.3. Wear safety glasses and a lab coat in all laboratory areas.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
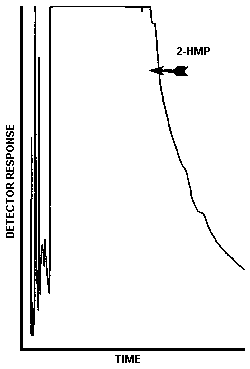
The injection size recommended in the analytical procedure (0.80 µL) was used in the determination of the detection limit of the analytical procedure. The detection limit of the analytical procedure was 2.7 ng per injection. This was the amount of phosgene which gave a peak whose height was sufficiently large to permit its visual detection in a sample chromatogram when it was compared to a blank sample chromatogram. This detection limit was determined by the analysis of a 3.4 µg/mL phosgene standard. Figure 4.1.1. is a chromatogram of the detection limit of the analytical procedure and Figure 4.1.2. is a chromatogram of a blank sample.
4.2. Detection limit of the overall procedure and reliable quantitation limit data
The injection size recommended in the analytical procedure (0.80
µL) was used in the determination of the detection limit of the
overall procedure and in the determination of the reliable
quantitation limit. Six samples were generated by sampling a
controlled test atmosphere containing 0.38
mg/m3 phosgene, in air at 55% relative
humidity and 27°C, for 18 min at 0.5 L/min. The average theoretical
amount collected was 3.4 µg/sample. Standards were prepared by
injecting phosgene gas into the headspace of several 2-mL glass vials,
each containing 1 mL of a solution of 15 mg/mL
Data for Detection Limit of the Overall
Procedure and the Reliable Quantitation Limit
|
| ||||
| sample | theoretical amount | amount recovered | percent | |
| number | (µg) | (µg) | recovered | |
|
| ||||
| 1 | 3.36 | 3.11 | 92.6 | |
| 2 | 3.32 | 3.40 | 102.4 | |
| 3 | 3.36 | 3.50 | 104.2 | |
| 4 | 3.42 | 3.42 | 100.0 | |
| 5 | 3.46 | 3.50 | 101.2 | |
| 6 | 3.36 | 3.50 | 104.2 | |
| 3.38 | 100.8 | |||
| ||||
|
| ||||
4.3. Precision (analytical method only)
The precision of the analytical method was evaluated by performing multiple injections of analytical standards. The standards were prepared by injecting appropriate amounts of phosgene gas into sealed 2-mL glass vials containing 150 mg of coated adsorbent. The standards were allowed to stand 16 h prior to desorption and analysis.
Phosgene Precision Data
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 49.4 | 98.8 | 197.7 |
| ppm | 0.05 | 0.1 | 0.2 |
|
| |||
| area counts | 61230 | 124600 | 257400 |
| 59680 | 125700 | 243700 | |
| 61770 | 125600 | 260700 | |
| 56620 | 116700 | 246000 | |
| 58730 | 122100 | 255100 | |
| 55830 | 115400 | 253600 | |
| 58977 | 121683 | 252750 | |
| SD | 2404 | 4571 | 6611 |
| CV | 0.0408 | 0.0376 | 0.0262 |
|
| |||
4.4. Instrument response to the analyte
The data in Table 4.3. are presented graphically in Figure 4.4. This figure is a calibration curve over the concentration range of 0.5 to 2 times the target concentration. The instrument response was linear over this range.
4.5. Breakthrough data
- 4.5.1. Several breakthrough studies were performed with the
recommended collection device by sampling controlled test
atmospheres containing phosgene in air. The average phosgene
concentration was 0.7 ppm (7 times the target concentration) and the
average relative humidity was 78% at 23°C. The sampling rate was 1
L/min. Breakthrough was defined as the amount of phosgene found on
the backup section divided by the total amount of phosgene collected
on the entire sampling tube. When the results of these breakthrough
studies were combined, 5% breakthrough occurred after sampling for
275 min. At the end of this time, 275 L of air had been sampled and
778 µg of phosgene had been collected. The results of these studies
are presented in Table 4.5.1.
Phosgene Breakthrough Data
|
| |||
| air volume | breakthrough | air volume | breakthrough |
| (L) | (percent) | (L) | (percent) |
|
| |||
| 242.1 | 2.7 | 273.5 | 4.8 |
| 252.9 | 5.5 | 291.8 | 7.9 |
| 257.4 | 3.4 | 294.3 | 5.1 |
| 256.1 | 4.4 | 357.1 | 8.4 |
| 267.5 | 6.6 | 379.8 | 8.7 |
| 270.5 | 4.3 | ||
|
| |||
4.5.2. The effects of reduced relative humidity on the sampling device were investigated by sampling test atmospheres containing phosgene in air with relative humidities of less than 80%. The average phosgene concentration of these atmospheres was 0.3 ppm (3 times the target concentration). Samples were taken at 0.1, 0.5 and 1 L/min. In addition to the recommended sampling device, Supelco sampling tubes were included in these studies. Breakthrough (bt) was defined as before. The sampling tube source (tube), the sampling rates (in L/min), the relative humidities (the average air temperature was 25 ± 2.2°C), the air volumes and the analytical results are presented in Table 4.5.2. Some of the test atmospheres were prepared using dilution air that was essentially dry (Section 4.9.). The relative humidities of these atmospheres were less than the lower limit of the operating range (12 to 100%) of the hygrometer. The relative humidities of these atmospheres are presented as BOR (below operating range).
Reduced Relative Humidity Study
|
| |||||
| tube | sampling | relative | air | percent of | percent |
| rate | humidity | volume(L) | theoretical | bt | |
|
| |||||
| SLCAL | 0.1 | 67 | 24.0 | 96.8 | 0.0 |
| SLCAL | 0.5 | 67 | 120.0 | 95.4 | 0.0 |
| SLCAL | 1.0 | 67 | 240.0 | 103.0 | 5.0 |
| Supelco | 0.1 | 67 | 24.0 | 73.0 | 0.0 |
| Supelco | 0.5 | 67 | 120.0 | 68.0 | 0.0 |
| Supelco | 1.0 | 67 | 240.0 | 58.4 | 19.0 |
| SLCAL | 0.1 | 34 | 24.0 | 109.8 | 0.0 |
| SLCAL | 0.5 | 34 | 120.0 | 102.4 | 0.0 |
| SLCAL | 1.0 | 34 | 240.0 | 105.5 | 3.6 |
| Supelco | 0.1 | 34 | 24.0 | 85.2 | 0.0 |
| Supelco | 0.5 | 34 | 120.0 | 83.7 | 0.0 |
| Supelco | 1.0 | 34 | 240.0 | 80.5 | 8.3 |
| SLCAL | 0.1 | BOR | 24.0 | 95.4 | 0.0 |
| SLCAL | 0.5 | BOR | 120.0 | 107.5 | 1.5 |
| SLCAL | 1.0 | BOR | 240.0 | 97.0 | 25.6 |
| Supelco | 0.1 | BOR | 24.0 | 87.9 | 0.0 |
| Supelco | 0.5 | BOR | 120.0 | 97.1 | 4.5 |
| Supelco | 1.0 | BOR | 240.0 | 76.9 | 34.0 |
|
| |||||
Two other studies were performed to determine if the Supelco tube recoveries could be improved. In one study, the Supelco adsorbent was repacked from the Supelco tubes (120/60-mg sections) into different glass tubing to SLCAL tube size specifications (150/75-mg sections). No improvement in recovery was observed, however, breakthrough was less for the repacked tubes than for the original tubes. In the other study, the Supelco tubes were stored for 3 days at ambient temperature before analysis. There was no improvement in recovery in these samples when they were compared to samples which were analyzed immediately.
The reason for the low phosgene recoveries obtained with the Supelco tubes is unknown. Analytical standards that were prepared using Supelco adsorbent gave similar results as those prepared using SLCAL adsorbent.
- 4.6. Desorption efficiency and stability of desorbed samples
- 4.6.1. Desorption efficiency
The desorption efficiency of phosgene was determined by spiking
the gas into sealed 2-mL glass vials containing 150-mg portions of
coated XAD-2 adsorbent. Six samples were prepared at each of three
concentrations. Analytical standards were prepared by injecting
equivalent amounts of phosgene into 1-mL aliquots of toluene
containing 15 mg/mL
The Desorption Efficiency
of Phosgene From XAD-2 Coated with 10%
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 53.0 | 97.7 | 196.0 |
|
| |||
| % desorption | 100.0 | 93.8 | 95.0 |
| efficiency | 94.4 | 93.1 | 91.8 |
| 97.4 | 93.4 | 93.9 | |
| 95.4 | 93.8 | 95.3 | |
| 94.9 | 94.2 | 95.7 | |
| 96.8 | 96.7 | 93.1 | |
| 96.5 | 94.2 | 94.1 | |
|
| |||
| The average desorption efficiency over the studied range was 94.9%. | |||
4.6.2. Stability of desorbed samples
The stability of desorbed samples was investigated by reanalyzing the target concentration desorption samples following 16 h of storage at room temperature. Fresh standards were used and the sample vials were resealed immediately after the first analysis. The average recovery, relative to the original analysis, was 98.0%.
The Stability of Desorbed Samples
|
| |||||||
| sample no. | 1 | 2 | 3 | 4 | 5 | 6 | |
|
| |||||||
| original analysis | (µg) | 91.6 | 91.0 | 91.3 | 91.6 | 92.0 | 94.5 |
| reanalysis | (µg) | 90.1 | 88.0 | 89.5 | 89.8 | 90.5 | 92.8 |
| percent of original | (%) | 98.4 | 96.7 | 98.0 | 98.0 | 98.4 | 98.2 |
|
| |||||||
- 4.7. Storage test
Thirty-six storage samples were generated by sampling a controlled test atmosphere containing 0.4 ppm of phosgene in air for 60 min at 1 L/min. The relative humidity of the sampled air was 61% at 23°C. The samples were stored either at ambient temperature or at -20°C and analyzed at periodic intervals. The results of the storage test are presented in Table 4.7. and are shown graphically in Figures 4.7.1. and 4.7.2.
Storage Tests
|
| |||||||
| storage time | ambient recovery | refrigerated recovery | |||||
| (days) | (percent) | (percent) | |||||
|
| |||||||
| 0 | 103.8 | 101.9 | 99.1 | 101.5 | 101.7 | 108.8 | |
| 4 | 101.9 | 106.4 | 105.9 | 100.1 | 103.3 | 106.6 | |
| 7 | 105.7 | 108.5 | 104.6 | 104.5 | 107.9 | 106.0 | |
| 11 | 109.8 | 105.8 | 106.8 | 103.2 | 105.8 | 105.1 | |
| 14 | 96.9 | 106.6 | 90.7 | 103.3 | 101.4 | 101.8 | |
| 19 | 100.4 | 102.1 | 102.1 | 103.0 | 102.3 | 102.6 | |
|
| |||||||
4.8. Reproducibility data
Reproducibility samples were generated by sampling a controlled test atmosphere containing 0.4 ppm of phosgene in air for 60 min at 1 L/min. The relative humidity of the sampled air was 45% at 26°C. The samples and a draft copy of this evaluation here given to a chemist unassociated with this evaluation. The samples were analyzed after 1 day of storage at ambient temperature. No individual sample deviated from its theoretical value by more than the precision (±13%) at the 95% confidence level for the 19-day storage test (Section 4.7.).
Reproducibility Results
|
| |||
| sample | theoretical | analytical | percent |
| no. | amount (µg) | results (µg) | recovery |
|
| |||
| 1 | 103.6 | 111.9 | 108.0 |
| 2 | 97.8 | 100.0 | 102.2 |
| 3 | 100.3 | 107.5 | 107.2 |
| 4 | 103.9 | 101.9 | 98.1 |
| 5 | 106.7 | 105.5 | 98.9 |
| 6 | 101.9 | 106.5 | 104.5 |
|
| |||
4.9. Generation of controlled test atmospheres
The controlled test atmospheres which were used in this evaluation were generated by diluting phosgene gas with air. The source of the phosgene was a compressed-gas cylinder which contained 105 ppm of phosgene in nitrogen. The gas cylinder was obtained from the Linde Division of Union Carbide. Phosgene and dilution air were introduced into the generation apparatus, which was constructed from glass and Teflon tubing, using precision, calibrated rotameters. The clean, dry dilution air was humidified, if desired, by passing it through a water bubbler prior to its entering the dilution stream. The water bubbler was contained in a temperature-controlled water bath. The relative humidity of the dilution air could be varied by changing the temperature of the water bath. If dry dilution air was required, the water bubbler was not used. The relative humidity of the test atmosphere was monitored, after mixing, with a YSI Model 91 Dew Point Hygrometer. The test atmosphere passed through a manifold from which samples could be collected.
The phosgene concentration of the test atmosphere was adjusted to the desired level by varying the gas flow rates from the calibrated rotameters.
The theoretical concentrations of the test atmospheres were determined using the manufacturer's concentration of the phosgene gas cylinder and the flow rates from the calibrated rotameters. The average analysis of "day zero" samples, used in the storage tests for this evaluation, was 102.8% of the theoretical concentration. The analytical standards used were prepared with a different, concentrated (99%) phosgene gas cylinder.
4.10. A procedure to coat XAD-2 adsorbent with
- 4.10.1. Apparatus
- 4.10.1.1. Soxhlet extractor
4.10.1.2. Rotary evaporator
4.10.1.3. Miscellaneous glassware: One-liter vacuum flask, 1-L round-bottomed evaporative flask, 1-L Erlenmeyer flask, 250-mL Buchner funnel with a coarse fritted disc, etc.
- 4.10.2. Reagents
- 4.10.2.1. Methanol, isooctane and toluene. Burdick and Jackson
solvents were used in this evaluation.
4.10.2.2. 2-(hydroxymethyl)piperidine. Technical grade
4.10.2.3. Amberlite XAD-2 non-ionic polymeric adsorbent, 20 to 60 mesh. Aldrich Chemical XAD-2 adsorbent was used in this evaluation.
- 4.10.3. Procedure
This procedure is very similar to the one described in Section 4.8. of OSHA Method 52: Acrolein and/or Formaldehyde (Ref. 5.1.). Coated adsorbent prepared by the procedure described below can be used for Method 52, if the formaldehyde blank level is determined to be sufficiently low.
Weigh 125 g of crude XAD-2 adsorbent into a 1-L Erlenmeyer flask.
Add about 200 mL of water to the flask and then swirl the mixture to
wash the adsorbent. Discard any adsorbent that floats to the top of
the water and then filter the mixture using a fritted Buchner
funnel. Transfer the adsorbent back to the Erlenmeyer flask and
repeat the water wash and the filtration. Air dry the adsorbent for
about 2 min. Transfer the adsorbent back to the Erlenmeyer flask and
add about 200 mL of methanol to the flask. Swirl and filter the
mixture as before. Transfer the washed adsorbent to a 1-L
evaporative flask and remove the methanol using the rotary
evaporation apparatus. Cool the flask to room temperature and add 13
g of
The coated adsorbent is now ready to be packed into sampling tubes. Currently, SLCAL is contracting with SKC, Inc. to pack sampling tubes with SLCAL-prepared adsorbent. The sampling tubes should be stored in the dark and segregated by lot number. A sufficient amount of each lot of coated adsorbent should be retained to prepare analytical standards for use with air samples from that lot number.