COAL TAR PITCH VOLATILES (CTPV)
COKE OVEN EMISSIONS
(COE)
SELECTED POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)
| Method no.: | 58 |
| Matrix: | Air |
| Procedure: | Air samples are collected by drawing known amounts of air
through cassettes containing glass fiber filters (GFF). The filters
are analyzed by extracting with benzene and gravimetrically
determining the |
| Recommended air volume and sampling rate: |
960 L at 2.0 L/min |
| Special requirements: | Each GFF must be transferred to a separate scintillation vial
after sampling and the vial sealed with a |
| Status of method: | Evaluated method. This method that has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: July 1986 | Chemist: Donald Burright |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
| Target concentrations: | 0.20 mg/m3 for Coal Tar Pitch
Volatiles (PEL) 0.15 mg/m3 for Coke Oven Emissions (PEL) 8.88 µg/m3 (1.22 ppm) for phenanthrene 0.79 µg/m3 (0.11 ppm) for anthracene 9.00 µg/m3 (1.09 ppm) for pyrene 3.27 µg/m3 (0.35 ppm) for chrysene 2.49 µg/m3 (0.24 ppm) for benzo(a)pyrene |
| Detection limits of the overall procedure: |
0.006 mg/m3 for BSF 0.427 µg/m3 (59 ppb) for phenanthrene (PHEN) 0.028 µg/m3 ( 4 ppb) for anthracene (ANTH) 0.260 µg/m3 (31 ppb) for pyrene (PYR) 0.073 µg/m3 ( 8 ppb) for chrysene (CHRY) 0.045 µg/m3 ( 4 ppb) for benzo(a)pyrene (BaP) |
| Reliable quantitation limits: | 0.034 mg/m3 for
BSF 0.740 µg/m3 (100 ppb) for PHEN 0.066 µg/m3 ( 9 ppb) for ANTH 1.13 µg/m3 (140 ppb) for PYR 0.273 µg/m3 ( 29 ppb) for CHRY 0.207 µg/m3 ( 20 ppb) for BaP |
| Standard errors of estimate at the target concentration: (Section 4.6.) |
8.3% for BSF 6.0% for PHEN 6.8% for ANTH 6.7% for PYR 6.3% for CHRY 5.8% for BaP |
1. General Discussion
-
Samples are collected closed-face with a two-piece cassette containing a GFF and a backup pad. A
three-piece cassette is not necessary. -
The GFF is removed from the cassette and placed in a glass vial which is sealed with a cap containing a polytetrafluoroethylene (PTFE) liner before shipment. This increases the recovery of the analytes over the old procedure.
-
The total extraction volume is reduced from 10 mL to 3 mL. This eliminates the concentration step of the old procedure (concentration to 1 mL) and greatly improves the recovery and precision.
-
The extracted samples are filtered through pure PTFE membrane filters instead of
fritted-glass filter funnels. Blank corrections, which were30-70 µg with the old procedure, are reduced to 5-20 µg.
1.1. Background
1.1.1. History
Coal tar pitch volatiles (CTPV) include the fused polycyclic hydrocarbons which volatilize from the distillation residues of coal, petroleum (excluding asphalt), wood, and other organic matter (Ref. 5.1.). Coke oven emissions (COE) are the benzene-soluble fraction (BSF) of total particulate matter present during the destructive distillation or carbonization of coal for the production of coke (Ref. 5.2.). Coal tar is obtained by the distillation of bituminous coal (Ref. 5.3.). Coal tar pitch is composed almost entirely of polynuclear aromatic compounds and constitutes 48-65% of the usual grades of coal tar (Ref. 5.3.)
The purpose of this work was to evaluate the sampling and
analytical method routinely used by OSHA, and to make appropriate
modifications if necessary. That method required samples be
collected with glass fiber filters (GFF) in three-piece polystyrene
cassettes. The sealed cassettes were shipped to the laboratory at
ambient temperature and upon receipt were stored in a refrigerator
until analyzed. The GFFs were placed in test tubes containing
benzene and sonicated for 20 min. The resulting solutions were
filtered with fine fritted glass filter funnels. The GFFs were then
rinsed twice with benzene and the filtered rinses combined with the
original extract. The benzene extracts were concentrated to 1 mL. A
Alternate samplers were not considered because the OSHA standard defines CTPV and COE as a function of those components that collected on a GFF. However, the following modifications were made to the previous procedure to reduce costs and improve the sensitivity and precision:
The modified procedure resulting from this evaluation requires
that the GFFs be removed from the polystyrene cassettes before
shipment and placed in sealed vials. Three milliliters of benzene
are added to the sample vials and then the vials are placed in a
mechanical shaker and shaken for 1 h. The resulting solutions are
filtered through pure PTFE membrane filters. One and
The selected PAHs used in this evaluation are phenanthrene (PHEN), anthracene (ANTH), pyrene (PYR), chrysene (CHRY), and benzo(a)pyrene (BaP). These compounds are analyzed by HPLC and are marker compounds to indicate the presence of PAHs. The presence of BaP, identified by GC/MS, is used to confirm the presence of CTPV or COE when the BSF exceeds the appropriate PEL.
1.1.2. Toxic effects (This section is for information only and should not be taken as a basis for OSHA policy.)
The following information was reported in "Occupational Health Guidelines for Chemical Hazards". (Ref. 5.4.)
Coal tar pitch volatiles (CTPV) are products of the destructive
distillation of bituminous coal and contain polynuclear aromatic
hydrocarbons (PNA's). These hydrocarbons sublime readily, thereby
increasing the amounts of carcinogenic compounds in the working
areas. Epidemiologic evidence suggests that workers intimately
exposed to the products of combustion or distillation of
bituminous coal are at risk of cancer at many sites. These include
cancer of the respiratory tract, kidney, bladder, and skin. In a
study of coke oven workers, the level of exposure to CTPV and the
length of time exposed were related to the development of cancer.
Coke oven workers with the highest risk of cancer were those
employed exclusively at topside jobs for 5 or more years, for whom
the increased risk of dying from lung cancer was
1.1.3. Operations where exposure may occur
In 1970, there were over 13,000 coke ovens in operation in the United States. It is estimated that approximately 10,000 persons are potentially exposed to COE. (Ref. 5.5.)
Coal tar pitch is used in metal and foundry operations, electrical equipment installations, pipe coating operations, and at construction sites. About 145,000 people are potentially exposed to CTPV. (Ref. 5.6.)
The PAHs that were studied in this evaluation have been found in many substances. These include coke oven emissions, coal tar pitch, creosote, exhaust of internal combustion engines, and cooked meats. benzo(a)pyrene and chrysene have also been isolated from cigarette smoke. (Refs. 5.5.-5.7.)
1.1.4. Physical properties (Ref.
5.8.)
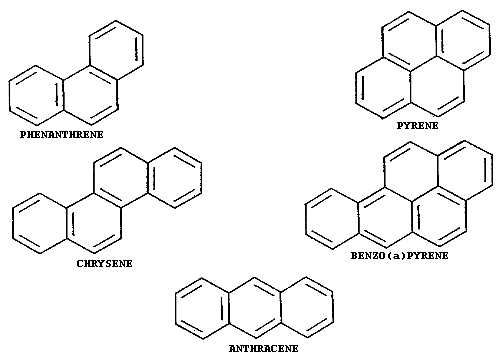
| Phenanthrene | |
| CAS no.: | 85-01-8 |
| MW: | 178.22 |
| bp: | 340°C at 760 mm Hg |
| mp: | 100°C |
| color: | white crystals |
| structure: | Figure 1.1.4. |
| Anthracene | |
| CAS no.: | 120-12-7 |
| MW: | 178.22 |
| bp: | 342°C at 760 mm Hg |
| mp: | 218°C |
| color: | colorless crystals |
| structure: | Figure 1.1.4. |
| Pyrene | |
| CAS no.: | 129-00-0 |
| MW: | 202.24 |
| bp: | 404°C at 760 mm Hg |
| mp: | 156°C |
| color: | colorless crystals |
| synonyms: | benzo(def)phenanthrene |
| structure: | Figure 1.1.4. |
| Chrysene | |
| CAS no.: | 218-01-9 |
| MW: | 228.28 |
| bp: | 448°C at 760 mm Hg |
| mp: | 254°C |
| color: | white crystals |
| synonyms: | 1,2-benzophenanthrene; benzo(a)phenanthrene |
| structure: | Figure 1.1.4. |
| benzo(a)pyrene | |
| CAS no.: | 50-32-8 |
| MW: | 252.30 |
| bp: | 311°C at 10 mm Hg |
| mp: | 179°C |
| color: | yellow needles |
| synonyms: | 3,4-benzopyrene; 6,7-benzopyrene |
| structure: | Figure 1.1.4. |
| Benzene-soluble fraction (The sum of those components collected on a GFF and soluble in benzene.) | |
| color: | brownish-yellow to black tar |
1.2. Limit defining parameters (The analyte air concentrations listed throughout this method are based on an air volume of 960 L and a solvent extraction volume of 3 mL. Air concentrations listed in ppm are referenced to 25°C and 760 mm Hg.)
1.2.1. Detection limits of the analytical procedure
1.2.1.1. Benzene-soluble fraction
The detection limit of the analytical procedure is 6 µg per
sample and is based on the precision of the analytical balance
used. This is the weight which corresponds to twice the standard
deviation of the precision data for a
1.2.1.2. Selected PAHs
The detection limits of the analytical procedure are listed
below. These are the amounts of analyte which will give a peak
whose height is about five times the height of the baseline noise.
(Section
4.1.2.)
Table 1.2.1.2.
Analytical Detection Limits
|
| ||
| compound | ng/injection | detector* |
|
| ||
| PHEN PHEN ANTH PYR CHRY BaP |
0.132 0.910 0.090 0.960 0.386 0.175 |
UV(254
nm) FL FL FL FL FL |
|
| ||
| * Fluorescence was more sensitive than UV for each PAH except PHEN | ||
1.2.2. Detection limits of the overall procedure
The detection limits of the overall procedure are listed below. These are the amounts of analyte, determined from Figures 4.2.1.-4.2.6., which when spiked onto the sampling device would allow recovery of an amount of analyte equivalent to the detection limits of the analytical procedure. (Section 4.2.)
Table 1.2.2.
Detection Limits of the Overall
Procedure
|
| ||||||
| BSF | PHEN | ANTH | PYR | CHRY | BaP | |
|
| ||||||
| µg/sample µg/m3 ppb |
6 6 -- |
0.41 0.43 59 |
0.027 0.028 4 |
0.25 0.26 31 |
0.070 0.073 8 |
0.043 0.045 4 |
|
| ||||||
1.2.3. Reliable quantitation limits
The reliable quantitation limits are listed below. These are the smallest amounts of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (±1.96 SD) of ±25% or better. (Section 4.3.)
Table 1.2.3.
Reliable Quantitation Limits
|
| ||||||
| BSF | PHEN | ANTH | PYR | CHRY | BaP | |
|
| ||||||
| µg/sample µg/m3 ppb |
33.1 34.5 -- |
0.71 0.74 100 |
0.064 0.066 9 |
1.08 1.13 140 |
0.262 0.273 29 |
0.199 0.207 20 |
|
| ||||||
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters
1.2.4. Sensitivities
The sensitivities of the analytical procedure over a concentration range representing about 0.5 to 2 times the target concentrations are listed below. These values were determined by the slope of the calibration curves. (Section 4.4.) The sensitivity will vary with the particular instrument used in the analysis. The values listed were obtained using an µL detector.
Table 1.2.4.
Sensitivities of Selected PAHs
|
| |
| compound | area counts/(µg/mL) |
|
| |
| PHEN ANTH PYR CHRY BaP |
19000 178000 21100 58900 125000 |
|
| |
1.2.5. Recoveries
The recovery of analytes from samples stored in vials used in the 15-day storage test remained above the percentages listed below. (Section 4.6.) The recovery of the analytes from the collection medium during storage must be 75% or greater.
Table 1.2.5.
Recoveries from Ambient Storage
|
| |
| compound | % recovery |
|
| |
| BSF PHEN ANTH PYR CHRY BaP |
89.4 92.2 90.7 86.9 96.2 99.9 |
|
| |
1.2.6. Precisions (analytical procedure)The pooled coefficients of variation obtained from replicate determinations of analytical standards at about 0.5 to 2 times the target concentration are shown below. The values were obtained using an µL detector. (Section 4.4.)
Table 1.2.6.
Analytical Precision
|
| |
| compound | CV |
|
| |
| PHEN ANTH PYR CHRY BaP |
0.0092 0.0051 0.0128 0.0094 0.0150 |
|
| |
1.2.7. Precisions (overall procedure)
The precisions at the 95% confidence level for the 15-day ambient storage tests are listed below. (Section 4.6.) These include an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
Table 1.2.7.
Precision of the Overall
Procedure
|
| |
| compound | percent |
|
| |
| BSF PHEN ANTH PYR CHRY BaP |
16.2 11.8 13.4 13.0 12.3 11.3 |
|
| |
1.2.8. Reproducibilities
Six samples, spiked with coal tar by liquid injection, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 21 days of storage at about 22°C. Another set of six samples, spiked with PAHs by liquid injection, and a draft copy of this procedure were given to another chemist unassociated with this evaluation. The samples were analyzed after 3 days of storage at about 22°C. The average recoveries are listed below. (Section 4.7.)
Table 1.2.8.
Reproducibilities
|
| ||
| compound | mean | percent |
|
| ||
| BSF PHEN ANTH PYR CHRY BaP |
94.2 98.0 90.4 101.4 98.7 100.6 |
5.4 3.4 2.4 3.4 2.7 3.0 |
|
| ||
1.3. Advantages
1.3.1. Recovery of the analytes is improved by placing the GFF in sealed glass vials before shipment.
1.3.2. The amount of benzene required for each sample is reduced from 10 mL to 3 mL per sample. This reduces the exposure to a suspected human carcinogen.
1.3.3. The reliable quantitation limits are much lower than those of the previously used procedure.
1.3.4. The use of pure PTFE membrane filters, instead of fritted glass filter funnels, lowers the blank correction and provides much better precision.
1.3.5. The amount of time samples spend in the nitrogen evaporator for the previous procedure is eliminated, a savings of about 2 h.
1.4. Disadvantages
The GFF must be transferred from the cassette to a scintillation vial by the industrial hygienist.
2. Sampling Procedure
2.1. Apparatus
2.1.1. A personal sampling pump that can be calibrated to within ±5% of the recommended flow rate with the sampling device in line.
2.1.2. A two-piece cassette containing a glass fiber filter is the sampling device.
2.1.3. Forceps to transfer the GFF to a scintillation vial.
2.1.4. Scintillation vials with PTFE-lined caps.
2.1.5. Aluminum foil or an opaque container to protect collected samples from light.
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
2.3.1. Attach the cassette to the sampling pump with flexible, plastic tubing so that the GFF in the sampling cassette is exposed directly to the atmosphere. Do not place any tubing in front of the sampler. The sampler should be attached vertically in the worker's breathing zone in such a manner that it does not impede work performance. The sampling device should be protected from direct sunlight (Ref. 5.9.).
2.3.2. After sampling for the appropriate time, remove the sampling device and install the two plastic plugs in the open ends of the cassette.
2.3.3. As soon as it is conveniently possible, but before the
sample is shipped, fold the filter into quarters (sampling surface
inside) and insert it into a scintillation vial (Figure
2.3.3.). Always handle the GFF with clean forceps. To avoid
losing any particulate material, the inside of the cassette should
be wiped with the folded filter. Install a cap that has a PTFE
liner, not a
2.3.4. Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.5. Submit at least one blank with each set of samples. The blank should be handled the same as the other samples except that no air is drawn through it.
2.4. Extraction efficiencies
The average extraction efficiencies of the analytes are listed below. The target concentrations were used for this determination. (Section 4.5.)
Table 2.4.
Extraction Efficiency from GFF
|
| |
| compound | percent |
|
| |
| BSF PHEN ANTH PYR CHRY BaP |
100.3 105.9 112.5 101.4 107.5 108.7 |
|
| |
2.5. Recommended air volume and sampling rate
2.5.1. The recommended air volume is 960 L.
2.5.2. The recommended air sampling rate is 2.0 L/min.
2.6. Interferences (sampling)
Suspected interferences should be reported to the laboratory with submitted samples.
2.7. Safety precautions (sampling)
The sampling equipment should be attached to the worker in such a manner that it will not interfere with work performance or safety.
3. Analytical Procedure
3.1. Apparatus
3.1.1. Benzene-soluble fraction
3.1.1.1. A calibrated microbalance capable of determining a
weight to the nearest microgram. A Mettler
3.1.1.2. Thirteen-millimeter stainless steel filter holder with a female Luer-Lok fitting.
3.1.1.3. Thirteen-millimeter pure PTFE membrane filters with 5-µm pores.
3.1.1.4. Two-milliliter PTFE cups, Cahn Scientific.
3.1.1.5. Two-milliliter disposable pipets.
3.1.1.6. Ten-milliliter glass syringe barrels with male Luer-Lok fittings.
3.1.1.7. Disposable culture tubes (13 × 100 mm).
3.1.1.8. Vacuum oven.
3.1.1.9. Mechanical shaker.
3.1.1.10. Forceps.
3.1.2. Selected PAHs
3.1.2.1. High performance liquid chromatograph equipped with a
fluorescence (µL) or an ultraviolet (UV) detector, manual or
automatic injector, gradient flow programmer and chart recorder. A
Waters
3.1.2.2. HPLC column capable of separating PAHs from any
interferences. A
3.1.2.3. An electronic integrator, or some other suitable method of measuring detector response.
3.1.2.4. Vials, 4-mL with PTFE-lined caps.
3.1.2.5. Volumetric flasks, pipets, and syringes.
3.2. Reagents
3.2.1. Acetonitrile (ACN), HPLG grade.
3.2.2. Water, HPLC grade. A Millipore Milli-Q system was used to prepare the water for this evaluation.
3.2.3. Benzene, HPLC grade.
3.2.4. Nitrogen gas.
3.2.5. Phenanthrene (PHEN).
3.2.6. Anthracene (ANTH).
3.2.7. Pyrene (PYR).
3.2.8. Chrysene (CHRY).
3.2.9. benzo(a)pyrene (BaP).
3.2.10. Tetrahydrofuran (THF), HPLC grade.
3.3. Standard preparation for selected PAHs
A stock standard solution is prepared by dissolving the PAHs in benzene. All dilutions of the stock solutions are made with benzene to arrive at the working range.
3.4. Sample preparation
3.4.1. Benzene-soluble fraction (CAUTION - All work with benzene must be done in a fume hood.)
3.4.1.1. Clean the PTFE cups by sonicating them in THF for a few minutes, and rinsing them twice with clean THF. Place the cups into a numbered holder. The cups are placed in a preheated oven (40°C under about 20 in. Hg vacuum) for 1 h. The cups are allowed to cool to room temperature and weighed to the nearest microgram. Handle the cups with clean, dry forceps.
3.4.1.2. Pipet 3.0 mL of benzene to each scintillation vial containing the sample filter.
3.4.1.3. Shake the vials for 60 min.
3.4.1.4. Insert a 13-mm pure PTFE membrane filter (5-µm) into the stainless steel holder and attach the holder to a syringe barrel. Add about 3 mL of benzene to the syringe and push the benzene through the filtering unit with nitrogen to check for leaks. A rubber stopper is used on the nitrogen line to pressurize the syringe barrel to 10 psig. Dry the filter by allowing the nitrogen to pass through the filter for 30 s.
3.4.1.5. Transfer the benzene extract from the vial into the syringe barrel, one sample per syringe. If the vial contains a considerable amount of particulate material, decant the extract into the syringe barrel. Push the benzene extract thru the filters into the disposable culture tube (13 × 100 mm) with nitrogen gas.
3.4.1.6. Pipet 1.5 mL of the benzene extract to a tared PTFE cup.
3.4.1.7. Place the PTFE cups in a preheated oven (40°C under about 15 in. Hg vacuum). Provide some air flow in the oven to sweep benzene vapor out of the oven. Heat the cups for about 3 to 4 h. Close the vent valve for the last hour of the drying period.
3.4.1.8. Remove the PTFE cups from the oven and allow them to cool to room temperature. Weigh the cups to the nearest microgram.
3.4.2. Selected PAHs
Transfer the remaining benzene solution from the culture tube to
a vial and seal with a
3.5. Analysis
3.5.1. Reverse phase HPLC conditions
| column: | |
| mobile phase: | 85:15 ACN/water (v/v) |
| flow rate: | 1.0 mL/min for 5 min, Curve 10 (flow program) for 5 min to 1.5 mL/min, then hold for 10 min |
| µL detector: | 254 nm excitation 370 nm emission |
| UV detector: | 254 nm |
| injection size: | 10 µL |
| retention time: | 7-18 min |
| chromatogram: | Figure 3.5.1. |
3.5.2. An external standard procedure is used to prepare a calibration curve using at least 2 stock solutions from which dilutions are made. The calibration curve is prepared daily. The samples are bracketed with analytical standards.
3.6. Interferences (analytical)
3.6.1. Benzene-soluble fraction
3.6.1.1. Any compound that is soluble in benzene and is not normally found in coal tar pitch volatiles or coke oven emissions is an interference. Anything that falls into or adheres to the PTFE cups during the time between weighings will give high results.
3.6.1.2. It has been reported that mineral oil is an
interference with the BSF determination in the aluminum industry
(Ref. 5.10.). The problem of separating mineral oil from the BSF
was not addressed in this evaluation but a status report from the
Aluminum Association Health Committee showed that the ANCAL
IATROSCAN
3.6.2. Selected PAHs
3.6.2.1. Any compound having a similar retention time as the PAHs is a potential interference. Generally, chromatographic conditions can be altered to separate an interference from the analyte.
3.6.2.2. Retention time on a single column is not proof of chemical identity. Analysis by an alternate HPLC column, absorbance response ratioing, and mass spectrometry are additional means of identification.
3.7. Calculations
3.7.1. Benzene-soluble fraction
The concentration in µg/m3 of BSF present in a sample is determined from the two weighings (in micrograms) of the PTFE cup. The factor "2" in the equation compensates for the fact that only 1/2 of the sample was used for the gravimetric procedure.
| µg/m3 = | 2[(final wt - tare wt) -
(blank final wt - tare wt)]
(air volume, m3) |
3.7.2. Selected PAHs
The concentration in µg/mL of the PAHs present in a sample is determined from the detector response of the analytes. Comparison of sample response with a least squares curve fit for standards allows the analyst to determine the concentration of the PAHs in µg/mL for the sample. Since the total sample volume was 3 mL, the results in µg/m3 of air are expressed by the following equation:
µg/m3 = 3 mL(µg/mL)/[(air vol., m3)(extrac. effic.)]
This value can be converted to an equivalent concentration in parts per million with the following equation:
ppm = (mg/m3)(24.46)/MW
| where | 24.46 MW |
= = |
molar volume at 25°C and 760 mm Hg molecular weight of PAH |
3.8. Safety precautions (analytical)
3.8.1. Avoid exposure to all standards.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
3.8.4. All work with benzene is to be performed in a fume hood. Benzene is a suspected human carcinogen.
4. Backup Data
4.1. Detection limit of the analytical procedure
4.1.1. Benzene-soluble fraction
The detection limit of the analytical procedure is 6 µg per
sample. This is the weight which corresponds to twice the standard
deviation of the precision data for a
4.1.2. Selected PAHs
The detection limits of the analytical procedure are listed below and were determined by injecting 10 µL of a standard. These amounts produced peaks whose heights were about 5 times the height of the baseline noise. The injection volume recommended in the analytical procedure (10 µL) was used in the determination of the detection limits for the analytical procedure. (Figures 4.1.2.1.-4.1.2.5. show chromatographs obtained with the µL detector.)
Table 4.1.2.
Analytical Detection Limits
|
| |||
| compound | µg/mL | ng/injection | detector |
|
| |||
| BSF PHEN ANTH PYR CHRY BaP |
0.0132 0.0910 0.0090 0.0960 0.0386 0.0175 |
0.132 0.910 0.090 0.960 0.386 0.175 |
UV (254
nm) FL FL FL FL FL |
|
| |||
4.2. Detection limit of the overall procedure
The detection limits of the overall procedure are listed in Table 4.2. The values were determined graphically (Figures 4.2.1.-4.2.6.) by plotting amount spiked versus amount recovered and determining the amount that corresponds to the analytical detection limit. The values listed are based on an µL detector.
Table 4.2.
Detection Limits of the Overall
Procedure
|
| ||||||
| BSF | PHEN | ANTH | PYR | CHRY | BaP | |
|
| ||||||
| µg/sample µg/m3 ppb |
6 6 -- |
0.41 0.43 59 |
0.027 0.028 4 |
0.25 0.26 31 |
0.070 0.073 8 |
0.043 0.045 4 |
|
| ||||||
4.3. Reliable quantitation limit
4.3.1. Benzene-soluble fraction
The reliable quantitation limit is 33.1 µg (34.5 µg/m3) of BSF per sample. Seven samples were prepared by injecting 4 µL of a coal tar solution (8.28 mg/mL) onto GFFs. The samples were analyzed the same day and the average results are reported in Table 4.3.1.
Table 4.3.1.
Reliable Quantitation Limit
|
| |
| Sample | % Recovery |
|
| |
| 1 2 3 4 5 6 7 SD = 8.7 |
99.7 93.7 86.6 99.7 105.7 81.6 87.6 1.96 SD = 17.1 |
|
| |
4.3.2. Selected PAHs
The reliable quantitation limits are listed below. Six samples were prepared by injecting several microliters of a benzene solution containing PAHs onto GFFs. The samples were analyzed the same day.
Table 4.3.2.
Reliable Quantitation Limit
|
| |||||
| analyte spike (µg) (µg/m3) (ppb) |
PHEN 0.71 0.74 100 |
ANTH 0.064 0.066 9 |
PYR 1.08 1.13 140 |
CHRY 0.262 0.273 29 |
BaP 0.199 0.207 20 |
|
| |||||
| %
recovery SD 1.96 SD |
94.7 92.7 91.1 89.9 91.0 97.9 92.9 3.0 5.8 |
90.1 91.2 89.4 86.4 87.0 87.0 88.5 2.0 3.9 |
91.1 102.0 92.2 93.9 82.3 86.9 91.4 6.7 13.1 |
93.3 96.3 97.5 94.9 93.8 97.0 95.5 1.7 3.4 |
97.0 105.4 102.5 98.6 99.3 95.7 99.8 3.6 7.1 |
|
| |||||
4.4. Sensitivity and precision (analytical method only)
4.4.1. Precision data for the benzene-soluble fraction
The following data were obtained from multiple weighings of calibration weights that are approximately 0.5 to 2 times the nominal weight of a PTFE cup. This was done to establish the precision of the analytical balance.
Table 4.4.1.
Precision Data
|
| |||
| 25 mg | 50 mg | 100 mg | |
|
| |||
SD CV |
25.005 25.003 25.007 25.007 25.005 25.006 25.005 25.006 25.008 25.006 25.006 0.0014 0.00006 |
49.991 49.990 49.993 49.993 49.993 49.994 49.992 49.992 49.994 49.994 49.993 0.0015 0.00003 |
99.998 100.001 100.001 100.001 100.002 100.002 100.000 100.000 100.000 100.003 100.001 0.0013 0.00001 |
|
| |||
4.4.2. Sensitivity and precision data for selected PAHs
The following data were obtained from multiple injections of analytical standards. This data was used to establish calibration curves for each analyte from which the sensitivity was determined. The data are also presented graphically in Figures 4.4.2.1.-4.4.2.5.
Table 4.4.2.1.
Precision and Sensitivity
Data
Approximately 0.5× Target Concentration
|
| |||||
| analyte µg/mL |
PHEN 2.49 |
ANTH 0.255 |
PYR 2.94 |
CHRY 1.27 |
BaP 0.525 |
|
| |||||
| areas SD CV |
45900.5 47374.6 47183.4 46965.1 46142.1 46512.1 46679.6 590.3 0.0126 |
51249.6 51970.1 52000.0 51575.7 51108.6 51627.2 51588.5 363.8 0.0071 |
62246.7 65309.6 64947.2 65054.5 63987.1 64048.0 64265.5 1129.4 0.0176 |
75163.5 77086.5 77164.0 77073.2 75900.5 76050.7 76406.4 825.6 0.0108 |
66750.5 69435.0 68508.0 68420.0 67441.5 67287.0 67973.7 987.0 0.0145 |
|
| |||||
Table 4.4.2.2.
Precision and Sensitivity
Data
Approximately 1× Target Concentration
|
| |||||
| analyte µg/mL |
PHEN 4.98 |
ANTH 0.51 |
PYR 5.88 |
CHRY 2.54 |
BaP 1.05 |
|
| |||||
| areas SD CV |
89773.1 89874.4 89365.4 89247.6 88542.6 89070.5 89312.3 487.1 0.0055 |
103477 103385 103311 103251 102573 103281 103213 324 0.0031 |
126795 127081 126379 125730 125370 126281 126273 630 0.0051 |
151961 152486 151748 150675 149400 150541 151135 1136 0.0075 |
136383 136615 135617 134451 134111 134593 135295 1061 0.0078 |
|
| |||||
Table 4.4.2.3.
Precision and Sensitivity
Data
Approximately 2× Target Concentration
|
| |||||
| analyte µg/mL |
PHEN 9.71 |
ANTH 0.99 |
PYR 11.76 |
CHRY 5.08 |
BaP 2.10 |
|
| |||||
| areas SD CV |
184607 180064 182561 182924 182992 183198 182724 1482 0.0081 |
182281 184202 183493 183448 183215 184599 183540 808 0.0044 |
248424 247112 246260 252779 253143 252296 250002 3088 0.0124 |
299267 297709 297519 302237 303651 303771 300692 2885 0.0096 |
262309 260233 259748 271134 269743 270109 265546 5329 0.0201 |
|
| |||||
Table 4.4.2.4.
The Pooled Coefficients of
Variation
|
| ||||
| PHEN | ANTH | PYR | CHRY | BaP |
|
| ||||
| 0.0092 | 0.0051 | 0.0128 | 0.0094 | 0.0150 |
|
| ||||
4.5. Extraction efficiency
4.5.1. Benzene-soluble fraction
The following data represent the analysis of GFFs that were liquid spiked with coal tar solution prepared by the procedure in Section 4.8. at the target concentration (207 µg/GFF). These data only show that compounds derived from the specially prepared coal tar pitch solution can be extracted from a GFF. Since the BSF is a collection of many compounds, the extraction efficiency is not applied to the calculations. The PTFE cups were reweighed 24 h later and the results were still valid.
Table 4.5.1.
Extraction Efficiency of
Benzene-solubles
|
| ||
| first day | 24 h later | |
|
| ||
| percent recovered SD |
98.1 98.1 100.0 100.0 97.1 105.8 102.9 100.3 3.1 |
102.9 101.0 100.0 99.0 99.0 106.8 107.7 102.3 3.6 |
|
| ||
4.5.2. Selected PAHs
The data listed below represent the results of the analysis of GFFs that were liquid spiked with PAHs at the target concentration. These samples were allowed to dry and then extracted with benzene and analyzed the same day. The samples were reanalyzed 24 hr later and found to be stable (Table 4.5.2.2.).
Table 4.5.2.1.
Extraction Efficiency of Selected
PAHs
|
| |||||
| analyte µg/sample |
PHEN 8.5 |
ANTH 0.76 |
PYR 8.6 |
CHRY 3.1 |
BaP 2.4 |
|
| |||||
| %
recovery SD |
107.6 108.9 104.8 104.6 106.8 106.0 104.4 104.0 105.9 1.8 |
117.7 117.0 110.0 109.9 112.0 113.0 111.5 108.7 112.5 3.3 |
106.3 110.0 100.9 96.6 98.7 100.3 101.0 97.0 101.4 4.6 |
111.6 112.0 105.2 103.8 107.9 108.0 106.7 104.7 107.5 3.1 |
110.9 113.2 105.9 105.2 109.3 110.4 108.2 106.5 108.7 2.8 |
|
| |||||
Table 4.5.2.2.
Extraction Efficiencies 24 Hours
Later
|
| |||||
| analyte µg/sample |
PHEN 8.5 |
ANTH 0.76 |
PYR 8.6 |
CHRY 3.1 |
BaP 2.4 |
|
| |||||
| %
recovery SD |
115.9 114.5 110.9 111.5 108.6 106.3 108.7 107.3 110.5 3.4 |
121.3 119.0 116.4 117.8 116.6 109.5 110.4 111.3 115.3 4.4 |
118.4 117.7 111.4 110.3 111.2 102.3 104.7 102.9 109.9 6.2 |
119.2 119.7 112.9 115.0 113.7 106.9 109.7 107.6 113.1 4.9 |
122.3 120.3 118.9 119.5 115.8 112.5 110.1 111.7 116.4 4.5 |
|
| |||||
4.6. Storage data
Storage samples were generated by liquid spiking 36 GFFs with coal tar and another 36 GFFs with PAHs. All of the spiked GFFs were stored in sealed glass vials. One-half of the vials were stored in a freezer at -20°C and the other half were stored in a closed drawer at ambient temperature (about 22°C). The results (percent recovery versus storage time) are given in Tables 4.6.2.-4.6.3. and shown graphically in Figures 4.6.1.-4.6.12.
Table 4.6.1.
Amount Spiked (µg/GFF)
|
| |||||
| BSF | PHEN | ANTH | PYR | CHRY | BaP |
|
| |||||
| 207 | 8.5 | 0.76 | 8.6 | 3.1 | 2.4 |
|
| |||||
Table 4.6.2.
Ambient Storage Test
(% recovery)
|
| ||||||
| day | BSF | PHEN | ANTH | PYR | CHRY | BaP |
|
| ||||||
| 0 3 6 9 12 15 |
100.5 89.9 90.8 91.8 78.3 88.9 90.8 99.5 73.4 87.4 90.3 87.4 86.5 91.3 90.3 100.5 87.0 93.7 91.8 85.0 85.0 |
108.8 107.8 102.4 103.7 100.8 102.9 98.5 97.5 98.3 99.6 104.1 103.0 94.2 101.2 97.5 88.1 93.1 91.7 92.0 94.9 95.2 |
113.0 113.4 108.5 108.5 110.4 109.6 104.3 103.2 100.2 99.3 104.7 105.9 94.6 104.6 98.4 84.3 88.9 88.7 93.1 99.0 95.3 |
105.9 105.4 100.3 102.6 101.1 100.1 91.3 95.3 91.5 99.4 101.1 105.3 90.4 91.5 91.5 82.5 93.2 89.6 85.5 89.0 88.8 |
108.8 108.3 105.0 105.3 104.4 102.8 97.9 100.3 98.8 102.8 108.7 110.5 102.0 101.4 99.2 92.4 96.6 96.7 94.4 100.1 95.3 |
110.2 109.5 105.0 110.2 105.5 107.3 101.4 102.7 100.6 105.3 108.9 108.4 104.0 103.5 101.5 97.3 104.6 101.0 97.3 101.9 100.0 |
|
| ||||||
Table 4.6.3.
Refrigerated Storage Test (%
recovery)
|
| ||||||
| day | BSF | PHEN | ANTH | PYR | CHRY | BaP |
|
| ||||||
| 0 3 6 9 12 15 |
100.5 89.9 90.8 91.8 78.3 88.9 86.0 84.1 92.8 90.3 92.3 98.1 88.4 86.5 93.2 94.7 92.8 108.2 90.8 90.8 84.1 |
108.8 107.8 102.4 103.7 100.8 102.9 98.9 99.0 98.3 105.0 101.7 99.3 97.6 95.4 95.4 95.8 96.3 98.3 96.5 95.2 101.6 |
113.0 113.4 108.5 108.5 110.4 109.6 103.3 102.6 101.3 110.6 106.6 104.4 100.3 99.9 97.5 96.7 99.4 99.0 100.6 101.1 105.2 |
105.9 105.4 100.3 102.6 101.1 100.1 92.7 95.9 92.8 100.2 95.5 95.6 91.3 88.1 87.0 89.6 96.0 90.1 94.8 90.9 98.5 |
108.8 108.3 105.0 105.3 104.4 102.8 100.7 98.9 99.0 105.0 102.9 102.2 99.3 98.8 96.1 96.0 99.6 95.4 100.6 98.9 102.8 |
110.2 109.5 105.0 110.2 105.5 107.3 104.5 102.3 104.1 107.5 106.1 105.6 101.0 101.3 98.6 107.6 103.2 101.8 97.7 100.7 107.9 |
|
| ||||||
4.7. Reproducibility data
Six samples, spiked with coal tar by liquid injection, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 21 days of storage at about 22°C. Another set of six samples, spiked with PAHs by liquid injection, and a draft copy of this procedure were given to another chemist unassociated with this evaluation. The samples were analyzed after 3 days of storage at about 22°C. All the results are corrected for extraction efficiency except for the BSF data and are listed below.
Table 4.7.
Reproducibility Results (percent of
theoretical amount)
|
| ||||||
| BSF | PHEN | ANTH | PYR | CHRY | BaP | |
|
| ||||||
SD |
101.4 90.8 87.0 92.8 99.5 93.9 94.2 5.4 |
99.1 97.8 91.8 101.8 99.8 97.8 98.0 3.4 |
91.7 93.0 86.1 90.9 90.9 89.6 90.4 2.4 |
105.4 104.8 96.5 101.7 100.4 99.3 101.4 3.4 |
102.2 101.6 95.5 98.4 98.1 96.5 98.7 2.7 |
105.0 103.4 97.5 100.4 99.2 98.3 100.6 3.0 |
|
| ||||||
4.8. Preparation of benzene-soluble standards used in evaluation
In this evaluation three different types of coal tar pitch were chosen at random from a collection of several confired coal tar pitch materials. Twenty grams of each pitch were placed in beakers containing 100 mL of benzene and sonicated for 1.5 h. The solutions were then combined and filtered twice with a fine fritted-glass filter funnel. The resultant solution was then passed through a glass fiber filter. The solution was concentrated with a stream of dry nitrogen and the gooey tar was placed in a heated oven (60°C under 20 in. Hg vacuum) for 4 h. A portion of the "dried" tar was used to prepare a stock solution in benzene. This was used to spike filters approximating a Coal Tar Pitch Volatile sample.
Figure 1.1.4. Structures of the selected
PAHs.
Figure 2.3.3. Folding procedure for the glass fiber
filter.
Figure 3.5.1. Chromatogram of selected PAHs at the target
concentration.
Figure 4.1.2.1. Analytical detection limit for
phenanthrene.
Figure 4.1.2.2. Analytical detection limit for
anthracene.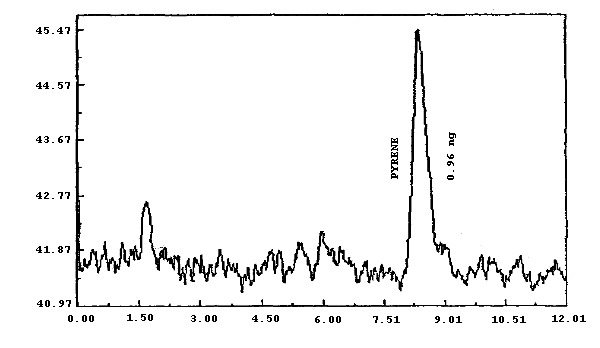
Figure 4.1.2.3. Analytical detection limit for
pyrene.
Figure 4.1.2.4. Analytical detection limit for
chrysene.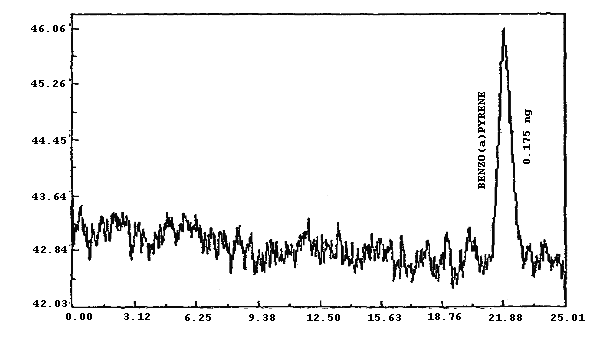
Figure 4.1.2.5. Analytical detection limit for benzo(a)pyrene.
Figure 4.2.1. Detection limit of the overall procedure for
benzene-soluble fraction.
Figure 4.2.2. Detection limit of the overall procedure for
phenanthrene.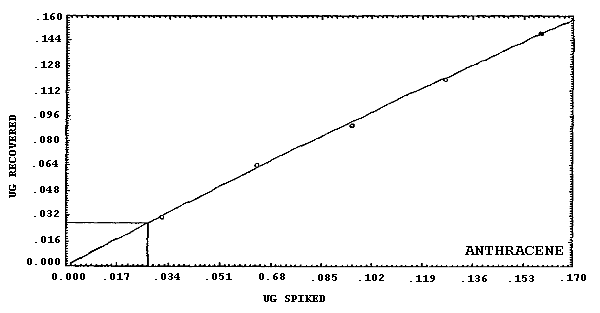
Figure 4.2.3. Detection limit of the overall procedure for
anthracene.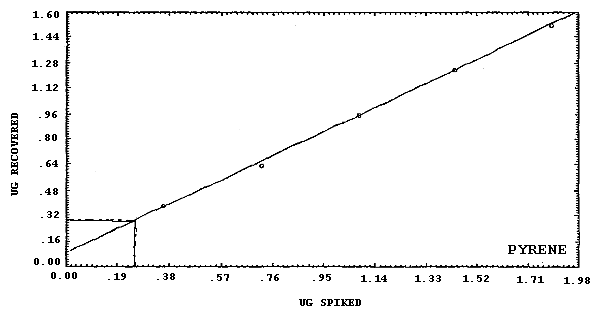
Figure 4.2.4. Detection limit of the overall procedure for
pyrene.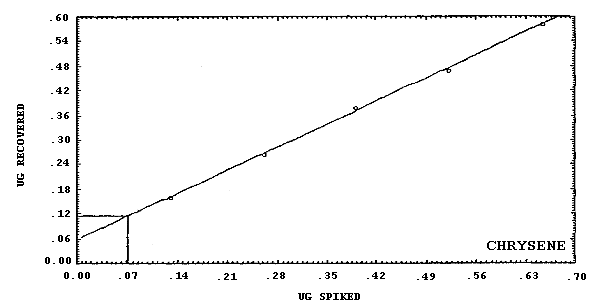
Figure 4.2.5. Detection limit of the overall procedure for
chrysene.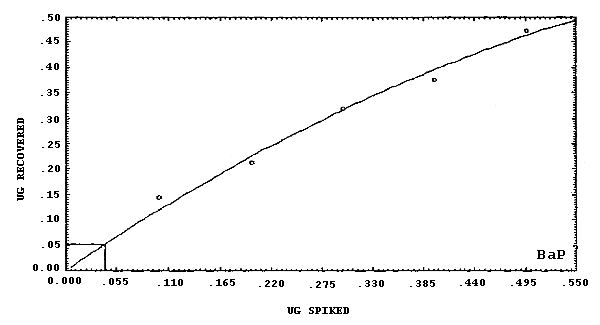
Figure 4.2.6. Detection limit of the overall procedure for
benzo(a)pyrene.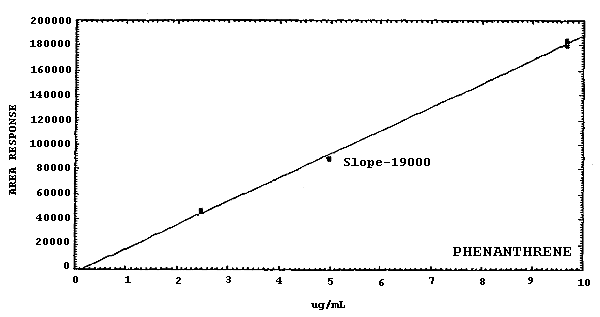
Figure 4.4.2.1. Calibration curve for
phenanthrene.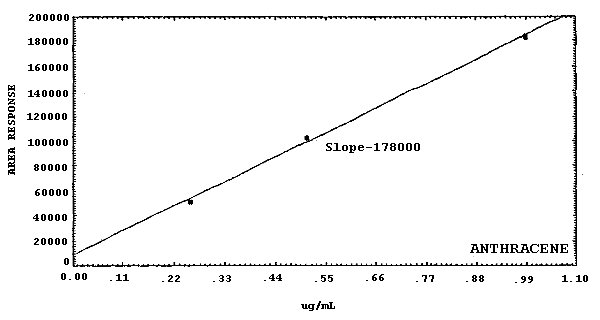
Figure 4.4.2.2. Calibration curve for
anthracene.
Figure 4.4.2.3. Calibration curve for
pyrene.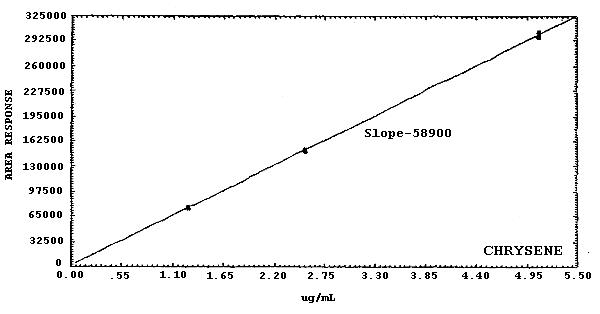
Figure 4.4.2.4. Calibration curve for
chrysene.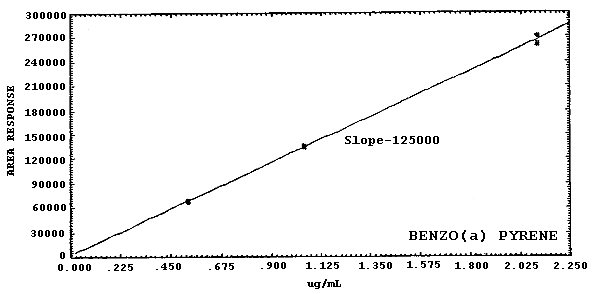
Figure 4.4.2.5. Calibration curve for benzo(a)pyrene.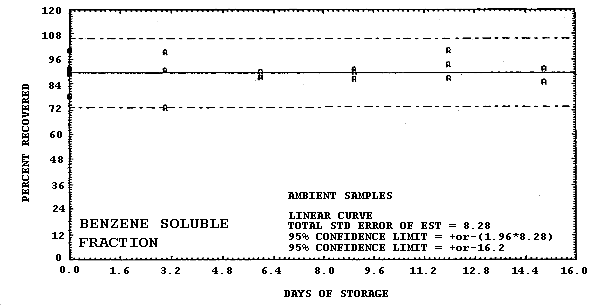
Figure 4.6.1. Ambient storage test for benzene-soluble
fraction.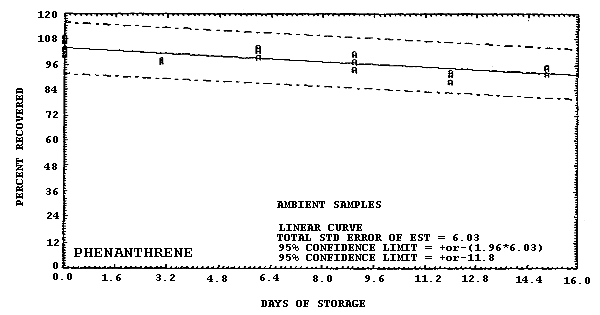
Figure 4.6.2. Ambient storage test for
phenanthrene.
Figure 4.6.3. Ambient storage test for
anthracene.
Figure 4.6.4. Ambient storage test for
pyrene.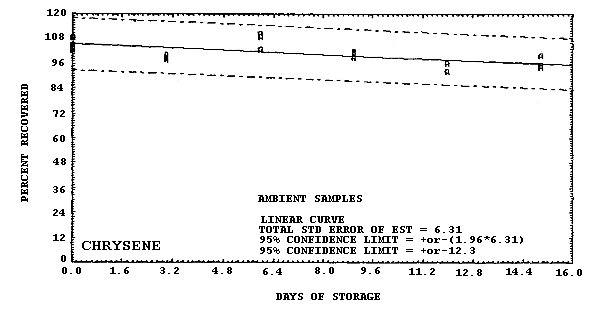
Figure 4.6.5. Ambient storage test for
chrysene.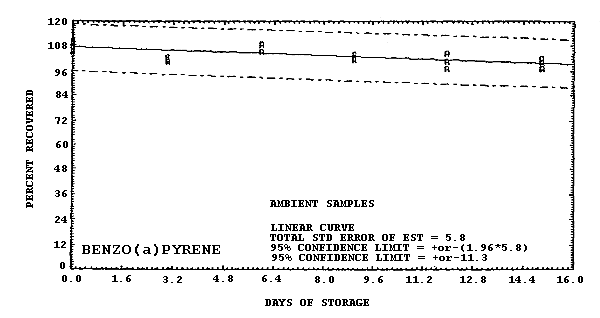
Figure 4.6.6. Ambient storage test for benzo(a)pyrene.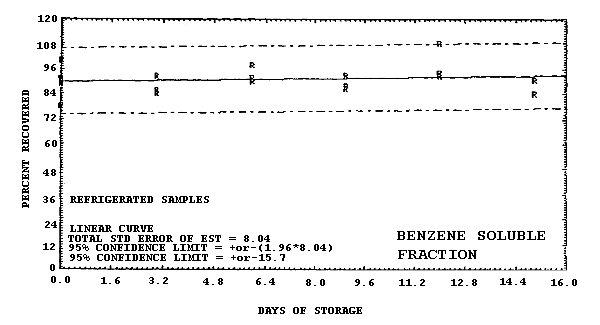
Figure 4.6.7. Refrigerated storage test for
benzene-soluble fraction.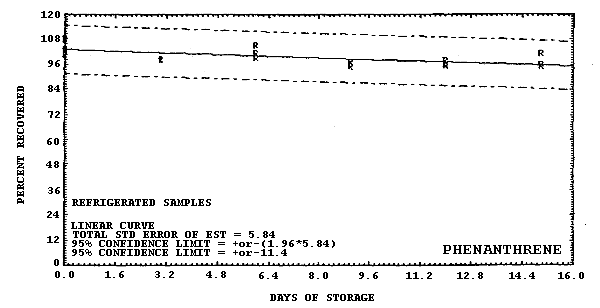
Figure 4.6.8. Refrigerated storage test for
phenanthrene.
Figure 4.6.9. Refrigerated storage test for
anthracene.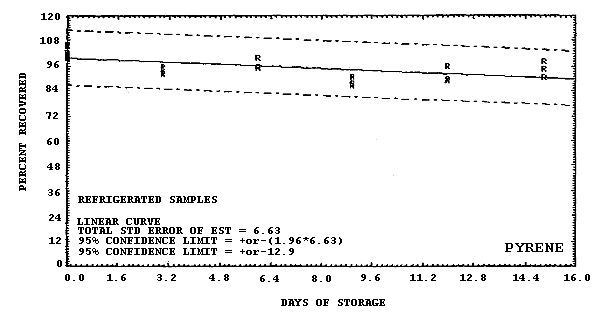
Figure 4.6.10. Refrigerated storage test for
pyrene.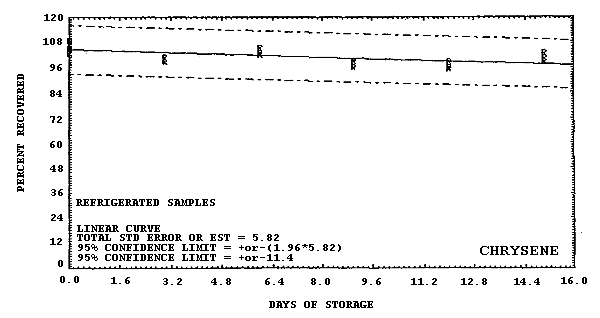
Figure 4.6.11. Refrigerated storage test for
chrysene.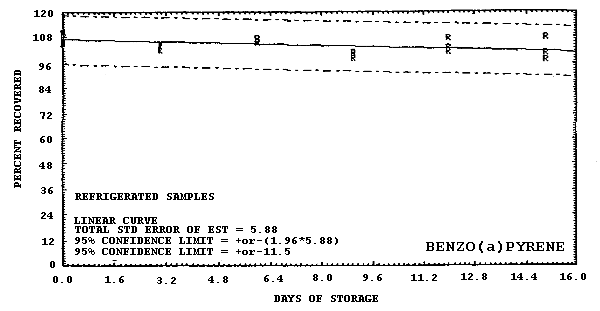
Figure 4.6.12. Refrigerated storage test for benzo(a)pyrene.
5. References
5.1. Code of Federal Regulations, Title 29; 1910.1002, p671, Washington, DC, 1984.
5.2. Code of Federal Regulations, Title 29; 1910.1029, p789, Washington, DC, 1984.
5.3. "Condensed Chemical Dictionary", 10th ed.; Van Nostrand Reinholt Co.: New York, 1981.
5.4. "Occupational Health Guidelines for Chemical Hazards", NIOSH/OSHA, Jan. 1981, DHHS(NIOSH) Publication No. 81-123.
5.5. "Criteria for a Recommended Standard...Occupational Exposure to Coke Oven Emissions", Department of Health, Education and Welfare; National Institute for Occupational Safety and Health: Cincinnati, OH, 1973; DHEW(NIOSH) Publication No. 73-11016.
5.6. "Criteria for a Recommended Standard...Occupational Exposure to Coal Tar Products", Department of Health, Education and Welfare; National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; DHEW(NIOSH) Publication No. 78-107.
5.7. "IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man, Certain Polycyclic Aromatic Hydrocarbons and Heterocyclic Compounds", Lyon, 1973, Vol. 3, 91-136 and 159-177.
5.8. Windholz, M., Ed. "Merck Index", 10th ed.; Merck and Co: Rahway, NJ, 1983.
5.9. Korfmacher, W.A.; Wehry, E.L.; Mamantov, G.; Natusch, D.F.S., Environ. Sci. Technol., 1980, 14(6), 1094.
5.10. Balya, D.R.; Danchik, R.S. Am. Ind. Assoc. J. 1984, 45(4), 260.
5.11. Danchik, R.S.; Balya, D.R., Aluminum Company of America, July, 1984, personal communication.