1. General Discussion
1.1. Background
1.1.1. History
The current OSHA
method for collecting acrolein vapor recommends the use of activated
13X molecular sieves. The samples must be stored in an ice bath during
and after sampling and they must be analyzed within 48 h of
collection. The current OSHA method for collecting formaldehyde vapor
recommends the use of bubblers containing 10% methanol in water as the
trapping solution (Ref. 5.1.).
This work was undertaken to
resolve the sample stability problems associated with acrolein and
also to eliminate the need to use bubblers to sample formaldehyde. A
goal of this work was to develop and evaluate a common sampling and
analytical procedure for acrolein and formaldehyde. The simultaneous
determination of these aldehydes was an appropriate goal because they
can be found together in industrial environments. Further, common
sampling and analytical procedures can reduce both field and
laboratory workloads.
NIOSH has developed independent
methodologies for acrolein (Ref. 5.2.) and formaldehyde (Ref. 5.3.)
which recommend the use of reagent-coated adsorbent tubes to collect
the aldehydes as stable derivatives. The formaldehyde sampling tubes
contain Chromosorb 102 adsorbent coated with
N-benzylethanolamine (BEA) which reacts with formaldehyde
vapor to form a stable oxazolidine compound. The acrolein sampling
tubes contain XAD-2 adsorbent coated with
2-(hydroxymethyl)piperidine (2-HMP) which
reacts with acrolein vapor to form a different, stable oxazolidine
derivative. Acrolein does not appear to react with BEA to give a
suitable reaction product (Ref. 5.2.), therefore, the formaldehyde
procedure cannot provide a common method for both aldehydes. However,
formaldehyde does react with 2-HMP to form a very
suitable reaction product. It is the quantitative reaction of acrolein
and formaldehyde with 2-HMP that provides the basis for this
evaluation.
This sampling and analytical procedure is very
similar to the method recommended by NIOSH for acrolein. Some changes
in the NIOSH methodology were necessary to permit the simultaneous
determination of both aldehydes and also to accommodate OSHA
Laboratory equipment and analytical techniques.
This
successfully evaluated method recommends the collection of acrolein
and formaldehyde vapors on pretreated XAD-2 adsorbent which has been
coated with 2-HMP. The goals of this work were attained
in that both aldehydes can be simultaneously determined without the
need to use bubblers and there are no sample stability
problems.
In June of 1989, this method was updated with
additional data which verified it would adequately accommodate the new
PELs for formaldehyde which went into effect in 1988. The new PELs for
formaldehyde are 1 ppm for the TWA and 2 ppm for the STEL. The
acrolein PEL remains a TWA of 0.1 ppm. The report for the update work
has been incorporated into the "Backup Data" section of this method as
Section 4.11.
1.1.2. Toxic effects (This section is for
information only and should not be taken as the basis of OSHA
policy.)
Acrolein: Human exposure to acrolein can occur through
inhalation of the vapors or percutaneous absorption of the liquid. The
results of exposure are intense irritation of the eyes, the
respiratory tract mucous membranes and finally pulmonary edema or
bronchitis. Skin and eye burns may result from prolonged and repeated
exposure or splashes of acrolein. Sensitization has been reported to
occur in some individuals. (Ref. 5.4.)
Acrolein has induced
mutagenic effects in various test systems. There is no evidence that
acrolein has carcinogenic or co-carcinogenic activity.
Acrolein has not been shown to have teratogenic or fetotoxic effects.
(Refs. 5.4. and 5.5.)
The International Agency for Research on
Cancer (IARC) did not make an evaluation regarding the mutagenicity of
acrolein because of the preliminary and conflicting nature of the
available data. Also, the absence of human data precluded an
evaluation of the carcinogenicity of acrolein by IARC. (Ref.
5.6.)
Formaldehyde: Symptoms of human exposure to formaldehyde
include irritation of the eyes, the nose and the throat which lead to
lachrymation, sneezing, shortness of breath, sleeplessness, tight
chest, nausea and excess phlegm. Formaldehyde has been shown to cause
dermatitis. Formaldehyde is an allergen and susceptible persons can
become sensitized to the agent. Formaldehyde has been reported to
cause menstrual disorders and secondary sterility in women.
Formaldehyde is mutagenic in a variety of test systems. IARC reports
that there is sufficient evidence that formaldehyde gas is
carcinogenic to rats. IARC also reports that epidemiological studies
provide inadequate evidence to assess the carcinogenicity of
formaldehyde to man. (Ref. 5.7.)
Formaldehyde can react with
hydrogen chloride to form bis-chloromethyl ether (BCME).
IARC reports that exposure to BCME may constitute a serious human lung
cancer hazard. (Ref. 5.8.)
NIOSH recommends that formaldehyde
be handled in the workplace as a potential occupational carcinogen.
The basis of this recommendation are two inhalation studies that
resulted in the same rare form of cancer in rats and in mice.
Formaldehyde has also demonstrated mutagenic activity in several test
systems. (Ref. 5.9.)
The Federal Panel on Formaldehyde has
concluded that formaldehyde should be presumed to pose a carcinogenic
risk to humans. The panel consisted of scientists from within the
federal government and was formed under the authority of the National
Toxicology Program. (Ref. 5.10.)
1.1.3. Potential workplace
exposure
Acrolein: Acrolein is produced by the catalytic vapor
phase oxidation of propylene with air. Acrolein production in the
United States was estimated to be 61 million pounds in 1974. This
figure does not include an additional 99 to 150 million pounds used as
a captive intermediate in the production of acrylic acid. The main
uses for acrolein are: fifty percent for the production of glycerin,
25% for the production of methionine (a poultry feed supplement) and
25% for other applications. Some of these applications are:
manufacturing of chemicals and chemical products including
glutaraldehyde and 1,2,6-hexanetriol, modification of
food starch and use as an aquatic herbicide, biocide and slimicide.
Acrolein has been used as a war gas and as a slimicide in the
manufacture of paper and paperboard for use to package food products.
(Ref. 5.6.)
In 1979, acrolein production was estimated to be 85
to 90 million pounds. Approximately 7500 workers are occupationally
exposed to acrolein annually. (Ref. 5.4.)
Formaldehyde:
Formaldehyde is produced by the catalytic vapor phase oxidation of
methanol with air. Most formaldehyde is marketed in a aqueous
solution, called formalin, which contains 37 to 50% formaldehyde by
weight. The United States produced about 6.4 billion pounds of aqueous
formaldehyde in 1978 and most of this amount was used domestically.
The United States consumption of formaldehyde was estimated to exceed
7.5 billion pounds in 1983. About half of the formaldehyde produced in
the U.S. is used to manufacture synthetic resins. These resins are
often used to produce particleboard, fiberboard and plywood.
Urea-formaldehyde resins are used to coat materials, to
produce paper products and to make foams for insulation. Other
important uses include textile treating and molding of plastic
materials. Formaldehyde is used in some medicines and also in
embalming fluids. It is used in fur and leather tanning and also in
the photographic industry. (Ref. 5.9.)
NIOSH estimated that 1.6
million workers were exposed to formaldehyde in a survey conducted
from 1972 to 1974. About one-third of this total was
employed in medical and health services occupations. Another
one-third of the total was employed in miscellaneous
occupations which included: chemicals and chemical products, printing
and publishing, paper, machinery, retail store, eating and drinking
places, automotive dealers and service stations, funeral services and
crematories, photographic studios and dry cleaning plants. (Ref.
5.9.)
Other jobs and/or occupations in which exposure to
formaldehyde may occur include: formaldehyde production workers,
seamstresses, hairdressers, glue workers, foundry employees, resin
manufacturing workers, wood laminating workers and fabric workers.
(Ref. 5.7.)
1.1.4. Physical properties
| Acrolein (Ref.
5.6.) |
| CAS no.: |
107-02-8 |
| molecular weight: |
56.1 |
| appearance: |
colorless liquid |
| boiling point: |
52.5 to 53.5°C |
| density: |
0.841 at 20°C |
| vapor pressure: |
200 mm Hg at 17.5°C |
| flash point: |
-26.1°C |
| molecular formula: |
CH2=CHCHO |
| synonyms: |
2-propenal; acraldehyde;
acrylaldehyde; acrylic aldehyde; allylaldehyde;
prop-2-en-1-al; 2-propen-1-one;
Aqualin; NSC 8819; propenal |
Acrolein polymerizes spontaneously, particularly in the
presence of light, alkali or strong acid.
| Formaldehyde
(Ref. 5.7.) |
| CAS no.: |
50-00-0 |
| molecular weight: |
30.0 |
| appearance: |
colorless gas |
| boiling point: |
-19°C |
| density: |
0.8153 at -20°C; 1.067
(air = 1.000) |
| vapor pressure: |
400 mm Hg at -33°C |
| ignition temp.: |
430°C |
| molecular formula: |
HCHO |
synonyms:
(including
polymeric forms from which formaldehyde can be generated) |
formaldehyde;
formaldehyde gas; formaldehyde solution; formalin 40; formalin
100%; formic aldehyde; methaldehyde; methanal; methyl aldehyde;
methylene glycol; methylene oxide; oxomethane; oxymethylene;
paraform; paraformaldehyde; polyoxymethylene glycols;
a-polyoxymethylene;
a-trioxane; ß-trioxymethylene; tetraoxymethylene;
a-polyoxymethylene;
trioxane |
Formaldehyde
polymerizes rapidly, especially under alkaline conditions.
1.2. Limit defining parameters (The
analyte air concentrations reported in this method are based on the
recommended air volume for each analyte collected separately and a
desorption volume of 1 mL. The amounts are presented as acrolein and/or
formaldehyde, even though the derivatives are the actual species
analyzed.)
1.2.1. Detection limits of the
analytical procedure
The detection limit of the analytical
procedure was 233 pg per injection for acrolein. This was the amount
of acrolein which gave a measurable response relative to the
interferences present in a standard. The detection limit of the
analytical procedure was 386 pg per injection for formaldehyde. This
was the amount of analyte which gave a peak whose height was about 5
times the height of the peak given by the residual formaldehyde
derivative (Section 4.8.) in a typical blank front section of the
recommended sampling tube (Section 4.1.).
1.2.2. Detection
limits of the overall procedure
The detection limits of the
overall procedure were 291 ng per sample (2.7 ppb or 6.1 µg/m³) for
acrolein and 482 ng per sample (16 ppb or 20 µg/m³) for formaldehyde.
These were the amounts of analyte spiked on the sampling device which
allowed recoveries approximately equal to the detection limits of the
analytical procedure (Section 4.2.).
1.2.3. Reliable
quantitation limits
The reliable quantitation limits were 291
ng per sample (2.7 ppb or 6.1 µg/m³) for acrolein and 482 ng per
sample (16 ppb or 20 µg/m³) for formaldehyde. These were the smallest
amounts of analyte which could be quantitated within the limits of a
recovery of at least 75% and a precision (±1.96 SD) of ±25% or better
(Section 4.2.).
The reliable quantitation limits and detection
limits reported in the method are based upon optimization of the
instrument for the smallest possible amount of analyte. When the target
concentration of an analyte is exceptionally higher than these limits,
they may not be attainable at the routine operating parameters.
1.2.4. Sensitivity
The
sensitivities of the analytical procedure over concentration ranges
representing 0.4 to 2 times the target concentration, based on the
recommended air volumes, were 9443 area units per µg/mL for acrolein
and 7589 area units per µg/mL for formaldehyde. These values were
determined from the slope of the calibration curves (Section 4.3.).
The sensitivity may vary with the particular instrument used in the
analysis.
1.2.5. Recovery
The recovery of acrolein from
samples used in a 19-day storage test remained above 88% when the
samples were stored at ambient temperature. The recovery of
formaldehyde from samples used in an 18-day storage test
remained above 92% when the samples were stored at ambient
temperature. These values were determined from regression lines which
were calculated from the storage data (Section 4.6.). The recovery of
the analyte from the collection device must be at least 75% following
storage.
1.2.6. Precision (analytical method only)
The
pooled coefficients of variation obtained from replicate
determinations of analytical standards over the range of 0.4 to 2
times the target concentration were 0.034 for acrolein and 0.0052 for
formaldehyde (Section 4.3.).
1.2.7. Precision (overall
procedure)
The precision at the 95% confidence level for the
ambient temperature storage tests were ±13.8% for acrolein and ±14.3%
for formaldehyde (Section 4.6.). These values each include an
additional ±5% for sampling error. The overall procedure must provide
results at the target concentrations that are ±25% at the 95%
confidence level.
1.2.8. Reproducibility
Samples
collected from controlled test atmospheres and a draft copy of this
procedure were given to a chemist unassociated with this evaluation.
The acrolein samples were analyzed following 7 days of storage at
ambient temperature. The average recovery was 99.0% and the standard
deviation was 10.5%. The formaldehyde samples were analyzed following
15 days of storage. The average recovery was 96.3% and the standard
deviation was 1.7% (Section 4.7.). 1.3. Advantages
1.3.1. The sampling and analytical
procedures permit the simultaneous determination of acrolein and
formaldehyde.
1.3.2. Samples are stable following storage at
ambient temperature for at least 18 days. 1.4. Disadvantage
None 2. Sampling Procedure
2.1. Apparatus
2.1.1. Samples are collected by use of
a personal sampling pump that can be calibrated to within ±5% of the
recommended sampling rate with the sampling tube in
line.
2.1.2. Samples are collected with laboratory prepared
sampling tubes. The sampling tube is constructed of
silane-treated glass and is about 8-cm long.
The i.d. is 4 mm and the o.d. is 6 mm. One end of the tube is tapered
so that a glass wool end plug will hold the contents of the tube in
place during sampling. The other end of the sampling tube is open to
its full 4-mm i.d. to facilitate packing of the tube. Both ends of the
tube are fire-polished for safety. The tube is packed
with a 75-mg backup section, located nearest the tapered
end and a 150-mg sampling section of pretreated
XAD-2 adsorbent which has been coated with
2-HMP. The two sections of coated adsorbent are separated
and retained with small plugs of silanized glass wool. Following
packing, the sampling tubes are sealed with two 7/32-in.
o.d. plastic end caps. Instructions for the pretreatment and the
coating of XAD-2 adsorbent are presented in Section 4.8.
of this method.
2.1.3. Sampling tubes, similar to those
recommended in this method, are marketed by Supelco, Inc. These tubes
were not available when this work was initiated, therefore, they were
not evaluated. 2.2.
Reagents
None required
2.3. Technique
2.3.1. Properly label the sampling
tube before sampling and then remove the plastic end
caps.
2.3.2. Attach the sampling tube to the pump using a
section of flexible, plastic tubing such that the large, front section
of the sampling tube is exposed directly to the atmosphere. Do not
place any tubing ahead of the sampling tube. The sampling tube should
be attached in the worker's breathing zone in a vertical manner such
that it does not impede work performance.
2.3.3. After sampling
for the appropriate time, remove the sampling from the pump and then
seal the tube with plastic end caps. Wrap the tube lengthwise with an
official OSHA seal (Form 21).
2.3.4. Include at least one blank
for each sampling set. The blank should be handled in the same manner
as the samples with the exception that air is not drawn through
it.
2.3.5. List any potential interferences on the sample data
sheet. 2.4. Breakthrough
(Breakthrough was defined as the relative amount of analyte found on a
backup sample in relation to the total amount of analyte collected on
the sampling train.)
2.4.1. Acrolein: When a test
atmosphere containing 3 times the PEL was sampled for 2 times the
recommended air volume, the breakthrough was 1% (Section 4.4.). No
breakthrough of acrolein from the 150-mg to the 75-mg adsorbent bed
was observed when the recommended sampling method was
followed.
2.4.2. Formaldehyde: For formaldehyde collected from
test atmospheres containing 2 times the PEL, the average 5%
breakthrough air volume was 41 L. The sampling rate was 0.1 L/min and
the average mass of formaldehyde collected was 250 µg (Section
4.4.). 2.5. Desorption
efficiency
No desorption efficiency corrections are necessary to
compute air sample results because analytical standards are prepared
using coated adsorbent. Desorption efficiencies were determined,
however, to investigate the recoveries of the analytes from the sampling
device. The average recoveries, over the range of 0.4 to 2 times the
target concentration, based on the recommended air volumes, were 102%
for acrolein and 96.2% for formaldehyde. The desorption efficiencies
were essentially constant over the ranges studied (Section
4.5.).
2.6. Recommended air volumes and sampling rate
2.6.1. The recommended air volume for
acrolein is 48 L collected at 0.1 L/min.
2.6.2 The, recommended
air volumes for formaldehyde are 24 L collected at 0.1 L/min for the
TWA and 3 L collected at 0.2 L/min for the STEL.
2.6.3. The
recommended air volume to be used when both aldehydes are sampled
together is 24 L collected at 0.1 L/min. 2.7. Interferences (sampling)
2.7.1. Any collected substance that is
capable of reacting with, and depleting the derivatizing reagent is a
potential interference. Chemicals which contain a carbonyl group, such
as acetone, may be capable of reacting with 2-HMP.
2.7.2. There
are no other known interferences to the sampling method.
2.8. Safety precautions
(sampling)
2.8.1. Attach the sampling equipment
to the worker in such a manner that it will not interfere with work
performance or safety.
2.8.2. Follow all safety practices that
apply to the work area being sampled.
3. Analytical
Procedure
3.1. Apparatus
3.1.1. A gas chromatography (GC),
equipped with a nitrogen selective detector. A
Hewlett-Packard Model 5840A GC fitted with a nitrogen
phosphorus flame ionization detector (NPD) was used for this
evaluation. Injections were performed using a
Hewlett-Packard Model 7671A automatic
sampler.
3.1.2. A GC column capable of resolving the analytes
from potential interferences. A 6-ft ×
1/4-in. o.d. (2-mm i.d.) glass GC column containing 10%
UCON 50-HB-5100 with 2% KOH on 80/100 mesh Chromosorb
W-AW was used for this evaluation. Injections were performed
on-column.
3.1.3. Vials, glass 2-mL with Teflon-lined
caps.
3.1.4. Volumetric flasks, pipets and syringes for
preparing standards, making dilutions and performing
injections. 3.2.
Reagents
3.2.1. Toluene and dimethylformamide.
Burdick and Jackson solvents were used in this
evaluation.
3.2.2. Helium, hydrogen and air, GC
grade.
3.2.3. Acrolein, of known high purity. Aldrich Chemical,
Gold Label Grade acrolein was used in this study.
3.2.4.
Formaldehyde, 37% by weight in water. Aldrich Chemical, A.C.S. Reagent
Grade formaldehyde was used in this evaluation.
3.2.5.
Amberlite XAD-2 adsorbent coated with 10%, by weight,
2-(hydroxymethyl)piperidine (2-HMP) (Section 4.8.).
3.2.6.
Desorbing solution with internal standard. This solution was prepared
by adding 20 µL of dimethylformamide to 100 mL of toluene.
3.3. Standard preparation
3.3.1. Acrolein: Prepare stock
standards by diluting known amounts of the aldehyde with methanol. A
standard containing 1 mg/mL acrolein was prepared by diluting 12 µL of
the 99% reagent to 10 mL with methanol.
3.3.2. Formaldehyde:
Prepare stock standards by diluting known volumes of 37% formaldehyde
solution with methanol. A procedure to determine the formaldehyde
content of these standards is presented in Section 4.9. A standard
containing 7.7 mg/mL formaldehyde was prepared by diluting 1 mL of the
37% reagent to 50 mL with methanol.
3.3.3. It is recommended
that analytical standards be prepared about 16 h before the air
samples are to be analyzed in order to ensure the complete reaction of
the analytes with 2-HMP. However, rate studies have shown
the reaction to be greater than 95% complete after 4 h. Therefore, one
or two standards can be analyzed after this reduced time if sample
results are outside the concentration range of the prepared
standards.
3.3.4. Place 150-mg portions of coated
XAD-2 adsorbent, from the same lot number as used to
collect the air samples, into each of several glass 2-mL
vials. Seal each vial with a Teflon-lined
cap.
3.3.5. Prepare fresh analytical standards each day by
injecting appropriate amounts of the diluted analytes directly onto
150-mg portions of coated adsorbent. It is permissible to
inject both acrolein and formaldehyde on the same adsorbent portion.
Allow the standards to stand at room temperature. A standard,
approximating the target levels, was prepared by injecting 11 µL of
the acrolein and 12 µL of the formaldehyde stock standards onto a
single coated XAD-2 adsorbent portion.
3.3.6. Prepare a
sufficient number of standards to generate the calibration curves.
Analytical standard concentrations should bracket sample
concentrations. Thus, if samples are not in the concentration range of
the prepared standard additional standards must be prepared to
determine detector response.
3.3.7. Desorb the standards in the
same manner as the samples following the 16-h reaction
time. 3.4. Sample
preparation
3.4.1. Transfer the 150-mg section of
the sampling tube to a 2-mL vial. Place the 75-mg section
in a separate vial. If the glass wool plugs contain a significant
number of adsorbent beads, place them with the appropriate sampling
tube section. Discard the glass wool plugs if they do not contain a
significant number of adsorbent beads.
3.4.2. Add 1 mL of
desorbing solution to each vial.
3.4.3. Seal the vials with
Teflon-lined caps and then allow them to desorb for 1 h. Shake the
vials by hand with vigorous force several times during the desorption
time.
3.4.4. Save the used sampling tubes to be cleaned and
recycled. 3.5.
Analysis
3.5.1. GC Conditions
| column temperature: |
bi-level temperature
program
first level - 100 to 140°C at 4°C/min upon
injection
second level - 140 to 180°C at 20°C/min following
completion of the first level
isothermal period - Hold column
at 180°C until the recorder pen returns to baseline (usually
about 25 min after injection) |
| injector temperature: |
180°C |
| helium flow rate: |
30 mL/min (detector response will
be reduced if nitrogen is substituted for helium carrier
gas) |
| injection volume: |
0.8 µL |
| GC column: |
6-ft × 1/4-in. o.d. (2-mm i.d.)
glass GC column containing 10% UCON 50-HB-5100 with 2% KOH on
80/100 Chromosorb W-AW |
| NPD conditions |
|
| hydrogen flow rate: |
3 mL/min |
| air flow rate: |
50 mL/min |
| detector temperature: |
275°C |
3.5.2. Chromatogram Figure 4.11.
3.5.3. Use a
suitable method, such as electronic integration, to measure detector
response.
3.5.4. Use an internal standard method to prepare the
calibration curve with several standard solutions of different
concentrations. Prepare the calibration curve daily. Program the
integrator to report results in µg/mL.
3.5.5. Bracket sample
concentrations with standards. 3.6. Interferences (analytical)
3.6.1. Any compound with the same
general retention time as the analytes and which also gives a detector
response is a potential interference. Possible interferences should be
reported to the laboratory with submitted samples by the industrial
hygienist.
3.6.2. GC parameters (temperature, column, etc.) may
be changed to circumvent interferences.
3.6.3. A useful means
of structure designation is GC/MS. It is recommended this procedure be
used to confirm samples whenever possible.
3.6.4. The coated
adsorbent usually contains a small amount of residual formaldehyde
derivative (Section 4.8.). 3.7.
Calculations
3.7.1. Results are obtained by use of
calibration curves. Calibration curves are prepared by plotting
detector response against concentration for each standard. The best
line through the data points is determined by curve
fitting.
3.7.2. The concentration, in µg/mL, for a particular
sample is determined by comparing its detector response to the
calibration curve. If either of the analytes is found on the backup
section, it is added to the amount found on the front section. Blank
corrections should be performed before adding the results together.
See Section 4.11. for additional information and suggestions on blank
determinations and corrections.
3.7.3. The acrolein and/or
formaldehyde air concentration can be expressed using the following
equation:
mg/m³ =
(A)(B)/C
|
| where |
A |
= |
µg/mL from Section 3.7.2. |
|
B |
= |
desorption volume |
|
C |
= |
liters of air
sampled |
No desorption
efficiency corrections are required.
3.7.4. The following
equation can be used to convert results in mg/m³ to
ppm.
ppm =
(mg/m³)(24.46)/MW
|
| where |
mg/m³ |
= |
result from Section 3.7.3. |
|
24.46 |
= |
molar volume of an ideal gas at 760
mm Hg and 25°C |
|
MW |
= |
molecular weight (acrolein = 56.1,
formaldehyde = 30.0) | 3.8. Safety precautions (analytical)
3.8.1. Avoid skin contact and
inhalation of all chemicals.
3.8.2. Restrict the use of all
chemicals to a fume hood whenever possible.
3.8.3. Wear safety
glasses and a lab coat in all laboratory areas.
4. Backup Data
(The
analyte concentrations are presented as acrolein and/or formaldehyde even
though the derivatives are the actual species analyzed.)
4.1. Detection limit data
The
injection size recommended in the analytical procedure (0.8 µL) was used
in the determination of the detection limits for the analytical
procedure. The detection limit of the analytical procedure was 233 pg
per injection for acrolein. This was the amount of acrolein which gave a
measurable response relative to interferences present in a standard. The
detection limit of the analytical procedure was 386 pg per injection for
formaldehyde. This was the amount of formaldehyde which gave a peak
whose height was about five times the height of the peak given by the
residual formaldehyde derivative in a typical blank front section of the
recommended sampling tube. These detection limits were determined by the
analysis of a sample containing 291 ng/mL of acrolein and 482 ng/mL of
formaldehyde. Figure 4.1. is a chromatogram of the detection limits of
the analytical procedure. The analysis was performed using a
Hewlett-Packard 5840A GC equipped with a NPD. The NPD offset was 75 mm
at attenuation 8. The chart speed was set at 0.25 cm/min.
4.2.
Detection limit of the overall procedure and reliable quantitation limit
data.
Six samples were used to determine the detection limit of
the overall procedure and the reliable quantitation limit. Individual
samples were prepared by injecting 291 ng of acrolein and 482 ng of
formaldehyde onto a single 150-mg portion of coated XAD-2
adsorbent. Analytical standards were prepared by injecting equivalent
amounts of the analytes into 1-mL aliquots of toluene
containing 15 mg/mL 2-HMP. The samples and standards were
stored about 16 h at room temperature before analysis. Since the
recoveries were high and approximately equivalent to the detection
limits of the analytical procedure, the detection limits of the overall
procedure and the reliable quantitation limit were 291 ng per sample
(2.7 ppb or 6.1 µg/m³) for acrolein and 482 ng per sample (16 ppb or 20
µg/m³) for formaldehyde.
Table 4.2.
Detection Limit of the
Overall
Procedure and Reliable Quantitation Limit Data
|
|
acrolein |
formaldehyde |
|
|
|
|
mass |
|
mass |
|
| sample |
recovered, |
recovery, |
recovered, |
recovery, |
| number |
ng |
% |
ng |
% |
|
| 1 |
287 |
98.6 |
471 |
97.7 |
| 2 |
299 |
103. |
453 |
94.0 |
| 3 |
284 |
97.6 |
478 |
99.2 |
| 4 |
269 |
92.4 |
464 |
96.3 |
| 5 |
310 |
106. |
477 |
99.0 |
| 6 |
284 |
97.6 |
450 |
93.4 |
|
 |
|
99.2 |
|
96.6 |
| SD |
|
4.7 |
|
2.5 |
| 1.96 SD |
|
9.3 |
|
4.9 |
|
4.3. Sensitivity and
precision (analytical method only)
The sensitivity and precision
of the analytical procedure were evaluated by performing multiple
injections of analytical standards. The standards were prepared by
injecting appropriate amounts of the aldehydes onto coated
XAD-2 adsorbent 16 h prior to desorption and analysis. The
data are presented in Tables 4.3.1. and 4.3.2. and also in Figures
4.3.1. and 4.3.2. The ISTD data are the results of an internal standard
calibration.
Table 4.3.1.
Acrolein Sensitivity and
Precision Data
|
| × target conc. |
0.4× |
1× |
2× |
| µg/sample |
4.0 |
10 |
20 |
|
|
|
|
|
ISTD |
area |
ISTD |
area |
ISTD |
area |
|
|
3.9 |
38600 |
10.8 |
98620 |
19.6 |
187400 |
|
4.0 |
40840 |
10.2 |
98820 |
20.0 |
190700 |
|
3.8 |
39260 |
10.1 |
98580 |
20.2 |
190300 |
|
4.0 |
40290 |
10.5 |
104300 |
19.9 |
178800 |
|
4.0 |
41360 |
9.2 |
102900 |
20.0 |
209500 |
|
3.9 |
38650 |
10.1 |
108900 |
20.2 |
194100 |
|
 |
3.93 |
|
10.15 |
|
19.98 |
|
| SD |
0.082 |
|
0.539 |
|
0.223 |
|
| CV |
0.021 |
|
0.053 |
|
0.011 |
|
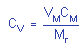 = 0.034 = 0.034 |
|
|
| The sensitivity
for acrolein was 9443 area counts per
µg/mL. |
Table 4.3.2.
Formaldehyde Sensitivity and
Precision Data
|
| × target conc. |
0.4× |
1× |
2× |
| µg/sample |
38.8 |
97 |
194 |
|
|
|
|
|
ISTD |
area |
ISTD |
area |
ISTD |
area |
|
|
38.7 |
284700 |
97.4 |
648800 |
194.6 |
1434000 |
|
38.8 |
292900 |
97.3 |
653800 |
193.8 |
1458000 |
|
39.0 |
301000 |
97.1 |
636100 |
192.4 |
1492000 |
|
38.8 |
283300 |
97.0 |
682200 |
192.0 |
1480000 |
|
38.7 |
289000 |
96.9 |
681800 |
195.5 |
1429000 |
|
38.6 |
294200 |
96.9 |
672400 |
195.7 |
1454000 |
|
 |
38.8 |
|
97.1 |
|
194.0 |
|
| SD |
0.137 |
|
0.210 |
|
1.56 |
|
| CV |
0.0035 |
|
0.0022 |
|
0.0080 |
|
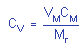 = 0.0052 = 0.0052 |
|
|
| The sensitivity
for formaldehyde was 7589 area counts per
µg/mL. |
4.4.
Breakthrough data
4.4.1. Acrolein: Acrolein test
atmospheres were generated in the manner discussed in Section 4.10. No
breakthrough from the 150-mg to the 75-mg
adsorbent bed was observed when the recommended sampling procedure was
followed. The most concentrated atmosphere studied contained 0.77
mg/m³ (about 3 times the PEL) acrolein. The relative humidity of this
atmosphere was 30% at 24°C. The atmosphere was sampled at 0.2 L/min
for 416 min. The total air volume was 83.2 L (1.7 times the
recommended volume). The amount of acrolein found on the backup
section of the sampling tube was 1% of the total amount found on the
tube.
Studies were performed in which the sampling tube was
reversed in order to evaluate breakthrough with a reduced adsorbent
bed. A short section of silane-treated glass tubing,
containing a small plug of silanized glass wool, was connected to the
adsorbent tube. Acrolein was injected onto the glass wool plug and
then humid air was drawn through the sampling train. The acrolein was
volatilized from the glass wool so that the sampling tube was
challenged with a vapor. This technique is known as vapor spiking. The
relative humidity of the air was 85% at 25°C. Air was drawn through
the tube at 0.2 L/mln 7 h. The total air volume was 84
L.
Table 4.4.1.
Acrolein Breakthrough with
the
Sampling Tube Connected in the Reverse Direction
|
| sample |
amt. on 75
mg |
amt. on
150 mg |
breakthrough, |
| number |
section µg |
section µg |
% |
|
| 1 |
46.4 |
4.4 |
8.7 |
| 2 |
48.0 |
6.4 |
11.8 |
| 3 |
47.0 |
4.8 |
9.3 |
| 4 |
41.6 |
6.0 |
12.6 |
|
The average
breakthrough was 10.6%. The amount of acrolein collected on each
sample was more than four times the target concentration based on the
recommended air volume. The air volume sampled was 1.8 times the
recommended volume. The high relative humidity of the sampled air did
not appear to affect breakthrough. These data plus the fact that the
recommended sampling tube contains a 150-mg front section show the
device to have more than adequate capacity for acrolein.
4.4.2.
Formaldehyde: Formaldehyde test atmospheres were generated in the
manner discussed in Section 4.10. Two breakthrough studies were
performed using test atmospheres. One study was conducted using only
150-mg coated adsorbent sections. Two sections were connected in
series. The second section was removed periodically and replaced with
a fresh section. The test atmosphere contained 5.3 mg/m³ formaldehyde,
the relative humidity of the air was 49% at 24°C and the sampling rate
was 0.1 L/min. Breakthrough was calculated using the amount of
formaldehyde found on the second section and the theoretical amount of
formaldehyde collected on the first tube. The theoretical amount of
formaldehyde was determined from the concentration of the test
atmosphere and the air volume sampled.
Table 4.4.2.1.
First Formaldehyde
Breakthrough Study
|
| elapsed time, |
cumulative |
| min |
breakthrough, % |
|
| 120 |
0.0 |
| 240 |
1.2 |
| 360 |
4.0 |
| 420 |
5.8 |
|
Five-percent
breakthrough occurred at 396 min (6.6 h), after 41.8 L of air had been
sampled and 284 µg of formaldehyde had been collected.
The
second breakthrough study was conducted using four sampling tubes. The
atmosphere was sampled for an appropriate time and then a tube was
removed for analysis. Sampling was continued using the remaining tubes
which were each removed at various intervals. The test atmosphere
contained 6.8 mg/m³ formaldehyde, the relative humidity of the air was
38% at 24°C and the sampling rate was 0.1 L/min. Breakthrough was
calculated using the amount of formaldehyde found on the 75-mg section
and the total amount of formaldehyde collected on both
sections.
Table 4.4.2.2.
Second Formaldehyde
Breakthrough Study
|
| sample |
sampling time, |
breakthrough, |
| number |
min |
% |
|
| 1 |
247 |
0.9 |
| 2 |
311 |
1.5 |
| 3 |
360 |
3.1 |
| 4 |
427 |
5.6 |
|
Five-percent
breakthrough occurred at 407 min (6.8 h), after 40.7 L of air had been
sampled and 214 µg of formaldehyde had been collected.
A vapor
spiking breakthrough study using formaldehyde and the recommended
sampling device was performed. The relative humidity of the sampled
air was 75% at 26°C. The sampling rate was 0.1 L/min. Two samples were
taken in this manner.
Table 4.4.2.3.
Vapor Spiked Formaldehyde
Breakthrough Study
|
|
amt. on 150 |
amt. on 75 |
|
| sample |
sample time, |
mg sect., |
mg sect., |
breakthrough, |
| number |
min |
µg |
µg |
% |
|
| 1 |
240 |
244.1 |
4.8 |
1.9 |
| 2 |
360 |
262.5 |
8.8 |
3.2 |
|
This study shows
that breakthrough is not a function of the relative humidity of the
sampled air. The data in Tables 4.4.2.1. - 4.4.2.3. indicate that the
coated adsorbent tube has adequate capacity for formaldehyde when the
recommended sampling method is followed. 4.5. Desorption Efficiency
The desorption
efficiency of acrolein and formaldehyde was determined by injecting the
analytes onto separate 150-mg portions of coated XAD-2
adsorbent. Analytical standards were prepared by injecting equivalent
amounts of the analytes into 1-mL aliquots of toluene containing 15
mg/mL 2-HMP. The samples and toluene solutions were spiked with the
analytes and then stored at room temperature overnight before
analysis.
Table 4.5.1.
Desorption Efficiency
of
Acrolein from XAD-2 Coated with 10% 2-HMP
|
| × target conc. |
0.4× |
0.7× |
0.9× |
1.1× |
1.4× |
1.8× |
| µg/sample |
4 |
8 |
10 |
12 |
16 |
20 |
|
| desorption |
92.2 |
96.2 |
99.6 |
109 |
97.4 |
93.1 |
| efficiency, % |
84.9 |
115 |
96.0 |
95.9 |
97.2 |
107 |
|
104 |
114 |
104 |
93.4 |
97.2 |
92.7 |
|
100 |
112 |
109 |
104 |
96.9 |
103 |
|
109 |
106 |
110 |
100 |
111 |
108 |
|
103 |
101 |
105 |
105 |
116 |
96.8 |
|
 |
98.8 |
107 |
104 |
101 |
103 |
100 |
|
| The average
desorption efficiency for acrolein was 102% and the standard
deviation was 7.3%. |
Table 4.5.2.
Desorption Efficiency
of
Formaldehyde from XAD-2 Coated with 10% 2-HMP
|
| × target conc. |
0.4× |
0.8× |
1.0× |
1.2× |
1.6× |
2.0× |
| µg/sample |
36.4 |
72.8 |
91.0 |
109.2 |
145.6 |
182 |
|
| desorption |
96.2 |
94.6 |
96.7 |
93.8 |
99.3 |
106 |
| efficiency, % |
93.0 |
99.3 |
97.0 |
101 |
97.1 |
97.5 |
|
90.5 |
94.8 |
92.7 |
97.7 |
98.0 |
96.5 |
|
99.2 |
96.4 |
86.3 |
99.2 |
92.1 |
93.4 |
|
97.8 |
98.8 |
91.0 |
91.0 |
97.5 |
95.9 |
|
103 |
98.7 |
93.8 |
93.8 |
99.7 |
94.5 |
|
 |
96.6 |
97.1 |
92.9 |
96.1 |
97.3 |
97.3 |
|
| The average
desorption efficiency for formaldehyde was 96.2% and the standard
deviation was 3.8%. |
4.6. Storage data
Test atmospheres were generated
in the manner discussed in Section 4.10. The acrolein samples were
collected from an atmosphere containing 0.35 mg/m³ acrolein. The
relative humidity of the air was 49% at 27°C. The sampling rate was 0.2
L/min and the sampling time was 150 min. The amount of acrolein thus
collected was equivalent to sampling a 0.22 mg/m³ atmosphere for 8 h at
0.1 L/min. The formaldehyde samples were collected from an atmosphere
containing 4.4 mg/m³ formaldehyde. The relative humidity of the air was
45% at 24°C. The sampling rate was 0.1 L/min and the sampling time was
215 min. The amount of formaldehyde thus collected was equivalent to
sampling a 3.9 mg/m³ atmosphere for 4 h at 0.1 L/min. The data in Tables
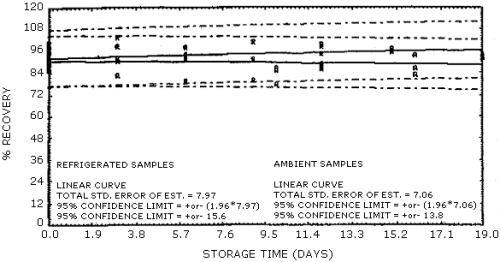
4.6.1. and 4.6.2. represent the effects of storage at ambient (21 to
26°C) and reduced (-20°C) temperatures on these samples. These data are
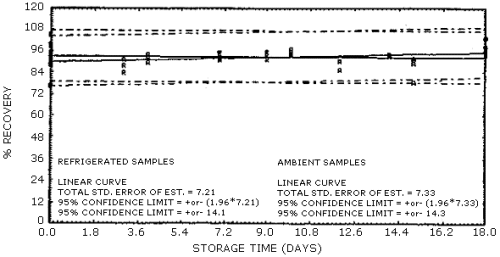
presented graphically in Figures 4.6.1. and 4.6.2.
Table 4.6.1.
Acrolein Storage Test
|
| storage |
ambient |
storage |
refrigerated |
| time, days |
recovery, % |
time, days |
recovery,
% |
|
| 0 |
85.7 |
95.7 |
99.0 |
0 |
90.8 |
87.7 |
97.2 |
| 0 |
89.8 |
92.9 |
90.9 |
0 |
87.3 |
87.4 |
91.5 |
| 3 |
92.3 |
82.7 |
90.4 |
0 |
90.8 |
92.9 |
101. |
| 6 |
94.2 |
91.6 |
92.9 |
3 |
98.7 |
104. |
92.0 |
| 10 |
85.0 |
86.8 |
77.6 |
6 |
98.0 |
79.2 |
93.1 |
| 12 |
86.8 |
85.9 |
88.9 |
9 |
79.6 |
91.1 |
101. |
| 16 |
82.7 |
86.1 |
93.8 |
12 |
94.7 |
99.6 |
98.3 |
| 19 |
93.2 |
94.7 |
92.2 |
15 |
97.4 |
95.2 |
96.2 |
|
Table 4.6.2.
Formaldehyde Storage
Test
|
| storage |
ambient |
storage |
refrigerated |
| time, days |
recovery, % |
time, days |
recovery,
% |
|
| 0 |
88.2 |
98.7 |
93.4 |
0 |
76.2 |
105. |
92.0 |
| 0 |
100. |
95.8 |
92.0 |
0 |
91.2 |
90.4 |
91.6 |
| 4 |
89.4 |
92.6 |
94.3 |
3 |
84.0 |
87.7 |
91.3 |
| 7 |
91.0 |
90.7 |
90.7 |
7 |
95.1 |
92.2 |
91.3 |
| 10 |
94.7 |
97.3 |
93.7 |
9 |
95.7 |
91.2 |
93.7 |
| 12 |
90.1 |
85.3 |
90.5 |
14 |
93.5 |
93.0 |
94.2 |
| 15 |
91.8 |
89.2 |
78.4 |
18 |
94.5 |
96.1 |
94.3 |
| 18 |
103. |
98.3 |
98.3 |
|
|
4.7. Reproducibility
data
Separate acrolein and formaldehyde samples were collected
from test atmospheres which were generated in the manner discussed in
Section 4.10. The samples and draft copies of this evaluation were given
to chemists unassociated with this work. The acrolein samples were
analyzed after 7 days of storage at ambient temperature. The
formaldehyde samples were analyzed following 15 days of
storage.
Table 4.6.2.
Formaldehyde Storage
Test
|
| analyte |
acrolein |
formaldehyde |
| µg/sample |
10.6 |
94.6 |
|
| % |
87.7 |
97.7 |
| recovered |
97.2 |
96.8 |
|
111. |
97.3 |
|
97.2 |
95.2 |
|
112. |
93.3 |
|
88.7 |
97.4 |
|
 |
99.0 |
96.3 |
| SD |
10.5 |
1.7 |
|
4.8. A procedure to
coat XAD-2 adsorbent with 2-HMP
4.8.1. Apparatus
4.8.1.1. Soxhlet extraction
apparatus.
4.8.1.2. Rotary evaporation
apparatus.
4.8.1.3. Vacuum desiccator.
4.8.1.4.
Miscellaneous glassware: 1-L vacuum flask, 1-L round-bottomed
evaporative flask, 1-L Erlenmeyer flask, 250-mL Buchner funnel with
a coarse fritted disc, etc. 4.8.2. Reagents
4.8.2.1. Methanol, isooctane and
toluene. Burdick and Jackson solvents were used in this
evaluation.
4.8.2.2. 2-(Hydroxymethyl)piperidine. The Aldrich
Chemical, Technical Grade was recrystallized from isooctane for use
in this evaluation.
4.8.2.3. Amberlite XAD-2 non-ionic
polymeric adsorbent, 20 to 60 mesh. Aldrich Chemical XAD-2 adsorbent
was used in this evaluation. 4.8.3. Procedure
Weigh 125 g of crude XAD-2
adsorbent into a 1-L Erlenmeyer flask. Add about 200 mL of water to
the flask and then swirl the mixture to wash the adsorbent. Discard
any adsorbent that floats to the top of the water and then filter the
mixture using a fritted Buchner funnel. Transfer the adsorbent back to
the Erlenmeyer flask and repeat the water wash and the filtration. Air
dry the adsorbent for about 2 min. Transfer the adsorbent back to the
Erlenmeyer flask and add about 200 mL of methanol to the flask. Swirl
and filter the mixture as before. Transfer the washed adsorbent to a
1-L evaporative flask and remove the methanol using the rotary
evaporation apparatus. Cool the flask to room temperature and add 13 g
of 2-HMP and 200 mL of toluene to the flask. Swirl the mixture and
allow it to stand for 1 h. Remove the toluene using rotary
evaporation. Seal the evaporative flask and allow the coated adsorbent
to stand overnight at ambient temperature. Transfer the coated
adsorbent to a Soxhlet extractor and extract the material with toluene
for about 24 h. Replace the contaminated toluene with fresh toluene
and continue the extraction for an additional 24 h. Replace the second
aliquot of contaminated toluene with methanol and continue the Soxhlet
extraction for 4 h. Transfer the adsorbent to a weighed 1-L
round-bottomed evaporative flask and remove the methanol using the
rotary evaporation apparatus. Determine the weight of the adsorbent
and then add an amount of 2-HMP, which is 10%, by weight, of the
adsorbent. Add 200 mL of toluene and then swirl the mixture. Allow the
flask to stand for 1 h. Remove the toluene using rotary evaporation.
If the last traces of toluene are difficult to remove, add about 100
mL of methanol to the flask, swirl the mixture and then remove the
solvents using rotary evaporation. XAD-2 adsorbent treated in this
manner will often contain residual formaldehyde derivative levels of
about 0.1 to 0.5 µg/150 mg of adsorbent. If the formaldehyde blank or
any other interference is determined to be too high, then the batch
should be returned to the Soxhlet extractor, extracted with toluene
again and then recoated with 2-HMP. This process can be repeated until
the desired blank and level of chromatographic interferences are
attained.
The coated adsorbent is now ready to be packed into
sampling tubes. The sampling tubes should be stored in a sealed
container to prevent contamination. Sampling tubes should be stored in
the dark at room temperature. The sampling tubes should be segregated
by coated adsorbent lot number. A sufficient amount of each lot number
of coated adsorbent should be retained to prepare analytical standards
for use with air samples from that lot number. 4.9. A procedure to determine formaldehyde by acid
titration
4.9.1. Apparatus
Miscellaneous
glassware. Fifty-milliliter burette, 250-mL Erlenmeyer flasks, 1-L
volumetric flasks, pipets, etc.
4.9.2. Reagents
4.9.2.1. Sodium sulfite, anhydrous.
Prepare a 0.1 M solution by dissolving 12.6 g of the salt in 1 L of
deionized water.
4.9.2.2. Hydrochloric acid, reagent grade.
Prepare a 0.1 N solution by diluting 7.9 mL of 38% HCl to 1 L with
deionized water.
4.9.2.3. Thymolphthalein indicator. Prepare
a 0.1% solution in ethanol.
4.9.2.4. Methyl orange indicator.
Prepare a 0.1% solution in ethanol.
4.9.2.5. Sodium
carbonate, ACS primary standard grade. 4.9.3. Procedure
Standardize the 0.1 N HCl
solution using sodium carbonate and methyl orange indicator. A
complete procedure for the standardization is presented in Ref.
5.11.
This procedure to determine formaldehyde was adapted from
the method presented in Ref. 5.12.
Place 50 mL of 0.1 M sodium
sulfite and three drops of thymophthalein indicator into a 250-mL
Erlenmeyer flask. Titrate the contents of the flask to a colorless
endpoint with 0.1 N HCl (usually one or two drops is sufficient).
Transfer 10 mL of the formaldehyde/methanol solution (prepared in
Section 3.3.2.) into the same flask and titrate the mixture with 0.1 N
HCl, again, to a colorless endpoint. The formaldehyde concentration of
the standard can be calculated by the following
equation:
| Formaldehyde, mg/mL = |
acid titer × acid normality ×
30.0
mL of sample |
This
method is based on the quantitative liberation of sodium hydroxide
when formaldehyde reacts with sodium sulfite to form the
formaldehyde-bisulfite addition product. The volume of
sample may be varied depending on the formaldehyde content but the
solution to be titrated must contain excess sodium sulfite.
Formaldehyde solutions containing substantial amounts of acid or base
must be neutralized before analysis. 4.10. Generation of test atmospheres
Controlled
test atmospheres of acrolein and formaldehyde were separately generated
using a Metronics Model 450 Dynacalibrator permeation apparatus. The
Metronics apparatus consists of a permeation device, usually a sealed
Teflon tube, containing the test material which is maintained at
constant temperature in a heated chamber. The permeation device provides
a constant flow of the test material into a carrier gas stream. The
carrier gas used in this evaluation was clean, dry nitrogen at a fixed
flow rate of 0.4 L/min. Certified permeation tubes were purchased from
Metronics. The effluent of the permeation chamber was diluted with humid
air which was introduced into the chamber stream using a calibrated
rotometer. The humid air was generated by bubbling clean, dry air
through a temperature controlled water bath. The relative humidity of
the combined chamber effluent and dilution air was determined, after
mixing, using a YSI Model 91 Dew Point Hygrometer. The relative humidity
of the test atmospheres was usually less than 80% because the permeation
chamber purge flow rate of dry gas was high in relation to the flow rate
of humid dilution air. Sampling was performed at a glass manifold
equipped with 6 ports.
The theoretical concentrations of the
acrolein test atmospheres were determined from the permeation rate of
the acrolein source tube and the sum of the purge flow rate and the
dilution air flow rate. The permeation rate of the tube was established
by maintaining the device in the constant temperature permeation chamber
with a purge flow of dry nitrogen and weighing the device periodically
until a constant weight loss per unit time was achieved. The permeation
rate of the acrolein tube was 444 ng/min at 30°C. The average assay of
"day zero" samples, used in storage tests for this evaluation (Section
4.6.), was 92.0% of the theoretical amount based on the gravimetric
permeation rate.
The theoretical concentrations of the
formaldehyde test atmospheres were also determined from the permeation
rate of the formaldehyde source tube and the sum of the purge flow rate
and the dilution flow rate. The permeation rate of the tube could not be
determined gravimetrically because the tube contained paraformaldehyde,
from which formaldehyde was generated by heating the tube. The
permeation rate of the tube was established by the use of two
independent sampling and analytical methods. One of the methods was the
aforementioned NIOSH adsorbent tube procedure for formaldehyde (Ref.
5.2.). The other method utilized bubblers containing
2,4-dinitrophenylhydrazine (DNPH) for sampling and then analysis by HPLC
(Ref. 5.13.). The permeation rate of the formaldehyde tube was 4711
ng/min at 100°C. The average assay of "day zero" samples, used in the
storage tests for this evaluation was 92.9% of the theoretical amount
based on the permeation rate as determined by the NIOSH and DNPH
methods. 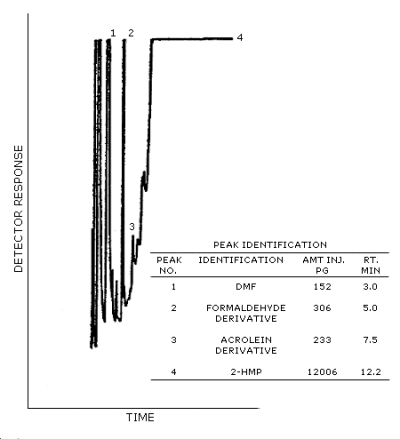
Figure 4.1. The detection limits of the analytical procedure
for formaldehyde and acrolein.

Figure 4.3.1.
Calibration curve for acrolein.

Figure 4.3.2.
Calibration curve for formaldehyde.
Figure
4.6.1. Storage tests for acrolein.
Figure
4.6.2. Storage tests for formaldehyde.

Figure 4.11. Typical chromatogram of a
standard containing DMF and the 2-HMP derivatives of acrolein and
formaldehyde.
4.11. Additional evaluation
data
This work was performed to verify that OSHA Method 52 (Ref.
5.14.) is suitable to monitor compliance with the new, reduced OSHA PELs
for formaldehyde. The previous PELs included an 8-h TWA of
3 ppm, a ceiling of 5 ppm and a 30 min peak of 10 ppm (Ref. 5.15.). The
new PELs consist of an 8-h TWA of 1 ppm, a 15-min STEL of 2
ppm and an "action level" of 0.5 ppm (Ref. 5.16.). The action level is
to be measured as an 8-h TWA. The action level is intended
to minimize the compliance burden for employers with workplaces in which
exposure to formaldehyde is low.
The areas of interest regarding
OSHA Method 52 which are addressed in this report include: (1) The
feasibility of increasing the sampling rate to monitor compliance with
the new OSHA STEL, (2) the determination of the residual (blank) amount
of formaldehyde derivative present in formaldehyde sampling tubes, (3)
the desorption efficiency of formaldehyde from samples prepared at 0.5,
1 and 2 times the new OSHA TWA, (4) the sensitivity and precision of the
analytical procedure at the new OSHA standard and (5) the ambient
temperature storage stability of samples collected at the new OSHA TWA.
An additional area of interest is also addressed; Supelco has recently
marketed a commercial version of the OSHA formaldehyde air sampling
tube. The Supelco sampling tubes were tested to determine if they are
suitable for use by OSHA.
Reagents. Formaldehyde sampling tubes, containing
XAD-2 adsorbent which has been coated with
2-(hydroxymethyl)piperidine (2-HMP), were
obtained from Supelco and also from the OSHA Analytical Laboratory.
2-HMP was purchased from Aldrich Chemical Company and was
recrystallized from isooctane prior to use. Toluene, methanol and
dimethylformamide were obtained from American Burdick and Jackson.
Permeation tubes, 0 Teflon, containing paraformaldehyde, were purchased
from VICI Metronics. Formaldehyde, 37% by weight in water, ACS Reagent
Grade, was purchased from Aldrich Chemical Company. The exact
concentration of the Aldrich formaldehyde solution was determined by
titration as specified in OSHA Method 52.
Instrumentation. The determinations were performed
using a Hewlett-Packard 5840A GC equipped with a nitrogen phosphorus
detector (NPD) . The NPD was set to give a 75-mm offset at
attenuation 8. Injections were made with a Hewlett-Packard
Model 7671A automatic sampler. A 6-ft ×
1/4-in. o.d. (2-mm i.d.) glass GC column
containing 10% UCON 50-HB-5100 with 2% KOH on 80/100 mesh
Chromosorb W-AW was purchased from Supelco to perform the separations.
Injections were made on-column. The GC column was temperature programmed
in two stages. First stage: 100°C to 140°C at 4°C/min. Second stage:
140°C to 180°C at 20°C/min. The column was then maintained at 180°C for
the balance of the determination. The total GC analysis time was about
30 min. The GC injector temperature was 180°C and the detector
temperature was 275°C. The GC carrier gas was helium and the flow rate
was 30 mL/min.
Apparatus. Controlled test
atmospheres of formaldehyde were generated using a Metronics Model 450
Dynacalibrator permeation apparatus. The apparatus contained a Teflon
permeation tube which was maintained at 100°C. The permeation device
provided a constant flow of formaldehyde into a carrier gas stream. The
carrier gas used in this work was clean, dry nitrogen at a fixed flow
rate of 0.4 L/min. The effluent of the permeation chamber was diluted
with humid air which was introduced into the chamber stream using a
calibrated rotameter. The humid air was generated by bubbling clean, dry
air through a temperature-controlled water bath. The relative humidity
of the combined chamber effluent and dilution air was determined, after
mixing, using a YSI Model 91 Dew Point Hygrometer. Sampling was
performed using calibrated, adjustable sampling tube flow holders (SKC,
Inc.) at a glass manifold equipped with six sampling ports.
Procedure. Air samples were generated by
sampling controlled test atmospheres with either OSHA or Supelco
sampling tubes. Formaldehyde stock standards were prepared by diluting
aqueous formaldehyde with methanol. Analytical standards and test
samples were prepared by spiking 150-mg portions of OSHA lot 12 coated
adsorbent with appropriate amounts of formaldehyde stock standards.
Additional analytical standards which did not utilize coated adsorbent
(solution standards) were prepared by spiking 1-mL aliquots of toluene,
which contained 15 mg/mL recrystallized 2-HMP, with appropriate amounts
of formaldehyde stock standards. Analytical standards and test samples
were prepared about 16 h prior to analysis to ensure the complete
reaction of formaldehyde with 2-HMP. Coated adsorbent standards and
samples were desorbed with 1-mL toluene for 1 h before analysis.
Dimethylformamide internal standard was added to the toluene which was
used for the desorption of samples, desorption of coated-adsorbent
standards and also for the dilution of recrystallized 2-HMP. Air samples
were analyzed using coated-adsorbent standards. Test samples and blanks
were analyzed with solution standards. The results are reported as
formaldehyde even though the actual analyzed species was the 2-HMP
derivative of formaldehyde.
Increasing the sampling rate. The 2-ppm formaldehyde
OSHA STEL requires a 15-min sample. OSHA's sampling method
specifies a 0.1 L/min sampling rate. A 15-min sample
collected at 0.1 L/min from a 2-ppm atmosphere would
contain only 4 µg of formaldehyde. An increase in the sampling rate
would cause more formaldehyde to be collected and this would result in a
potentially more accurate and precise determination.
Sampling
tube capacity must be considered when determining a sampling rate.
Sampling tube capacity was evaluated by determining breakthrough from
the front to the back sections of sampling tubes which were used to
sample a test atmosphere for increasing periods of time. Excessive
breakthrough could indicate that either the 2-HMP had been depleted or
that the formaldehyde residence time was not long enough for the
derivatization reaction to be complete. A limited number of samples were
collected from a 2-ppm formaldehyde test atmosphere at 0.5 L/min. This
sampling rate was found to be unacceptable because of the high
breakthrough observed after sampling for only 15 min. Because excess
2-HMP was present in these samples, sampling at 0.5 L/min failed
apparently because of inadequate formaldehyde residence time in the
sampling tube. Sampling tube capacity was therefore evaluated at 0.2
L/min using OSHA and Supelco tubes by sampling a test atmosphere. The
formaldehyde concentration was 2 ppm and the relative humidity was 64%
at 25°C. The results of this study are presented in Table
1.
Table 1
Sampling Tube Capacity
|
| OSHA lot 12 |
Supelco lot
673-30 |
Supelco lot
673-40 |
| air vol (L) |
BT (%) |
air vol (L) |
BT (%) |
air vol (L) |
BT (%) |
|
| 24.0 |
1.9 |
24.6 |
1.0 |
24.9 |
5.9 |
| 27.6 |
2.1 |
27.6 |
1.0 |
29.4 |
7.2 |
| 37.2 |
5.4 |
35.5 |
1.2 |
37.1 |
10.0 |
| 41.6 |
7.3 |
|
41.9 |
12.3 |
| 46.8 |
9.2 |
46.2 |
2.6 |
|
| 50.9 |
10.8 |
|
51.6 |
15.2 |
| 55.8 |
11.7 |
|
56.2 |
16.9 |
| 66.1 |
12.2 |
65.6 |
9.1 |
|
|
| BT =
breakthrough |
The air
volumes at which 5% breakthrough occurred were determined graphically by
plotting the data in Table 1. The breakthrough plots are shown in
Figures 1, 2 and 3. The 5% breakthrough air volumes were: OSHA lot 12 =
35 L, Supelco lot 673-30 = 50 L and Supelco lot 673-40 = 23 L. The data
show that the sampling rate can be increased to 0.2 L/min to monitor
compliance with the 15-min OSHA STEL. These data also show that there
are capacity variations between lots of formaldehyde sampling tubes. The
sampling rate should not be increased from 0.1 L/min to monitor
compliance with the TWA.
Blank
determinations. The sampling and analytical procedure for
formaldehyde is unique in that a significant blank-amount subtraction
must be performed. All XAD-2 adsorbent coated with 2-HMP will contain
some amount of residual formaldehyde derivative which must be determined
so that the blank subtractions can be made. Blank subtractions should be
performed both on standards and on samples. The blank correction is
especially important at low formaldehyde levels or when the blank amount
is high.
The amount of residual derivative present in the front
sections of ten OSHA lot 12 formaldehyde sampling tubes was determined.
The ten sampling tubes were selected at random. The results of this
study are presented in Table 2.
Table 2
Formaldehyde Blank
Determinations
|
| sample no. |
amount (µg) |
Sample no. |
amount (µg) |
|
| 1 |
0.74 |
6 |
0.70 |
| 2 |
0.66 |
7 |
0.76 |
| 3 |
0.59 |
8 |
0.62 |
| 4 |
0.74 |
9 |
0.56 |
| 5 |
0.74 |
10 |
0.71 |
 = 0.68 = 0.68 |
SD = 0.07 |
CV =
0.10 | |
|
The blank amounts
determined for field-blank samples may be different from those shown in
Table 2. This may be due to differences in sampling-tube lots, minor
differences in instrument calibration, field-blank sample contamination
and other indeterminate causes. The field-blank amount should be used to
perform blank subtractions from the associated field
samples.
Because of the imprecision of the blank-amount
determination, errors can inadvertently be introduced into the analysis
of field samples. It is, therefore, essential to take precautions to
assure that the presence of formaldehyde is not reported when it is
absent. One such precaution to minimize the possibility of this error
occurring would be to utilize an arbitrary parameter called the "minimum
reportable amount" (MRA). The MRA is based on the assumption that the
precision of all blank-amount determinations is similar to that in Table
2. No field-sample result (after blank subtraction) less than the MRA
should be used in subsequent calculations. Field samples containing less
formaldehyde than the MRA should be reported simply as "less than MRA".
The MRA should not be confused with the reliable quantitation limit. A
field-sample result lower than the reliable quantitation limit can be
reported with confidence because it is the difference of two sample
results which were each larger than the reliable quantitation limit. The
MRA is calculated as follows:
MRA = 1.96 × 0.10 ×
B
|
| where |
1.96 |
= |
z-statistic from the normal
distribution at the 95% confidence level |
|
0.10 |
= |
coefficient of variation from Table
2 |
|
B |
= |
field-blank amount determined from
the blank sample submitted with the set of field
samples |
Because the MRA
is a precaution against reporting false positive field-sample results,
it has significance only when the field-sample result is similar to the
field-blank amount.
Desorption
efficiency. No desorption efficiency corrections are necessary to
compute sample results because analytical standards are prepared using
coated adsorbent. Desorption efficiencies were determined, however, to
investigate formaldehyde recovery from the sampling medium. The results
of this study are presented in Table 3. The average desorption
efficiency over the studied range was 101.4% and the SD was
2.6%.
Table 3
Percent Desorption
Efficiency
|
|
15.4 µg |
24.1 µg |
30.9 µg |
34.4 µg |
55.0 µg |
61.8 µg |
|
0.5× |
0.8× |
1.0× |
1.2× |
1.9× |
2.1× |
|
|
98.3 |
99.8 |
98.2 |
101.6 |
102.8 |
98.5 |
|
98.1 |
101.2 |
99.6 |
106.4 |
102.2 |
101.8 |
|
101.1 |
101.4 |
96.9 |
105.0 |
105.2 |
98.7 |
|
99.0 |
104.7 |
99.9 |
99.0 |
103.6 |
99.5 |
|
102.1 |
104.8 |
99.0 |
103.5 |
106.0 |
103.5 |
|
102.1 |
103.9 |
98.8 |
101.4 |
102.6 |
98.4 |
| |
 |
100.1 |
102.6 |
98.7 |
102.8 |
103.7 |
100.0 |
|
Sensitivity and precision. The sensitivity and
precision of the analytical method was evaluated by performing multiple
determinations of coated-adsorbent standards which were prepared at 0.5,
1 and 2 ppm. The results of the sensitivity and precision study are
presented in Table 4. ISTD data are results from an internal standard
calibration.
Table 4
Sensitivity and Precision
Data
|
|
0.52 ppm |
1.0 ppm |
2.1 ppm |
|
15.4
µg/sample |
30.9
µg/sample |
61.8
µg/sample |
|
ISTD |
area |
ISTD |
area |
ISTD |
area |
|
|
15.4 |
3144000 |
31.2 |
6365000 |
62.2 |
12690000 |
|
15.3 |
3132000 |
31.2 |
6372000 |
61.9 |
12631000 |
|
15.4 |
3154000 |
31.2 |
6374000 |
61.8 |
12614000 |
|
15.4 |
3152000 |
30.4 |
6216000 |
61.9 |
12645000 |
|
15.5 |
3170000 |
30.8 |
6294000 |
61.5 |
12559000 |
|
15.6 |
3183000 |
30.5 |
6235000 |
61.5 |
12563000 |
|
 |
15.43 |
|
30.88 |
|
61.80 |
|
| SD |
0.1033 |
|
0.3708 |
|
0.2681 |
|
| CV |
0.00670 |
|
0.01201 |
|
0.00433 |
|
|
The pooled coefficient
of variation at the new OSHA standard is 0.0083. It is similar to that
obtained at the previous standard which was 0.0052. The sensitivity of
the analytical method is defined as the slope of the calibration curve.
The calibration curve was prepared by plotting the data in Table 4 and
it is shown in Figure 4. The sensitivity of the analytical method is
203936 area units per µg/mL.
Ambient temperature
storage stability. Storage samples were generated by sampling a
test atmosphere containing 2-ppm formaldehyde at 0.1 L/min for 2 h. The
relative humidity of the test atmosphere was 58% at 28°C. The results of
the ambient temperature storage study are presented in Table 5 and in
Figures 5 and 6.
Table 5
Ambient Temperature Storage
Test
|
| storage time |
OSHA lot 12 |
storage time |
Supelco lot
673-30 |
| (days) |
(%
recovered) |
(days) |
(%
recovered) |
|
| 0 |
100.4 |
96.8 |
99.6 |
0 |
104.4 |
106.0 |
105.2 |
| 4 |
104.8 |
97.6 |
101.2 |
3 |
102.8 |
99.2 |
97.6 |
| 7 |
95.4 |
100.9 |
100.0 |
6 |
95.1 |
99.2 |
97.4 |
| 11 |
98.8 |
101.2 |
100.0 |
10 |
102.0 |
98.8 |
98.4 |
| 14 |
103.2 |
102.4 |
100.4 |
13 |
102.0 |
96.8 |
97.2 |
| 19 |
97.6 |
98.8 |
104.4 |
18 |
97.6 |
97.6 |
99.6 |
|
These data show that
samples containing the equivalent of 1 ppm formaldehyde are stable for
at least 19 days of storage at ambient temperature.
The present sampling and analytical method used by OSHA to
monitor occupational exposure to formaldehyde is suitable for use at the
new OSHA TWA and action level. The recommended sampling rate for STEL
samples is 0.2 L/min. The sampling tubes which were purchased from
Supelco gave acceptable sample results. 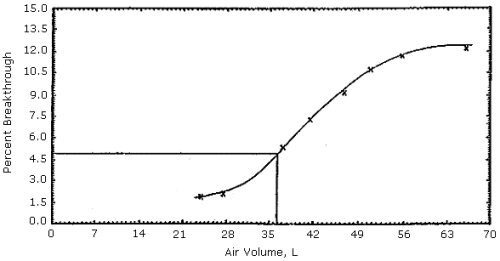
Figure 1. OSHA sampling tubes (lot
12) capacity test.
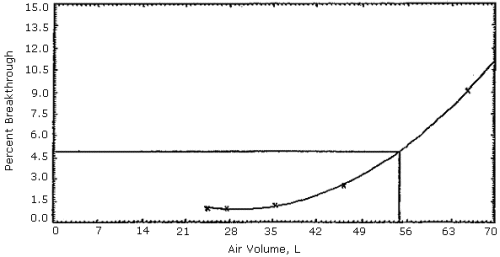
Figure 2. Supelco
sampling tubes (lot 673-30) capacity test.
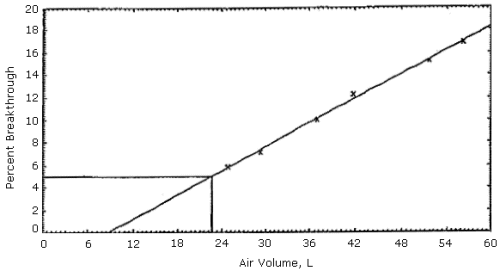
Figure 3.
Supelco sampling tubes (lot 673-40) capacity test.

Figure 4. Calibration curve for
formaldehyde.
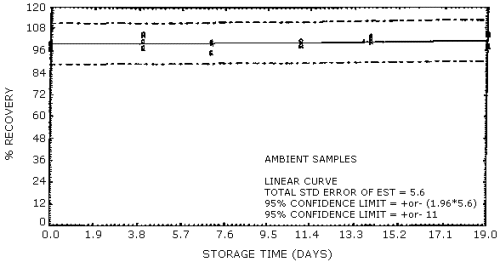
Figure 5. OSHA sampling
tubes (lot 12) ambient temperature storage test.
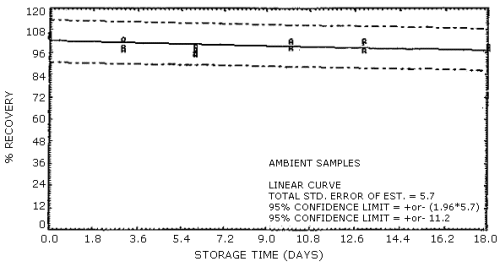
Figure 6. Supelco sampling tubes (lot 673-40) ambient
temperature storage test.
5. References
5.1. "Industrial Hygiene Technical
Manual", U.S. Department of Labor-Occupational Safety and
Health Administration, March 30, 1984, Appendix A-Chemical
Information Tables.
5.2. Kennedy, E.R.; O'Connor, P.F. and
Gagnon, Y.T. Anal. Chem. (1984), (56),
2120-2123.
5.3. Kennedy, E.R. and Hill, R.H. Anal. Chem. (1982),(54), 1739-1742.
5.4.
"Information Profiles on Potential Occupational Hazards, Vol. I. Single
Chemicals. Acrolein", Syracuse Research Corporation, U.S. Dept. of
Commerce, NTIS, Springfield, VA., PB 81-147951.
5.5.
"Acrolein Health Effects", Midwest Research Inst., U.S. Dept. of
Commerce, NTIS, Springfield, VA., PB 82-161282.
5.6. "IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Some Monomers, Plastics and Synthetic Elastomers, and Acrolein"
International Agency for Research on Cancer: Lyon, 1979, Vol. 19,
479-494.
5.7. "IARC Monograph on the Evaluation of
the Carcinogenic Risk of Chemical to Humans, Some Industrial Chemicals
and Dyestuffs" International Agency for Research on Cancer: Lyon, 1982,
Vol. 29, 345-389.
5.8. "IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Man, some Aromatic
Amines Hydrazine and Related Substances, N-nitroso
Compounds and Miscellaneous Alkylating Agents", International Agency for
Research on Cancer: Lyon, 1973, Vol. 4,
231-237.
5.9. "Current Intelligence Bulletin No. 34,
Formaldehyde: Evidence of Carcinogenicity", April 15, 1981, U.S. Dept.
of Health and Human Services, Public Health Service, Center for Disease
Control, NIOSH .
5.10. "Environmental Health Perspectives"
(1982),(43), 139-168.
5.11. Treadwell, F.P. and
Hall, W.T. "Analytical Chemistry", John Wiley and Sons, Inc.: New York,
1948, Vol. II, 481-483.
5.12. Walker, J.F.
"Formaldehyde", Reinhold Publ. Corp.: New York, 1953, 382.
5.13.
Kurvata, K.; Vebori, M.; Yamasaki, H.; Kuge, Y. and Kiso, Y. Anal. Chem. (1983), (55), 2013-2016.
5.14.
"OSHA Analytical Methods Manual", U.S. Department of Labor, Occupational
Safety and Health Administration, OSHA Analytical Laboratory: Salt Lake
City, UT, Method 52, American Conference of Governmental Industrial
Hygienists (ACGIH): Cincinnati, 1985, ISBN:
0-936712-66-X.
5.15. "Code of Federal Regulations";
Office of the Federal Register, National Archives and Records Service
Administration; U.S. Government Printing Office:, Washington, DC, 1985,
29 CFR 1901.1 Ch. XVII (7-1-85 Edition) 1910.1000, Table
Z-2.
5.16. "Fed. Regist.", 1987, 52 (December 4),
46168-46313.
|













