CYCLOHEXANONE
| Method no.: | 01 |
| Matrix: | Air |
| Target concentration: | 50 ppm (200 mg/m3) (OSHA PEL) |
| Procedure: | Collection of vapors on Chromosorb 106, desorption with carbon disulfide, analysis by gas chromatography with flame ionization detection. |
| Detection limit based on recommended air volume: (For analytical procedure only) |
0.05 ppm |
| Recommended air volume and sampling rate: |
10 L at 0.05 to 0.2 L/min |
| Standard error of estimate at the target concentration: (Section 4.3.) |
5.2% |
| Status of Method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1979 | Chemist: Carl J. Elskamp |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In the past, cyclohexanone has been determined by collection on activated charcoal, desorption with carbon disulfide and analysis by gas chromatography (Ref. 5.1.). It has now been found that once cyclohexanone is collected on charcoal, the recovery drops off severely with time (Ref. 5.2.). This new method requires collection of cyclohexanone vapors on Chromosorb 106 (Ref. 5.3.) instead of charcoal. The cyclohexanone on Chromosorb 106 remains stable for at least 16 days, even at room temperature. The OSHA Analytical Laboratory, Salt Lake City, Utah, has analyzed from 400 to 500 cyclohexanone samples per year for the last two years.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy)
Breathing vapors constitute the prime route of absorption by the body. The most important effects of inhalation of concentrations exceeding the PEL (50 ppm, 200 mg/m3) are eye, nose, and throat irritation and narcosis (Ref. 5.4. and 5.5.). It can cause a dermatitis upon skin contact and also can cause damage to the liver and kidneys (Ref. 5.6.). A concentration of 75 ppm inhaled by a human was considered an irritant (Ref. 5.7.).
1.1.3. Operations where exposures occur
The most important use for cyclohexanone is a chemical intermediate in the production of adipic acid (Ref. 5.4.). It is also used as a solvent for cellulose esters and ethers, dyes, resins, lacquers, shellac, oil, and fats. Its properties make it desirable as a degreaser, a spotting agent for removing stains in the dry cleaning and textile industries, a solvent in paint removers and printing inks, and in the plastics industry (Ref. 5.5.).
1.1.4. Size of work population that face exposure - unknown.
1.1.5. Physical properties (Ref. 5.4.)
| CAS number: | 108-94-1 |
| molecular weight: | 98.14 |
| boiling point: | 155.6°C |
| specific gravity: | 0.9478 at 20°C/4°C |
| flash point: | 111°F (43.6°C) Closed Cup 129°F (54°C) Open Cup |
| lower explosive limit: | 1.1% |
| evaporation rate (ether = 1): | 22.2 |
| color: | colorless to pale yellow |
| odor: | ketone-type odor, similar to acetone and peppermint |
| other names: | pimelic ketone, cyclohexyl ketone, ketohexamethylene, Anone, Hytrol O, Nadone, Sextone |
| structural formula: | 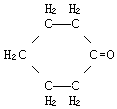 |
1.2. Detection limit, precision, sensitivity, and working range
- 1.2.1. The detection limit for the analytical procedure is 0.8
ng. The coefficient of variation at this level is 1.17%. This is
equivalent to 0.05 ppm for a
1.2.2. The pooled coefficient of variation for 1 to 4 mg of cyclohexanone per sample is 0.33% for the analytical method. This range is equivalent to 25 to 100 ppm cyclohexanone for a 10-L air sample. (Section 4.2.)
1.2.3. The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the PEL based on the recommended air volume is 396,000 area counts per mg/mL. The sensitivity is determined by the slope of the calibration curve. The sensitivity will vary somewhat with the particular instrumentation and parameters used in the analysis. (Section 4.2.)
1.2.4. The lower limit of the estimated working range, assuming adequate desorption efficiency, is 1 ppm. The upper limit of the working range is dependent on the capacity of the Chromosorb 106.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are at
least ±25% of the true values at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium must be 75% or greater.
1.3.3. The method meets the required criteria at the PEL
concentration for a
1.4. Advantages
- 1.4.1. The sampling procedure is convenient.
1.4.2. The analytical procedure is quick, sensitive and reproducible.
1.4.3. Reanalysis of samples is possible.
1.4.4. Samples are stable, even at room temperature.
1.4.5. It may be possible to analyze other compounds simultaneously.
1.4.6. Interferences can be circumvented by proper selection of GC parameters.
1.5. Disadvantages
Some interfering compounds are extracted from untreated Chromosorb 106. (Section 3.6.3.)
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated to within
±5% of the recommended flow rate with the sampling tube in line.
2.1.2. Chromosorb 106 tubes: Glass tube, with both ends heat
sealed, 85 mm ×
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break open the ends of the
Chromosorb 106 tube. All tubes must be from the same lot.
2.3.2. Connect the Chromosorb 106 tube to the sampling pump with flexible tubing. The short section of the Chromosorb 106 tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the Chromosorb 106 tube.
2.3.5. Seal the Chromosorb 106 tube with plastic caps immediately after sampling.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be
transported in glass containers with
2.4. Breakthrough
The average breakthrough (5% breakthrough) volume for three
separate samples from a
2.5. Desorption efficiency
- 2.5.1. The desorption efficiency, from liquid injections onto
the front section of the tubes, averaged 97.7% from 25 to 100 ppm
for a
2.5.2. The desorption efficiency of a particular compound may vary from one laboratory to another and also from one batch of Chromosorb 106 to another. Thus, it is necessary to determine the desorption efficiency for a particular batch of Chromosorb 106.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The large pressure drops across the Chromosorb 106 tubes limit the maximum flowrate. The acceptable flowrate range is 0.05 to 0.2 L/min.
2.7. Interferences
- 2.7.1. It is unknown if any other compound would severely
interfere with the collection of cyclohexanone on Chromosorb 106. In
general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Suspected interferences should be listed on the sample data sheets.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment on the employee so that it
does not interfere with work performance.
2.8.2. Wear safety glasses when breaking the ends of the sampling tubes.
2.8.3. Place the sampling tube in a holder so the sharp end is not exposed while sampling.
3. Analytical procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. GC column capable of separating cyclohexanone and an
internal standard from any interferences and carbon disulfide. The
column used for validation studies was:
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 10-µL or other convenient sizes for
preparing standards and
3.1.6. Pipets for dispensing carbon disulfide. The Glenco 1-mL dispenser is adequate and convenient.
3.1.7. Volumetric flasks, 5-mL and other convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Cyclohexanone, reagent grade.
3.2.2. Chromatographic grade carbon disulfide.
3.2.3. A reagent grade internal standard, such as ethyl benzene.
3.2.4. Desorbing reagent - 1 µL internal standard/1 mL CS2.
3.2.5. Purified GC grade helium, hydrogen, and air.
3.3. Sample preparation
- 3.3.1. The front and back sections of each sample are
transferred to separate
3.3.2. Each section is desorbed with 1.0 mL of desorbing reagent.
3.3.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.4. Standard preparation
- 3.4.1. Standards are prepared by diluting the pure cyclohexanone
with the desorbing reagent.
3.4.2. A concentration of 1 µL of cyclohexanone per 1 mL of
desorbing reagent equals 26.28 ppm for a
3.5. Analysis
- 3.5.1. GC conditions
| flow rates (mL/min) | zone temperatures (°C) | |||
| helium: | 25 | injector: | 200 | |
| hydrogen: | 35 | detector: | 250 | |
| air: | 250 | column: | 125 | |
| injection size: 1 µL | ||||
| cyclohexanone elution time: 5.7 min | ||||
3.5.2. Peak areas are measured by an integrator or other suitable means.
3.5.3. An internal standard procedure is used. The integrator is
calibrated to report results in ppm for a
3.6. Interferences
- 3.6.1. Any compound eluting in the same general time as
cyclohexanone or the internal standard is a potential interference.
Possible interferences are listed on the sample data sheets. GC
parameters should be chosen so these interferences will pose no
problems.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. When Chromosorb 106 is desorbed with CS2, some late eluting peaks appear on the chromatograms. These do not interfere with the analysis but the analysis time is increased. It may be possible to pretreat the Chromosorb 106 by heating it under a flow of an inert gas or by extracting it with a solvent prior to preparing the tubes for sampling.
3.6.4. Retention time data on a single column is not considered proof of chemical identity. Samples over the PEL should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Since the integrator is programmed to report results in ppm for a
| ppm cyclohexanone = | (ppm on report) (10)
(liters of air sampled) |
3.8. Safety precautions
- 3.8.1. All work done with the solvents (preparation of
standards, desorption of samples, etc.) should be done in a hood.
3.8.2. Avoid any skin contact with all of the solvents.
3.8.3. Wear safety glasses at all times.
4. Backup Data
- 4.1. Detection limit
A standard containing 0.002 µL cyclohexanone/mL CS2 (1.90 µg/mL) was used. Peak heights were used since the integrator could not pick up the peaks.
|
| |||
| injection | peak height (mm) | ||
|
| |||
| 1 | 34.5 | 35.0 | |
| 2 | 35.5 | SD = | 0.408 |
| 3 | 35 | CV = | 1.17% |
| 4 | 35 | ||
|
| |||
GC conditions used for determination of detection limit:
| Hewlett-Packard 5840A, flame ionization detector | ||||
| column: 10-ft × 1/8-in. stainless steel, 20% SP2100/0.1% CW1500 | ||||
| attenuation: 0 | ||||
| injection size: 0.4 µL | ||||
| temperatures (°C) | flows (mL/min) | |||
| injector: | 200 | helium: | 24 | |
| detector: | 200 | hydrogen: | 35 | |
| column: | 125 | air: | 240 | |
| Detection Limit = 1.90 ng/µL × 0.4 µL = 0.8 ng | ||||
| This amount is equivalent to 0.05 ppm for a
| ||||
4.2. Instrument response to the analyte and analytical precision
|
| |||
| injection | 0.5× PEL 948 µg |
1× PEL 1896 µg |
2× 3792 µg |
|
| |||
| 1 | 971 | 1889 | 3772 |
| 2 | 966 | 1897 | 3781 |
| 3 | 964 | 1899 | 3787 |
| 4 | (Lost) | 1891 | 3775 |
| 5 | 976 | 1900 | 3787 |
| 6 | 970 | 1902 | 3791 |
| 969.4 | 1896.3 | 3782.2 | |
| SD | 4.67 | 5.20 | 7.49 |
| CV | 0.00482 | 0.0027 | 0.0020 |
| | |||
|
| |||
See Figure 4.2. for calibration curve.
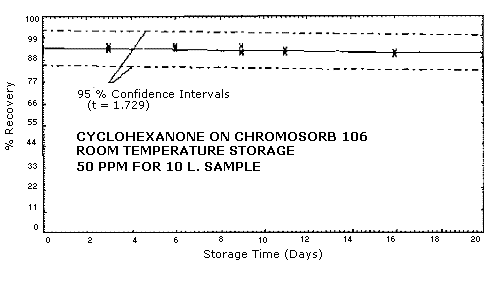
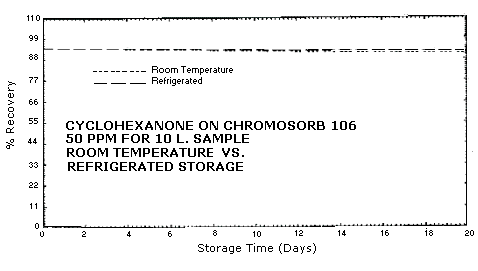
4.3. Storage Study
Samples were collected on Chromosorb 106 sampling tubes at 0.18
L/min for 55 min from a
|
| ||||||||
| storage time | % recovery | |||||||
| (days) | (refrigerated) | (ambient) | ||||||
|
| ||||||||
| 0 | 92.4 | 94.3 | 93.9 | 92.4 | 94.3 | 93.9 | ||
| 0 | 92.5 | 93.8 | 92.5 | 93.8 | ||||
| 3 | 93.6 | 93.7 | 97.7 | 93.9 | 93.7 | 95.3 | ||
| 6 | 95.5 | 93.0 | 94.8 | 94.3 | 93.9 | 95.2 | ||
| 9 | 98.8 | 94.1 | 90.2 | 95.0 | 91.2 | 92.1 | ||
| 11 | 93.6 | 93.2 | 92.5 | 93.0 | 91.3 | 93.1 | ||
| 16 | 91.2 | 91.6 | 89.7 | 90.2 | 89.8 | 91.6 | ||
|
| ||||||||
The standard error of estimate for room temperature samples is 5.2%. This includes a sampling error of ±5%. See Figures 4.3.1., 4.3.2, and 4.3.3.
4.4. Desorption efficiency
|
| |||
| × target conc. µg/sample |
0.5× 0.9952 |
1× 1.990 |
2× 3.981 |
|
| |||
| desorption | 97.2 | 98.1 | 96.7 |
| efficiency, | 96.4 | 98.1 | 96.9 |
| % | 98.2 | 98.1 | 98.2 |
| 96.5 | 97.4 | 97.3 | |
| 97.6 | 100.1 | 99.1 | |
| 96.4 | 97.9 | 97.8 | |
| 97.0 | 98.3 | 97.7 | |
|
| |||
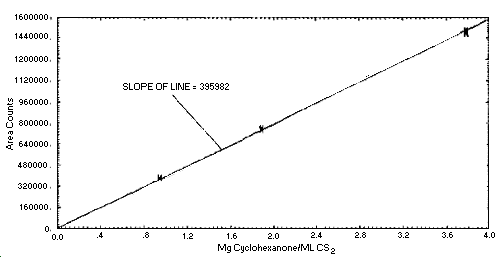
Figure 4.2. Calibration
curve.
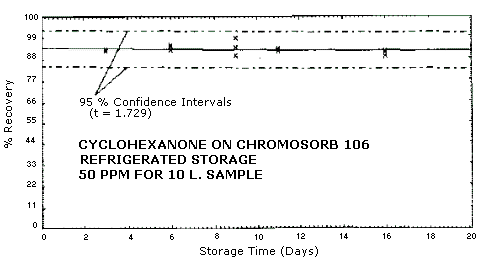
Figure 4.3.1. Refrigerated
storage samples.