SULFUR DIOXIDE IN WORKPLACE ATMOSHPERES
(IMPREGNATED
ACTIVATED BEADED CARBON)
| Method Number: | ID-200 | |
| Matrix: | Air | |
| OSHA Permissible Exposure Limits: Final Rule Limits: |
2 ppm Time Weighted Average (TWA) 5 ppm | |
| Transitional Limit: | 5 ppm TWA | |
| Collection Device: | An air sample is collected using a calibrated sampling pump and a glass tube containing impregnated activated beaded carbon (IABC). A prefilter/cassette assembly can be used to collect particulate, if necessary. | |
| Recommended Sampling Rate: TWA & STEL: |
0.1 liter per minute (L/min) | |
| Recommended Air Volume: TWA: STEL: |
12 L (0.1 L/min for 120 min) 1.5 L (0.1 L/min for 15 min) | |
| Analytical Procedure: | The sampling medium is desorbed in 15 mM sodium
hydroxide which contains 0.3 N ( | |
| Detection Limit: Qualitative: Quantitative: |
0.004 ppm (12-L air sample) 0.032 ppm (1.5-L air sample) 0.013 ppm (12-L air sample) 0.104 ppm (1.5-L air sample) | |
| Precision and Accuracy: | TWA
|
STEL
|
| Validation Range: CVT (pooled): Bias: Overall Error: |
1.36 to 4.16
ppm 0.048 -0.033 ±12.9% |
5.79 ppm 0.028 (CV2) +0.006 ±6.2% |
| Method Classification: | Validated Method | |
| Chemist: | James C. Ku | |
| Date: | April, 1992 | |
OSHA Salt Lake Technical Center
Salt Lake City, Utah
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by
USDOL-OSHA.
Similar products from other sources can be substituted.
1. Introduction
This method describes the sample collection and analysis of airborne sulfur dioxide (SO2). Samples are taken in the breathing zone of workplace personnel, and analysis is performed by ion chromatography (IC).
- 1.1. History
Previously, OSHA collected compliance samples for
SO2 exposure determinations in
![]() 1%) hydrogen peroxide
(H2O2) which
converted the SO2 to sulfuric acid
(H2SO4). The
amount of sulfate
(SO42-) in the
peroxide solution was measured by IC and gravimetrically converted to
represent the amount of SO2 collected (5.1.).
Because bubblers are inconvenient to use as personal samplers due to
spillage or breakage, it was desirable to develop a
1%) hydrogen peroxide
(H2O2) which
converted the SO2 to sulfuric acid
(H2SO4). The
amount of sulfate
(SO42-) in the
peroxide solution was measured by IC and gravimetrically converted to
represent the amount of SO2 collected (5.1.).
Because bubblers are inconvenient to use as personal samplers due to
spillage or breakage, it was desirable to develop a
Using the principle applied for the impregnated charcoal collection of SO2, a new material, impregnated activated beaded carbon (IABC) was developed. The IABC has a significantly lower background level of SO42- (< 3 µg). This current method was evaluated using the IABC as the collection media.
1.2. Principle
Sulfur dioxide is collected using IABC sorbent which is contained
in a glass tube. The collected SO2 is
converted to sulfite
(SO32-) by the
sorbent and then slowly oxidized to
SO42-. This
oxidation is augmented at the laboratory by addition of a desorbing
solution containing 0.3 N (![]() 1%)
H2O2 in 15 mM
sodium hydroxide (NaOH) to each IABC sample. The resultant
SO42- is analyzed
by IC using a conductivity detector; a gravimetric conversion is used
to calculate the amount of SO2 collected.
1%)
H2O2 in 15 mM
sodium hydroxide (NaOH) to each IABC sample. The resultant
SO42- is analyzed
by IC using a conductivity detector; a gravimetric conversion is used
to calculate the amount of SO2 collected.
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for determining
compliance with the OSHA
1.3.2. The method is simple, rapid, and easily automated.
1.3.3. The
SO42-
contaminant (background) levels of the IABC sorbent are very low
(< 3 µg), especially when compared to the impregnated
charcoal previously used in OSHA Method No.
1.3.4. A disadvantage is the need for a desorption efficiency
(DE) correction which is
1.4. Method Performance
A synopsis of method performance is presented below. Further information can be found in Section 4.
- 1.4.1. This method was validated over the concentration range of
1.36 to 4.16 ppm. An air volume of 12 L and a flow rate of 0.1 L/min
were used.
1.4.2. The qualitative detection limit was 0.0187 µg/mL or
0.187 µg (as
SO42-) when
using a
1.4.3. The quantitative detection limit was 0.0624 µg/mL
or 0.624 µg (as
SO42-) when
using a
1.4.4. The sensitivity of the analytical method, whenusing the instrumental parameters listed in Section 3.6., was calculated from the slope of a linear working range curve (0.5 to 10 µg/mL SO42-). The sensitivity was 2.2 × 107 area units per 1 µg/mL. A Dionex Series 4500i ion chromatograph with AI450 computer software was used (Dionex, Sunnyvale, CA).
1.4.5. This method compared favorably to OSHA Method no. ID-104 (modified) for SO2 (5.1.) which served as the reference method.
1.4.6. A desorption efficiency (DE) correction is required at mass loadings up to 400 µg SO2 (see Sections 3.7.3. and 4.1.).
1.4.7. The total pooled coefficient of variation
(CVT) for samples taken at about 0.5, 1,
and 2 times the OSHA PEL (1 to 4 ppm for
| TWA | STEL | LOW | |
| CV | 0.048 | 0.028 | 0.032 |
| Bias | -3.3% | +0.6% | -6.5% |
| OE | ±12.9% | ±6.2% | ±12.9% |
For the STEL evaluation, 5.32 ppm was used for test atmospheres. For sampling at a concentration (LOW) near what may be expected in indoor air monitoring, approximately 0.3 ppm SO2 was used. Bias and overall error (OE) values were calculated from those found analytically versus theoretical (known) values. The theoretical concentrations were calculated from flows of a certified cylinder of SO2 and dilution air.
1.4.8. The collection efficiency at 2 times the PEL was 100%. Samples were collected from a generated test atmosphere of 4 ppm SO2 for 120 min.
1.4.9. Breakthrough tests were performed at concentrations of 7.20 and 14.8 ppm SO2. No breakthrough was found for a sampling time of 240 min and an average sample flow rate of 0.1 L/min.
1.4.10. Samples can be stored at ambient (20 to 25 °C) temperature for a period of at least 30 days. Storage stability results show the mean sample recovery after 30 days was within ±10% of the theoretical calculations. Samples were stored on a laboratory bench.
1.5. Interferences
- 1.5.1. Other particulate sulfate compounds and
H2SO4 will
interfere in the analysis of SO2 if they
are collected in the IABC. Particulate and
H2SO4 mist can
be removed from the air during sampling using a modified sampling
device which contains a Teflon®
1.5.2. Sulfur trioxide gas (SO3), if present in a dry atmosphere, can give a positive bias in the SO2 determination.
1.5.3. Any substance that has the same retention time as SO42-, when using the ion chromatographic operating conditions described in this method, is an interference. If the possibility of an interference exists, changing the separation conditions (column, eluent flow rate and strength, etc.) may circumvent the problem.
1.6. Source of Exposure
Sulfur dioxide is generated as a
1.7. Physical and Chemical Properties (5.4., 5.5.)
| Sulfur dioxide (CAS No.
7446-09-5) | |
| Chemical formula | SO2 |
| Formula weight | 64.07 |
| Melting point | -72.7 °C |
| Boiling point | -10.0 °C |
| Vapor density | 2.3 (air = 1) |
SO2 is a colorless, nonflammable gas with a characteristic, strong, and suffocating odor. It is soluble in water, methanol, ethanol, chloroform, ethyl ether, acetic acid, and sulfuric acid.
1.8. Toxicology (5.6.)
Information listed within this section is a synopsis of current knowledge of the physiological effects of SO2 and is not intended to be used as a basis for OSHA policy.
Sulfur dioxide is intensely irritating to the eyes and respiratory tract. Workplace exposure to SO2 can cause both chronic and acute effects. The chronic effects of exposure include permanent pulmonary impairment, which is caused by repeated episodes of bronchoconstriction. It has been reported that workers' exposure to high concentrations of SO2 (80 to 100 ppm) may cause an increased incidence of nasopharyngitis, shortness of breath on exertion (dyspnea), and chronic fatigue. Concentrations of SO2 from 2 to 36 ppm produced a significantly higher frequency of respiratory disease symptoms, including chronic coughing, expectoration, and dyspnea.
The acute effects include upper respiratory tract irritation, rhinorrhea, choking, and coughing. Within 5 to 15 minutes from the onset of exposure, workers develop temporary reflex bronchoconstriction and increased airway resistance.
2. Sampling
- Filter for particulate collection, Teflon® (PTFE),
0.45 µm pore size,
25-mm diameter (part no. 130620, Nucleopore Corp., Pleasanton, CA) - Carbon-filled polypropylene cassette,
25-mm diameter, (part no. 300075, Nucleopore) (See Section 4.10. for further details regarding this cassette) - Porous plastic support pad (part no. 220600, Nucleopore)
- 2.1. Equipment
- 2.1.1. Calibrated personal sampling pumps capable of sampling
within ±5% of the recommended flow rate of 0.1 L/min are used.
2.1.2. Solid sorbent sampling tubes are prepared using glass tubes, glass wool plugs, and IABC.
Sampling tubes can be commercially obtained. Two types of sampling tubes are commercially available:
Type I is a glass tube packed with a
Type II, a combination sampling device (Forest
Biomedical, Salt Lake City, UT) can be used to remove particulate
and collect
H2SO4 mist
during SO2 sampling. The combination
device, as shown below, consists of two different glass tubes
connected together. The front part of the tube contains a
Teflon® filter, retaining rings, foam, and a glass wool
plug. The Teflon® filter is used to trap any particulate
and H2SO4. The
dimensions of the front portion of the sampling device are
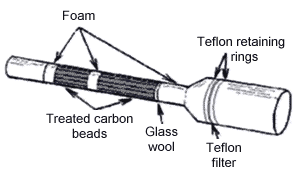
<---<-----<
Sample Flow <---<-----
Type II -
Combination Sampling Device
If commercial tubes are unavailable, sampling tubes can be
prepared using carbon bead impregnated in the same fashion as
discussed in OSHA method
Note: The grade of carbon bead appears less significant for SO2 when compared to phosphine (5.7.).
Prepare each tube for SO2 collection
with a
2.1.3. A stopwatch and bubble tube or meter are used to calibrate pumps.
2.1.4. Various lengths of polyvinyl chloride tubing are used to connect sampling tubes to pumps.
2.1.5. If the workplace air being sampled is suspected of containing particulate which could interfere (i.e. H2SO4 or sulfates), the prefilter/cassette assembly listed below or a Type II sampling tube should be used.
Note: Do not use glass fiber prefilters for particulate collection during sampling for SO2. Loss of SO2 can occur due to the slightly basic properties of these filters. See Section 4.10. for further details.
Assemble the prefilter assembly such that sampled air enters the Teflon® filter first and the plastic support pad faces the sampling tube. Use a minimum amount of tubing to connect the Type I sampling tube to the prefilter assembly.
2.2. Sampling Procedure (Bulk or wipe samples are not applicable)
- 2.2.1. Connect the sampling tube (Type I or II) to the
calibrated sampling pump, making sure sampled air enters the large
section (100 mg) of IABC first. Place the sampling device on the
employee such that air is sampled from the breathing zone.
2.2.2. For STEL samples, use a flow rate of 0.1 L/min and a
minimum sampling time of 15 min. For TWA determinations, take
consecutive
2.2.3. After sampling, place plastic end caps tightly on both ends of the tube and apply OSHA Form 21 seals. Record the sampling conditions.
2.2.4. Use the same lot of IABC tubes for blank and collected samples. Handle the blank sorbent tube in exactly the same manner as the sample tubes except that no air is drawn through it. Submit at least one blank tube for each batch of ten samples.
2.2.5. When other compounds are known or suspected to be present in the air, such information should be transmitted with the sample.
2.2.6. Specify SO2 analysis and ship
samples to the laboratory. If necessary, any Teflon®
3. Analysis
- 3.1. Safety Precautions
- 3.1.1. Refer to appropriate IC instrument manuals and the
Standard Operating Procedure (SOP) for proper instrument operation
(5.8.).
3.1.2. Observe laboratory safety regulations and practices.
3.1.3. Sulfuric acid, sodium hydroxide, and hydrogen peroxide are corrosive. Use appropriate personal protective equipment such as safety glasses, gloves, and lab coat when handling corrosive chemicals. Prepare solutions in an exhaust hood.
3.2. Equipment
- 3.2.1. Ion chromatograph (Model 4000i or 4500i Dionex,
Sunnyvale, CA) equipped with a conductivity detector.
3.2.2. Automatic sampler (Dionex Model
3.2.3. Laboratory automation system: Ion chromatograph interfaced with a data reduction system (AI450, Dionex).
3.2.4. Micromembrane suppressor, anion (Model AMMS-1, Dionex).
3.2.5. Separator and guard columns, anion (Model HPIC-AS4A and AG4A, Dionex).
3.2.6. Disposable syringes (1 mL).
3.2.7. Syringe
Note: Some syringe
3.2.8. Miscellaneous volumetric glassware: Micropipettes, volumetric flasks, Erlenmeyer flasks, graduated cylinders, and beakers.
3.2.9. Scintillation vials, glass,
3.2.10. Equipment for eluent degassing (vacuum pump, ultrasonic bath).
3.2.11. Analytical balance (0.01 mg).
3.3. Reagents - All chemicals should be at least reagent grade.
- 3.3.1. Principal reagents:
CAUTION: NaOH, H2SO4, or 30% H2O2 can cause skin irritation or burns.
Sodium carbonate
(Na2CO3)
Sodium
bicarbonate (NaHCO3)
Sodium hydroxide
(NaOH)
Sulfuric acid
(H2SO4),
concentrated, 98%
Hydrogen peroxide
(H2O2),
30%
Sodium sulfate
(Na2SO4),
anhydrous
Deionized water (DI H2O) with
a conductance of <10 µS.
3.3.2. Eluent (1.0 mM Na2CO3 + 1.0 mM NaHCO3):
Dissolve 0.212 g Na2CO3 and 0.168 g NaHCO3 in 2.0 L DI H2O, Sonicate this solution and degas under vacuum for 15 min.
3.3.3. Suppressor regenerant solution (0.02 N H2SO4):
Carefully transfer 1.14 mL concentrated
H2SO4 into a
3.3.4. Desorbing solution [0.3 N (![]() 1%)
H2O2 in 15 mM
NaOH]:
1%)
H2O2 in 15 mM
NaOH]:
Dissolve ![]() 0.6 g NaOH in
approximately 500 mL of DI H2O contained
in a
0.6 g NaOH in
approximately 500 mL of DI H2O contained
in a
3.3.5. Sulfate (SO42-) stock standard (1,000 µg/mL):
Dissolve and dilute 1.4792 g of Na2SO4 to 1.0 L with DI H2O. Prepare yearly.
3.3.6. Sulfate (SO42-) standard solutions, 100, 10, and 1 µg/mL:
Pipette appropriate volumes of the 1,000 µg/mL SO42- stock standard into volumetric flasks and dilute to the mark with eluent. Prepare monthly.
3.4. Working Standard Preparation
- 3.4.1. Prepare
SO42- working
standards in eluent. A method for preparing a series of working
standards using
| Working Std (µg/mL) |
Std Solution (µg/mL) |
Aliquot (mL) |
Eluent Added (mL) |
| 0.5 1 2 5 10 20 30 50 |
1 1 10 10 10 100 100 100 |
5 * 2 5 * 2 3 5 |
5 * 8 5 * 8 7 5 |
| * Already prepared in Section 3.3.6. | |||
3.4.2. To prepare each working standard listed above, pipette an appropriate aliquot of the specified standard solution (prepared in Section 3.3.6.) and add the specified amount of eluent.
3.4.3. As an alternative, pipette each aliquot into a
3.5. Sample Preparation
Note: If
H2SO4 is a
requested analyte and a Type II sampling device or a PTFE prefilter
was used, see OSHA Stopgap Method
- 3.5.1. Carefully remove and discard the rear glass wool plug (or
foam for the Type II sampler) without losing any beaded
carbon.
Note: The sorbent should always be removed from the glass
tube via the opposite end of collection (i.e.
3.5.2. Carefully transfer each IABC section from a sample tube
and place in separate
3.5.3. Pipette 10 mL of desorbing solution into each flask. Cap each flask tightly and allow each solution to sit for at least 60 min. Occasionally swirl each solution.
3.6. Analysis
- 3.6.1. Pipette a 0.5- to 0.6-mL portion of each standard or
sample solution into separate automatic sampler vials. Place a
filtercap into each vial. The large filter portion of the cap should
face the solution.
3.6.2. Load the automatic sampler with labeled samples, standards, and blanks.
3.6.3. Set up the ion chromatograph in accordance with the SOP (5.8.)
Note: An SOP is a written procedure for a specific instrument. It is suggested that SOPs be prepared for each type of instrument used in a lab to enhance safe and effective operation.
Typical operating conditions for a Dionex 4000i or 4500i with a conductivity detector and an automated sampler are listed below:
| Ion Chromatograph
| |
| Eluent: | 1.0 mM Na2CO3/1.0 mM NaHCO3 |
| Column temperature: | ambient |
| Anion precolumn: | AG4A |
| Anion separator column: | AS4A |
| Anion suppressor: | AMMS-1 |
| Conductivity Output range: | 1 µS |
| Sample injection loop: | 50 µL |
| Pump
| |
| Pump pressure: | |
| Flow rate: | 2 mL/min |
| Chromatogram
| |
| Run time: | 10 min |
| Peak retention time: |
3.6.4. Follow the SOP for further instructions regarding analysis (5.8.).
3.7. Calculations
- 3.7.1. After the analysis is completed, retrieve the peak areas
or heights. Obtain hard copies of chromatograms from a printer.
3.7.2. Prepare a
3.7.3. Perform a blank correction for each IABC front and backup sections. Subtract the µg/mL SO42- blank value (if any) from each sample reading if blank and sample solution volumes are the same. If a different solution volume is used, subtract the total µg blank value from total µg sample values.
3.7.4. Calculate the air concentration of SO2 (in ppm) for each air sample:
A = (µg/mL
SO42-) × (Sol
Vol) × (GF)
| corr µg SO2 = | DE |
| ppm SO2 = | (AV) × (Mol Wt) |
| Where: | ||
| A | = | uncorrected µg SO2 |
| corr µg SO2 | = | µg SO2 with DE correction applied |
| µg/mL SO42- | = | Amount found (from calibration curve) |
| Sol Vol | = | Solution volume (mL) from Section 3.5.3. |
| GF, SO2/SO42- | = | Gravimetric factor = 0.667 |
| Mol Vol | = | Molar volume (L/mol) = 24.45 (25 °C and 760 mmHg) |
| AV | = | Air volume (L) |
| Mol Wt | = | Molecular weight for SO2 = 64.0 (g/mol) |
| DE | = | Desorption efficiency ( |
| Amt of SO2 (µg)
|
DE Factor
| |||
| <
30 31 51 76 101 201 >400 |
to to to to to |
50 75 100 200 400 |
0.800 0.825 0.850 0.875 0.900 0.950 1.000 | |
3.7.5. An alternative to the DE correction sliding scale above is the following equation:
DE = -1.1386 × 10-6 (A)2 + 1.0037 × 10-3 (A) + 7.81 × 10-1
Where: A = uncorrected µg SO2
3.7.6. The DE correction may be
3.8. Reporting Results
Add the backup section ppm SO2 result (if any) to the front section result for each sample. Report results to the industrial hygienist as ppm SO2.
This method has been validated for a
The validation consisted of the following experiments:
- An analysis of 19 samples (6 samples each at 2 × and 1
×
TWA-PEL, and 7 samples at 0.5 ×TWA-PEL) for the DE study. - A sampling and analysis of 16 samples (6 samples each at 2
× and 1 ×
TWA-PEL, and 4 samples at 0.5 ×TWA-PEL) collected from dynamically generated test atmospheres at 50% RH. Samples at a concentration near the STEL and at concentrations expected during indoor air quality investigations ( 0.3 ppm) were also
taken.
0.3 ppm) were also
taken.
- A determination of the sampling media collection efficiency at
approximately 4 ppm (2 ×
TWA-PEL) . - A determination of breakthrough.
- An evaluation of storage stability at 20 to 25 °C for 24 collected samples.
- A determination of any significant effects on results when sampling at different humidities.
- A determination of the qualitative and quantitative detection limits.
- A comparison of methods.
- Evaluation of the Type II sampling tube for collecting SO2.
- Evaluation of a prefilter/cassette assembly for use with Type I samplers.
- Summary.
All theoretical (known) concentrations of generated test atmospheres
were calculated from controlled flows of a cylinder of 303 ppm
SO2 in nitrogen (certified concn, Alphagaz,
LaPorte, TX) and dilution air. An analysis of the cylinder concentration
using OSHA Method No.
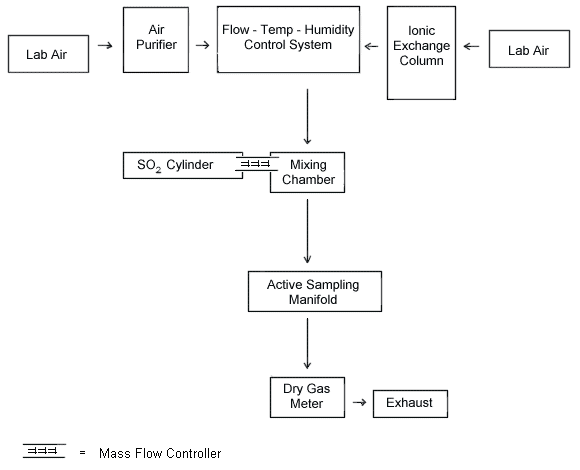
A generation system was assembled, as shown in Figure
1, and used for all experiments except detection limit determinations.
Samples using OSHA Method
All results were calculated from concentration-response curves and statistically examined for outliers. In addition, the analysis (Section 4.1.) and sampling and analysis results (Section 4.2.) were tested for homogeneity of variance. Possible outliers were determined using the Treatment of Outliers test (5.9.). Homogeneity of variance was determined using the Bartlett's test (5.10.). Statistical evaluation was conducted according to Inorganic Methods Evaluation Protocol (5.11.). The overall error (OE) (5.11.) was calculated using the equation:
OEi = ± (
|biasi| +
2CVi) × 100% (95% confidence level)
Where i is the respective sample pool being examined.
- The SO2 source mentioned previously was used to generate test atmospheres of SO2. This source was diluted with filtered, humidified air using the system shown in Figure 1.
- Dynamic generation system
AMiller-Nelson Research Inc. flow, temperature, and humidity control system (ModelHCS-301, Monterey, CA) was used for air flow control and conditioning. All generation system fittings and connections were Teflon®. A glass mixing chamber was used to mix the tempered, filtered air with the contaminant gas. The system was set to generate test atmospheres at 50% RH and 25 °C. - The SO2 and diluent air flow rates were adjusted using mass flow controllers. Flow rates were also measured using a dry test meter (for diluent air) and a soap bubble flow meter (for SO2 gas).
- Samples were taken from the sampling manifold using
constant-flow pumps. Calibrated P125 and Alpha 2 pumps (E.I. duPont de Nemours & Co., Wilmington, DE) were used. Pump flow rates were approximately 0.1 L/min and sampling time was 120 min. Sample concentrations were approximately 0.5, 1, and 2 times the OSHATWA-PEL for12-L air samples. For samples taken near the STEL ( 5.8 ppm SO2 was used), a 0.1
L/min sampling rate for 15 min was used. For low concentration
samples, a test atmosphere of approximately 0.3 ppm was used.
5.8 ppm SO2 was used), a 0.1
L/min sampling rate for 15 min was used. For low concentration
samples, a test atmosphere of approximately 0.3 ppm was used.
- The concentration was increased to a level approximately 4 times
the
TWA-PEL (7.20 ppm SO2). - Samples were collected at 0.1 L/min for 240 min.
- the hydrophilic nature of the impregnated base,
- the hydrophobicity of the beaded carbon, and
- complications arising from limited temperature control during sample generations at 80% RH.
- 4.1. Analysis
Nineteen samples were prepared by adding known amounts of
SO2 to the IABC tubes to determine
recoveries (DE) for the analytical portion of the method. For this
experiment, an active method of spiking with
- 4.1.1. Procedure: Sampling tubes containing IABC
were spiked using the low flow pumps and generation system as
described in references 5.7.
and 5.12.
The SO2 source mentioned previously was
diluted to approximately 30 ppm using the generation system shown in
Figure
1. Calibrated
4.1.2. Results: Desorption
efficiencies, presented in Table
1, varied depending on the amount of
SO2 collected. For 0.5 times the PEL (32
µg SO2), the DE was close to 0.8;
the DE was 1.0 for 6 × PEL (![]() 400 µg SO2). The DE
corrections are similar (although larger) to those found during a
previous validation of a sorbent tube (Method no. ID-107, see
reference 5.3.)
for SO2 collection. The previous method
used charcoal impregnated in a fashion similar to the IABC sorbent.
The average DE correction for the lot of treated charcoal specified
in Method no.
400 µg SO2). The DE
corrections are similar (although larger) to those found during a
previous validation of a sorbent tube (Method no. ID-107, see
reference 5.3.)
for SO2 collection. The previous method
used charcoal impregnated in a fashion similar to the IABC sorbent.
The average DE correction for the lot of treated charcoal specified
in Method no.
A mechanism to explain the decrease in DE at low mass loadings has not been found; however, it is possible that a portion of the initial SO2 entering the sampling tube strongly bonds with the surface of the carbon bead matrix and becomes unrecoverable. A strong and weak bonding mechanism for SO2 has been noted on active carbon surfaces (5.13.). Once the strong bonding sites are occupied, a DE closer to what is expected is achieved. At larger mass loadings the amount of strongly bonded SO2 would remain the same, and become insignificant when compared to the total amount collected; thus the DE approaches unity.
A test of two different grades of beaded carbon (impregnated) was also conducted and a significant difference in DE was not noted:
- (Sampling Time = 120 min, flow rate
SO2 concentration = 1.19 ppm, 25 °C, 50% RH)
| SKC Bulk
|
SKC lot no. 745*
| |
| Grade Sample Type N Mean (ppm SO2) Std Dev (ppm SO2) CV Recovery |
General II 3 1.19** 0.015 0.013 100% |
MU-AZ II 3 1.20** 0.006 0.005 101% |
| * | SKC Catalog no. |
| ** | DE corrected using scale shown in Section 3.7.3. |
Grades listed above are assigned by the manufacturer of the untreated beaded carbon (Kureha Chemical, NY) to designate bead type and manufacturing process used. The general grade is a more common bead which was found to have poor retention efficiency for collection of phosphine (5.7.).
Although the data shows comparable DEs, even for different grades, future lots or grades of beaded carbon should be evaluated for DE corrections.
To determine the precision and accuracy of the method, known concentrations of SO2 were generated, samples were collected, prepared, and analyzed.
- 4.2.1. Procedure:
4.2.2. Results: The results for TWA, STEL, and low
| TWA | STEL | LOW | |
| CV | 0.048* | 0.028** | 0.032** |
| Bias | -3.3% | +0.6% | -6.5% |
| OE | ±12.9% | ±6.2% | ±12.9% |
| * | CVT(pooled), see reference 5.11. for further details. The CV1 is taken from 0.5, 1, and 2 × PEL results. The 6 × PEL level is not included in statistical calculations. |
| ** | CV2 only |
4.3. Collection Efficiency
Procedure: Six
Results: The results in Table 4 show a CE of 100%. No SO2 was found in the second sorbent section for the CE experiment.
4.4. Breakthrough Study
(Note: Breakthrough is defined as >5% loss of analyte through the sampling media at 50% RH)
Procedure: Two separate experiments were conducted to test for breakthrough. For the first experiment, the same procedure as the CE test was used with two exceptions:
The second experiment was conducted using the Type II sampling tube
(Section
2.1.2.) at a concentration larger than expected during routine
sampling of industrial hygiene operations (14.8 ppm
SO2). Experimental parameters for this test
were: Four Type II sampling tubes, 25 °C, 50% RH,
The amount of breakthrough for each sampling tube was calculated by dividing the amount collected in the second section by the total amount of SO2 collected in the first and second sections.
Results: No breakthrough of SO2 into the second section was found. Results for both concentrations are shown in Table 5.
4.5. Storage Stability
Procedure: A study was conducted to assess the
storage stability at 20 to 25 °C of the sampling media after
SO2 collection.
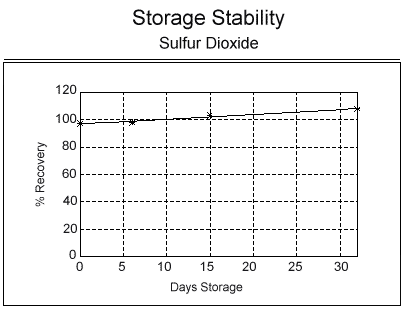
Results: The mean of samples analyzed after 32 days was within 10% of the theoretical value as shown in Table 6. A slight increase in recovery over time was noted as shown in the figure below.
Note: Prior to the validation, preliminary humidity tests
using a beaded carbon impregnated with a 10% (w/w) load of a metal
hydroxide base indicated acceptable SO2
collection at 25 and 50% RH (assuming 25 °C). Although data was also
acceptable at 80% RH
Factor (1) appeared to be the main factor in producing a slurry. The "wetting" effect apparently was assisted by differences in temperature during generation. The high humidity tests were conducted during the winter season and the ambient temperature in the laboratory was about 18 °C. The ambient temperature is normally near the test atmosphere temperature of 25 °C; due to a malfunctioning room thermostat it was somewhat cooler during these tests. The temperature of the sampling tubes are ambient because of the sampling manifold design. The test atmosphere condensation inside the tube probably was accelerated when entering the cooler sampling tube.
The amount of base impregnation was lowered to 1% (w/w) to alleviate potential formation of a slurry.
Procedure: A study was conducted to determine any
effect on results when samples are collected in different humidities.
Samples were taken using the generation system and procedure described
in Section
4.2. Test atmospheres were generated at 25 °C and at approximately
0.5, 1, and 2 times the OSHA
Results: Results of the humidity tests are listed in Table 7. An F test was used to determine if any significant effect occurred when sampling at different humidities. As shown, the calculated F values exceeded critical F values (5.14.) for all the concentrations tested and a significant difference in results occurred across the humidity ranges tested. An examination of the data indicates a decrease in recoveries as humidity increases; however, a correction for humidity effect was not instituted because results at higher humidities were considered acceptable in terms of overall error (< ±25%).
The finding that increasing humidity produces a decrease in the amount of sulfate found appears to contradict previous findings in the literature (5.15.). The previous study (5.15.) indicated the conversion of SO2 to sulfate on an active carbon surface was facilitated by the presence of an aqueous environment; however, testing carbons impregnated with a metal hydroxide base was not performed. The base appears to significantly alter the adsorption characteristics of the beaded carbon for SO2.
4.7. Qualitative and Quantitative Detection Limit Study
Procedure: Low concentration samples were prepared by
spiking desorbing solutions (Section
3.3.4.) with aliquots of aqueous standards prepared from sodium
sulfate. These samples were analyzed using a
Results: The results are shown in Table
8 for qualitative and quantitative detection limits, respectively.
The qualitative limit is 0.0187 µg/mL as
SO42- at the
99.86% confidence level. The quantitative limit (99.99% confidence) is
0.0642 µg/mL as
SO42-. Using a
4.8. Comparison of Methods
Procedure: In order to compare the performance of
this method and to confirm the theoretical
SO2 concentrations, an independent method
(OSHA Method no.
Results: Table 9 shows the results for different SO2 concentrations. As shown, the theoretical concentration of the generation system, the IABC, and impinger results are in good agreement.
4.9. Type II Tube Study
Procedure: Eight Type II sampling tubes (see Section
2.1.2. for a description of this tube) were chosen for a
statistical study using the generation system described in Section
4.2. These tubes contained PTFE membranes as prefilters. Samples
were collected
Results: Results are listed in Table 10. As shown, the Type II sampling device can be used to collect SO2 without significantly altering the concentration. A slight increase in recovery was noted when compared to the earlier Type I tube results at 80% RH (Section 4.6.).
Procedure: Past research regarding aerosols (5.17.) has indicated that particulate in the air sampled may penetrate any glass wool plugs and deposit on the sorbent when using conventional sampling tubes. To remedy this, a prefilter is generally used to stop the particulate before entry into the sampling tube. A preliminary experiment was conducted using the Type II sampling tube with glass fiber prefilters instead of PTFE. Significant losses were noted and were apparently caused by the slightly basic glass fiber filters reacting with some of the SO2.
To further evaluate the possibility of
SO2 reacting with a prefilter/cassette
sampling device, an experiment was performed using six Type I sampling
tubes with prefilter sampling assemblies consisting of PTFE
filter/polypropylene backup
The test was conducted by taking six IABC samples without
prefilters
Results: The results of the comparison of IABC samples taken with and without prefilters is shown in Table 11. As shown, a difference in the amount of SO2 collected was not noted between the prefilter/IABC and IABC sampling assembly. The PTFE prefilter/cassette assembly does not appear to inhibit the collection of SO2 when using the stated sampling conditions.
4.11. Summary
The validation results indicate the method meets both the NIOSH and
OSHA criteria for accuracy and precision (5.10.,
5.11.).
Collection efficiency, breakthrough, and storage stability are
adequate. Although it appears that humidity effects are significant
when sampling at different humidities, the results are within an
acceptable range (OE < ±25%). Detection limits are adequate when
samples are taken for 120 min at 0.1 L/min, or for
The contaminant levels of blank IABC samples, when compared to
levels found in the sorbent used in OSHA Method no.
5. References
- 5.1. Occupational Safety and Health
Administration Salt Lake Technical Center: Sulfur Dioxide in
Workplace Atmospheres (Bubbler) (USDOL/OSHA Method No.
5.2. Smith, D.L., W.S. Kim, and R.E.
Kupel: Determination of Sulfur Dioxide by Adsorption on A Solid
Sorbent Followed by Ion Chromatography Analysis. Am. Ind. Hyg.
Assoc. J.
5.3. Occupational Safety and Health
Administration Analytical Laboratory: Sulfur Dioxide in
Workplace Atmospheres (Solid Sorbent) (USDOL/OSHA Method No.
5.4. Patty, F.A.; Ed. Industrial Hygiene and Toxicology, 2nd rev. ed. Vol. 2. New York: Interscience, 1963.
5.5. National Institute for Occupational
Safety and Health: Criteria for a Recommended Standard -
Occupational Exposure to Sulfur Dioxide [DHEW (NIOSH) Publication
No.
5.6. "Sulfur Dioxide" Federal Register
54:12 (19 Jan. 1989). pp
5.7. Occupational Safety and Health
Administration Salt Lake Technical Center: Phosphine in Workplace
Atmospheres (and Backup Data Report)(USDOL/OSHA Method No.
5.8. Occupational Safety and Health Administration Salt Lake Technical Center: Ion Chromatography Standard Operating Procedure (Ion Chromatographic Committee). Salt Lake City, UT. In progress.
5.9. Mandel, J.: Accuracy and Precision,
Evaluation and Interpretation of Analytical Results, The Treatment of
Outliers. In Treatise On Analytical Chemistry, 2nd ed., Vol.1,
edited by I. M. Kolthoff and P. J. Elving. New York: John Wiley and
Sons, 1978. pp.
5.10. National Institute for Occupational
Safety and Health: Documentation of the NIOSH Validation
Tests by D. Taylor, R. Kupel, and J. Bryant (DHEW/NIOSH Pub. No.
5.11. Occupational Safety and Health
Administration Analytical Laboratory: Precision and Accuracy
Data Protocol for Laboratory Validations. In OSHA Analytical Methods
Manual 1st ed. Cincinnati, OH: American Conference of Governmental
Industrial Hygienists (Pub. No. ISBN:
5.12. National Institute for Occupational Safety and Health: Backup Data Report No. S332 for Phosphine, Attachment A. Cincinnati, OH: National Institute for Occupational Safety and Health, 1977 (unpublished).
5.13. Davini, P.: Adsorption and
Desorption of SO2 on Active Carbon: The
Effect of Surface Basic Groups. Carbon
5.14. Dowdy, S. and S. Wearden: Statistics for Research. New York: John Wiley and Sons, 1983. Chapter 8.
5.15. Halstead, J.A., R. Armstrong, B.
Pohlman, S. Sibley, and R. Maier: Nonaqueous Heterogeneous
Oxidation of Sulfur Dioxide. J. Phys. Chem.
5.16. Long, G.L. and J.D. Winefordner:
Limit of Detection -- A Closer Look at the IUPAC Definition.
Anal.Chem.
5.17. Fairchild, C.I., and M.I. Tillery:
The Filtration Efficiency of Organic Vapor Sampling Tubes against
Particulates. Am. Ind. Hyg. Assoc.J.
5.18. Occupational Safety and Health
Administration Salt Lake Technical Center: Asbestos (USDOL/OSHA
Method No.
5.19. Cassenelli, M.E.: Development of a
Solid Sorbent Monitoring Method for Chlorine and Bromine in Air with
Determination by Ion Chromatography. Appl. Occup. Environ. Hyg.
|
| |||||||
| (OSHA-PEL) Taken (µg SO2) |
Found (µg SO2) |
Recovery (F/T) |
N | Mean DE | Std Dev | CV1 | |
|
| |||||||
| (0.5 × PEL) | |||||||
| 31.850 32.090 32.090 32.090 32.090 32.090 32.090 |
25.330 24.280 26.730 28.290 24.930 25.530 25.610 |
0.795 0.757 0.833 0.882 0.777 0.796 0.798 | |||||
| 7 | 0.805 | 0.041 | 0.051 | ||||
| (1 × PEL) | |||||||
| 64.200 64.200 64.200 64.200 64.350 65.820 |
52.080 52.850 54.580 54.210 53.680 54.430 |
0.811 0.823 0.850 0.844 0.834 0.827 | |||||
| 6 | 0.832 | 0.014 | 0.017 | ||||
| (2 × PEL) | |||||||
| 131.340 131.340 131.340 131.340 131.340 128.400 |
113.960 116.950 111.350 116.130 120.030 115.780 |
0.868 0.890 0.848 0.884 0.914 0.902 | |||||
| 6 | 0.884 | 0.024 | 0.027 | ||||
| (6 × PEL) | |||||||
| 386.130 394.960 403.320 450.430 409.050 435.440 |
374.590 408.760 407.880 380.990 395.910 439.590 |
0.970 1.035 1.011 0.846* 0.968 1.010 | |||||
| 5 | 0.999 | 0.030 | 0.030 | ||||
| * Outlier, was deleted from final statistical calculations F/T = Found/Taken DE = Desorption Efficiency CV1 (Pooled) = 0.036 (Calculated from pooling 0.5, 1, and 2 × PEL data only) The mean DEs were significantly less than 1.0; therefore, a DE correction is needed. No corrections are necessary for SO2 >400 µg. | |||||||
|
| ||||||||
| (OSHA-PEL) Taken (ppm SO2) |
Found (ppm SO2) |
Recovery (F/T) |
N | Mean DE | Std Dev | CV2 | OE | |
|
| ||||||||
| (0.5 × PEL) | ||||||||
| 1.360 1.360 1.360 1.360 |
1.340 1.420 1.280 1.350 |
0.985 1.044 0.941 0.993 | ||||||
| 4 | 0.991 | 0.042 | 0.043 | 9.4 | ||||
| (1 × PEL) | ||||||||
| 2.400 2.400 2.400 2.400 2.400 2.400 |
2.190 2.390 2.400 2.360 2.420 2.280 |
0.912 0.996 1.000 0.983 1.008 0.950 | ||||||
| 6 | 0.975 | 0.037 | 0.038 | 10.0 | ||||
| (2 × PEL) | ||||||||
| 4.160 4.160 4.160 4.160 4.160 4.160 |
4.040 3.690 4.070 3.870 3.690 4.210 |
0.971 0.887 0.978 0.930 0.887 1.012 | ||||||
| 6 | 0.944 | 0.051 | 0.054 | 16.5 | ||||
| | ||||||||
| F/T = Found/Taken | OE = | Overall error (±%) |
| Bias | = | -0.033 |
| CV2 (Pooled) | = | 0.046 |
| CVT (Pooled) | = | 0.048 |
| Overall Error (Total) | = | 12.9% |
| * Samples were taken for 2 h. | ||
|
| ||||||||
| Sample No. | Air Vol | Found | Taken | ---------------- Statistical Analysis ----------------- | ||||
| (L) | (---ppm SO2---) | N | Mean | Std Dev | CV | Recovery (%) | ||
|
| ||||||||
| 1 2 3 4 |
1.33 1.32 1.50 1.33 |
5.26 5.35 5.56 5.22 |
5.32 5.32 5.32 5.32 | |||||
| 4 | 5.35 | 0.15 | 0.028 | 100.6 | ||||
|
| ||||||||
| Table 3b
Sampling and Analysis - Low Concentration (25 °C and 50% RH) |
||||||||
|
| ||||||||
| Sample No. | Air Vol | Found | Taken | ---------------- Statistical Analysis ----------------- | ||||
| (L) | (---ppm SO2---) | N | Mean | Std Dev | CV2 | Recovery (%) | ||
|
| ||||||||
| 1 2 3 4 5 6 |
12.0 12.6 11.0 12.0 12.6 11.3 |
0.288 0.288 0.300 0.288 0.300 0.275 |
0.310 0.310 0.310 0.310 0.310 0.310 | |||||
| 6 | 0.290 | 0.009 | 0.032 | 93.5* | ||||
|
| ||||||||
| * | A DE correction of 0.8 was used from the scale listed in Section 3.7.4. Low mass loadings (<30 µg) may be more accurately corrected using the equation discussed in Section 3.7.5. | |||||||
|
| |||
| ------- ppm SO2 Found -------- | |||
| Sample No. | First Section | Second Section | % Collection Efficiency |
|
| |||
| 1 2 3 4 5 6 |
4.04 3.69 4.07 3.87 3.69 4.21 |
ND ND ND ND ND ND |
100.0 100.0 100.0 100.0 100.0 100.0 |
| Notes: (a) | Sampled at 0.1 L/min for 120 min. |
| (b) | Samples were desorbed using a sample solution volume = 10.0 mL |
| (c) | ND = None detectable (<0.2 µg/mL as SO42-) |
|
| |
|
| |||
| ------- ppm SO2 Found -------- | |||
| Sample No. | First Section | Second Section | % Breakthrough |
|
| |||
| 1 2 3 4 5 6 7 8 |
7.28 6.09 6.97 7.27 15.08 15.10 14.28 13.64 |
ND ND ND ND ND ND ND ND |
0 0 0 0 0 0 0 0 |
| Notes: (a) | Sampled at 0.1 L/min for 240 min. |
| (b) | Samples were desorbed using a sample solution volume
= 10.0 mL. A |
| (c) | ND = None detectable (<0.004 ppm SO2) |
| (d) | Samples |
|
| |
|
| ||||||||
| Day | Air Vol | Found | Taken | ---------------- Statistical Analysis ----------------- | ||||
| (L) | (---ppm SO2---) | N | Mean | Std Dev | CV | Recovery (%) | ||
|
| ||||||||
| 0 | 11.5 10.6 10.7 11.5 10.6 11.5 |
2.19 2.39 2.40 2.36 2.42 2.28 |
2.40 2.40 2.40 2.40 2.40 2.40 | |||||
| 6 | 2.34 | 0.088 | 0.038 | 97.5 | ||||
| 6 | 11.9 10.5 10.6 11.7 10.3 10.4 |
2.21 2.44 2.26 2.33 2.43 2.34 |
2.39 2.39 2.39 2.39 2.39 2.39 | |||||
| 6 | 2.34 | 0.091 | 0.039 | 97.9 | ||||
| 15 | 11.8 10.5 10.6 11.8 10.5 10.6 |
2.34 2.64 2.32 2.31 2.51 2.43 |
2.35 2.35 2.35 2.35 2.35 2.35 | |||||
| 6 | 2.43 | 0.130 | 0.054 | 103 | ||||
| 32* | 8.2 7.9 8.0 8.2 7.9 8.0 |
2.62 2.46 2.52 2.58 2.56 2.45 |
2.35 2.35 2.35 2.35 2.35 2.35 | |||||
| 6 | 2.53 | 0.068 | 0.027 | 108 | ||||
| * | 90-min sampling time was used for this test only. The rest of the samples were taken for 120 min. |
|
| ||||
| % RH
|
||||
| ppm SO2 Taken | 0.990 | 1.48 | 1.19 | |
| ppm SO2 Found | 1.08 0.994 0.998 1.13 1.05 1.11 |
1.33 1.41 1.27 1.35 |
1.02 1.04 1.05 1.05 1.10 1.02 | |
| N Mean (ppm) Std Dev (ppm) CV Ave Recovery |
6 1.06 0.057 0.054 107% |
4 1.34 0.058 0.043 90.5% |
6 1.05 0.029 0.028 88.0% | |
|
| ||||
| At the 99% confidence level: | ||||
| Fcrit = 6.70 | Fcalc = 33.81 (2, 13 degrees of freedom) | |||
| Fcrit < Fcalc; therefore, a significant difference in results was noted across the humidity levels tested. | ||||
|
| ||||
| % RH
|
||||
| ppm SO2 Taken | 2.33 | 2.40 | 2.48 | |
| ppm SO2 Found | 2.44 2.50 2.48 2.48 2.32 2.51 |
2.19 2.39 2.40 2.36 2.42 2.28 |
2.25 2.26 2.20 2.40 2.19 2.36 | |
| N Mean (ppm) Std Dev (ppm) CV Ave Recovery |
6 2.46 0.070 0.029 105% |
6 2.34 0.088 0.038 97.5% |
6 2.28 0.085 0.038 91.8% | |
|
| ||||
| At the 99% confidence level: | ||||
| Fcrit = 6.36 | Fcalc = 24.22 (2, 15 degrees of freedom) | |||
| Fcrit < Fcalc; therefore, a significant difference in results was noted across the humidity levels tested. | ||||
|
| ||||
| % RH
|
||||
| ppm SO2 Taken | 4.44 | 4.40 | 4.40 | |
| ppm SO2 Found | 4.28 4.32 4.25 4.41 4.72 4.59 |
4.04 3.69 4.07 3.87 3.57 4.21 |
4.03 3.67 3.94 3.81 3.96 | |
| N Mean (ppm) Std Dev (ppm) CV Ave Recovery |
6 4.43 0.188 0.042 99.7% |
6 3.91 0.244 0.062 88.8% |
6 3.88 0.143 0.037 88.2% | |
|
| ||||
| At the 99% confidence level: | ||||
| Fcrit = 6.52 | Fcalc = 11.88 (2, 14 degrees of freedom) | |||
| Fcrit < Fcalc; therefore, a significant difference in results was noted across the humidity levels tested. | ||||
|
| |||
| ----------- SO2 (as SO42-) Level -------------- | |||
| Sample No. | Rbl PA |
0.05 µg/mL PA |
0.10 µg/mL PA |
|
| |||
| 1 2 3 4 5 6 |
1.07 1.14 0.87 1.24 1.25 1.21 |
6.32 5.91 6.39 5.98 5.81 6.38 |
13.66 13.04 13.19 13.32 13.35 13.96 |
| N Mean Std Dev CV |
6 1.13 0.14 0.128 |
6 6.13 0.26 0.042 |
6 13.42 0.34 0.025 |
| PA = Integrated Peak Area
(SO42-) /
1,000,000 Rbl = Reagent Blank | |||
|
| |||
Using the equation: Cld = k(sd) / m
Where:
| Cld | = | the smallest reliable detectable concentration an analytical instrument can determine at a given confidence level. |
| k | = = | 3 (Qualitative Detection Limit, 99.86%
Confidence) 10 (Quantitative Detection Limit, 99.99% Confidence) |
| sd | = | standard deviation of the reagent blank (Rbl) readings. |
| m | = | analytical sensitivity or slope as calculated by linear regression. |
| Cld | = | 3(0.14) / 22.45 = 0.0187 µg/mL as SO42- for the qualitative limit. |
| Cld | = | 10(0.14) / 22.45 = 0.0624 µg/mL as SO42- for the quantitative limit. |
| Qualitative detection limit | = | 0.187 µg
SO42-
( 0.004 ppm SO2 ( |
| Quantitative detection limit | = | 0.624 µg
SO42-
( 0.013 ppm SO2 ( |
|
| ||||
| Set#
|
Method
|
SO2 Concn (ppm)
|
N
|
Std Dev
|
| 1 | THE 104M IABC |
1.36 1.36 1.35 |
6 4 |
0.156 0.057 |
| 2 | THE 104M IABC |
2.40 2.31 2.34 |
6 6 |
0.058 0.058 |
| 3 | THE 104M IABC |
4.40 4.16 3.93 |
5 6 |
0.358 0.214 |
| 4 | THE 104M IABC |
5.32 5.79 5.35 |
6 4 |
0.497 0.152 |
| Notes: (a) | THE = | Theoretical (Taken) value, calculated from certified SO2 cylinder and gas generation system flows. |
| (b) | 104M = | Impinger samples taken using OSHA Method No.
|
| (c) | IABC = | Impregnated activated beaded carbon |
|
| ||||||||
| Sample No. | Air Vol | Found | Taken | ---------------- Statistical Analysis ----------------- | ||||
| (L) | (---ppm SO2---) | N | Mean | Std Dev | CV | Recovery (%) | ||
|
| ||||||||
| 1 2 3 4 5 6 7 8 |
10.6 10.3 11.3 12.7 10.6 10.3 11.3 12.7 |
1.65* 2.08 2.23 2.16 1.91 2.14 1.98 2.10 |
2.23 2.23 2.23 2.23 2.23 2.23 2.23 2.23 | |||||
| 7 | 2.09 | 0.11 | 0.052 | 93.6 | ||||
| * Outlier, not used in statistical analysis | ||||||||
| Sample Set # |
|||||
| Air Vol, L | ppm SO2 Found | Air Vol, L | ppm SO2 Found | ||
|
| |||||
| 1 2 3 4 5 6 |
12.1 12.1 11.2 11.8 11.1 12.3 |
1.80 1.62 2.04 1.92 1.79 1.89 |
11.8 11.1 12.3 * 12.1 11.2 |
1.79 1.71 1.96 1.84 1.95 | |
| N Mean Std Dev CV |
6 1.84 0.14 0.077 |
5 1.85 0.11 0.058 | |||
* Pump stopped during sampling
| Notes: (a) | Sampling Time = 120 min |
| (b) | Flow Rate |
| (c) | Sample Solution Volume for Desorption = 10 mL |
The system shown above provided a means for generating dynamic test atmospheres. The system consists of four essential elements:
- a flow-temperature-humidity control system,
- an SO2 vapor generating system,
- a mixing chamber, and
- an active sampling manifold.
Figure 1