SOLID SORBENT |
| Method No.: | ID-188 |
| Control No.: | T-ID188-FV-02-0201-M |
| Matrix: | Air |
| OSHA Permissible Exposure
Limits* Final Rule Limit (ammonia): Final Rule Limits (ammonium chloride fume): (ammonium chloride fume or ammonium sulfamate):* |
35 ppm [Short-Term Exposure Limit
(STEL)] 20 mg/m3 STEL* 10 mg/m3 [Time Weighted Average (TWA)]* |
| Transitional Limit: | 50 ppm TWA |
| Collection Device: | For ammonia collection, a personal sampling pump is used to draw a known volume of air through a glass tube containing carbon beads impregnated with sulfuric acid (CISA). |
| Recommended Sampling
Rates TWA Determinations: STEL Determinations: |
Ammonia 0.10 liter per minute (L/min) 0.5 L/min |
| Recommended Air
Volume TWA: STEL: |
Ammonia 24 L 7.5 L |
| Analytical Procedure: | The sample is desorbed with deionized water and analyzed as ammonium ion using an ion chromatograph. |
| Detection Limits Qualitative: | Ammonia 0.60 ppm (24-L air sample) 1.9 ppm (7.5-L air sample) |
| Quantitative: | 1.5 ppm (24-L air sample) 4.8 ppm (7.5-L air sample) |
| Precision and
Accuracy Validation Range: CVT: Bias: Overall Error: |
Ammonia 30.7 to 101.8 ppm 0.050 -0.009 ±10.9% |
| Method Classification: | Validated Method |
| Chemist: Date (Date Revised): |
Robert G. Adler 1987 (January 2002) |
| * Note: | Ammonium chloride fume or ammonium sulfamate can be sampled and analyzed using this method. A mixed-cellulose ester filter, polystyrene cassette, and personal sampling pump (2 L/min) are used to collect the sample. Samples are analyzed by ion chromatography after resorption in deionized water. |
Commercial manufacturers and products mentioned in this method are for descriptive use only and do not constitute endorsements by USDOL-OSHA. Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
- Introduction
This method describes the sample collection and analysis of airborne ammonia. Ammonium chloride fume or ammonium sulfamate can also be analyzed using this method. Samples are taken in the breathing zone of workplace personnel and are analyzed by ion chromatography (IC).
1.1 History
1.1.1 Sampling:
The previous OSHA sampling procedure for ammonia involved the use of a midget fritted glass bubbler containing 0.1 N sulfuric acid (H2SO4) (8.1, 8.2). Bubbler sampling is inconvenient to use. It involves the use of a liquid which if spilled may be irritating to the skin or may damage sampling pumps. Also, the sample solutions may leak during shipment.
The present method employs glass tubes containing CISA which avoids liquid sampling media problems. It is based on a procedure described by Bishop, et. al. (8.3).
1.1.2 Analysis:
Two analytical procedures have previously been used by OSHA. In the earliest procedure, ammonia was analyzed by a colorimetric method using Nessler reagent (8.2, 8.4). This method has significant interferences. The most recent method involved the use of the ammonia ion specific electrode (ISE) which does not discriminate between ammonia and amines (8.1).
The present method provides an analytical procedure which is easily set up and automated. Partial processing of the data is performed while the analysis is in progress.
1.1.3. An alternate screening technique for measuring ammonia exposures in the workplace involves the use of detector tubes (8.5). Other methods are needed to determine long-term ammonia concentrations since short-term detector tubes offer only spot checks of the environment.
1.2 Principle
A known volume of air is drawn through a sampling tube containing carbon beads impregnated with sulfuric acid (CISA). Ammonia is collected and converted to ammonium sulfate. Samples are desorbed using a known volume of deionized water (DI H2O) and analyzed as ammonium ion by IC. For ammonium chloride fume or ammonium sulfamate, samples are collected on 0.8-µm mixed-cellulose ester filters, desorbed in DI H2O, and also analyzed as ammonium ion by IC.
1.3 Advantages and Disadvantages
1.3.1 This method has adequate sensitivity for determining compliance with the OSHA permissible exposure limit (PEL) for workplace exposures to ammonia.
1.3.2 The method is simple, rapid, and easily automated.
1.3.3 Previous IC methods for ammonia have described rapid loss of peak resolution resulting primarily from metal-column binding. Using equivalent equipment described in Section 6.2 eliminates eluent contact with metal surfaces, subsequent corrosion and rapid loss of resolution due to metals binding on the separator column.
1.3.4 Previous studies have also indicated changes in ammonium peak characteristics with changes in pH. When using the equipment and conditions described herein, retention times or peak shapes were not significantly affected when the diluent concentration was from 0.0001 to 0.02 N H2SO4. The peak characteristics were significantly different when a diluent of 0.1 N H2SO4 was used. (Note: due to the H2SO4 on the beads, a 25 mL solution volume = 0.02 N H2SO4).
1.3.5 Potential exposure to H2SO4 is reduced in comparison to previous methods for ammonia.
1.3.6. The analysis is specific for the ammonium ion (NH4+).
1.3.7 After sample preparation (and acidification with additional H2SO4), ammonia can also be determined by the ISE analytical technique (8.1) or a calorimetric procedure (8.2).
1.3.8 One disadvantage is that ammonium salts present in the air as dust would constitute a positive interference; however, particulate will be captured in the glass wool plug preceding the acid-treated beads. A polystyrene cassette containing a mixed-cellulose ester filter can also be used as a prefilter to collect any particulate.
1.3.9 Another disadvantage is the positive interference from monoethanolamine, isopropanolamine, or propanolamine. If present, these compounds will produce peaks in the vicinity of the ammonium ion when using this method. Mobile phase ion chromatography (8.6) can be used for confirmation of ammonia if these compounds are present.
| CAS No. | 7664-41-7 |
| Chemical formula | NH3 |
| Formula weight | 17.03 |
| Boiling point | -33.35°C |
| Melting point | -77.7°C |
| Density, gas (air = 1) | 0.5967 |
| Density, liquid | 0.6818 (-33.35°C) |
| Critical temperature | 132.4°C |
| Critical pressure | 11.3 × 103 kPa |
| Autoignition temperature | 651°C |
| Flammable limits | 16-25% (by volume in air) |
| Solubility Cold water (0°C) Hot water (100°C) |
89.9 g/100 cc 7.4 g/100 cc |
| Color | Colorless |
| Lower limit of perception | Approximately 20 ppm |
|
| |
| Ammonium chloride CAS No. | 12125-02-9 |
| Ammonium sulfamate CAS No. | 7773-06-0 |
| Chemical formula: Ammonium chloride Ammonium sulfamate |
NH4Cl NH4OSO2NH2 |
1.5 Prevalence and Use
Ammonia is a widely used chemical, being involved in the manufacture of fertilizers, nitric acid, explosives, and synthetic fibers. It is also used in refrigeration (8.8). Occupations with the potential for exposure to ammonia include the following (8.7):
| Amine workers Ammonia workers Ammonium salt makers Aniline makers Case hardeners Chemical laboratory workers Chemical manufacturers Coal tar workers Color makers Compressed gas workers Cyanide makers Dye makers Explosive makers Farmers |
Fertilizer workers Glass cleaners Maintenance workers (janitors) Manure handlers Nitric acid makers Organic chemical synthesizers Petroleum refinery workers Refrigeration workers Rocket fuel makers Sewer workers Soda ash makers Solvay process workers Tanners Urea makers |
| Note: | Information contained within this section is a synopsis of present knowledge of the physiological effects of ammonia and is not intended to be used as a basis for OSHA policy. |
Ammonia forms a strong alkaline solution in water, and the high solubility and strong alkalinity make it especially irritating to the upper respiratory system. Exposure to ammonia can occur not only from the vapor but also from the liquid and from concentrated aqueous solutions. Depending upon the exposure, symptoms can range from mild upper respiratory irritation to inflammatory processes of the entire respiratory tract with complications of pulmonary edema and bronchopneumonia. Symptoms may also include hoarseness and tightness in the throat. The odor threshold for ammonia varies among the reports received; 50 ppm is known to produce a strong odor. Brief exposure to 100 ppm increases nasal air flow resistance, possibly from vascular congestion, edema and increased mucus secretion. Mild irritation of the eyes, nose and throat is produced by 50 ppm but not by 25 ppm. Acclimation appears to develop to 50 ppm within one week, and to 100 ppm within 2 to 3 weeks of repeated exposure. Volunteers exposed to 500 ppm for 30 minutes experienced hyperventilation and an increase in respiratory rate. Exposure to 1,000 ppm produced immediate coughing. Exposures to 700 to 1,700 ppm can be incapacitating due to extreme lacrimation and coughing. The eyes, skin and respiratory tract may be severely inflamed. Massive accidental exposure can be quickly fatal; autopsies of individuals who have died from exposure have indicated severe damage at every level of the respiratory system, including edema and hemorrhage. Skin burns from exposure to liquid ammonia can also occur. Ammonia is irritating to the eyes; failure to irrigate the eyes with a considerable amount of water following heavy exposure may lead to blindness.
- Range, Detection Limit and Sensitivity (8.11)
2.1 This method was validated over the concentration range of 30.7 to 101.8 ppm. Air volumes of about 21 L and flow rates of about 0.1 L/min were used. The average sampling time was 210 min.
2.2 The qualitative detection limit was 0.2 µg/mL or 10.0 µg (as NH3) when using a 50-mL solution volume. This corresponds to 0.60 ppm NH3 for a 24-L air volume. The quantitative detection limit was 0.50 µg/mL or 25 µg (as NH3) when using a 50-mL solution volume. This corresponds to 1.5 ppm NH3 for a 24-L air volume. A 50-µL sample loop and a 30 microsiemens detector setting were used for both IC detection limit determinations.
2.3 The sensitivity of the analytical method, when using the instrumentation specified in Section 6.2, was calculated from the slope of a linear working range curve (1 to 10 µg/mL ammonium ion). The sensitivity was 12,380 area counts per 1 µg/mL ammonium ion (a Dionex AutoIon 400 data reduction system was used). Data manipulation was also performed using a Hewlett-Packard 3357 Laboratory Automation System. The sensitivity for this system was 361,000 area counts per 1 µg/mL ammonium ion (1 area count = 0.25 microvolt-second for the Hewlett-Packard system).
- Method Performance (8.11)
Test results are based on samples collected from an in-house dynamic generation system at flow rates of approximately 0.1 L/min and sampling times of 180 to 240 min. Exceptions are noted below.
3.1 The pooled coefficient of variation (CVT) for samples taken in the range of 30.7 to 101.8 ppm was 0.050. The method exhibited slight negative bias (-0.009). overall error was within acceptable limits at ±10.9%.
3.2 The collection efficiency at about 2 times the PEL was 100%.
3.3 Breakthrough tests were performed at a concentration of 258 ppm, 50% RH, and 25°C. Breakthrough of ammonia into backup sections of sorbent was undetectable. Samples were collected for 335 min.
3.4 Samples can be stored at ambient (20 to 25°C) laboratory conditions for at least 29 days. The mean recovery of samples analyzed after 29 days was within 5% of the mean recovery of samples analyzed after 1 day of storage. Samples were stored in an office desk.
3.5 Sampling tubes stored 11 months before use gave satisfactory results during validation experiments.
- Interferences
4.1 When other compounds are known or suspected to be present in the air, such information should be transmitted with the sample.
4.2 Any compound having the same retention time as the ammonium ion, is an interference. The following compounds were noted as potential interferences with ammonium ion when using the equipment and conditions stated in Section 6:
Methyl- and dimethylamine, mono- and diethanolamine, iso- and propanolamine.
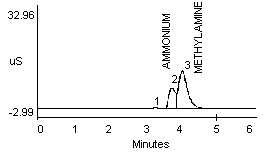
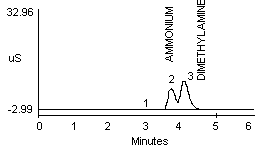
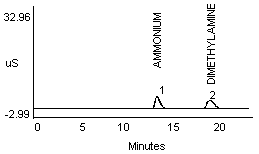
4.2.1 Methylamine and ammonium are not separated well in a 1:1 mixture (Figure 1a and 1b). Dimethylamine and ammonium in a 1:1 mixture show better resolution (Figure 1c).
- 1) Both mixtures displayed diminished peak areas for the ammonium ion; however} ammonium peak heights were similar to the 10 µg/mL standard shown in Figure 1a. If an interference of this type is present, peak heights can be used for calculations instead of peak areas.
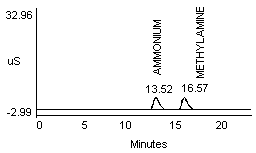
- 2) An alternate eluent (0.012 M HCl) offered sufficient resolution between ammonia and methyl- or dimethylamine (Figure 1d and 1e). This eluent can be used for confirmation if necessary.
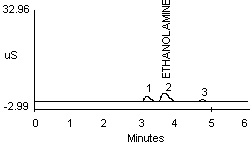
4.2.2 A peak in the same vicinity as ammonia was noted when a dilute monoethanolamine (MEA) solution was analyzed (see Figure 1f). The detector response for MEA is about one-half that seen for ammonia at a concentration of approximately 10 µg/mL (Figure 1a and 1f). Separation of ammonium and MEA was not noted when a 1:1 mixture (10 µg/mL for each) was analyzed when using either the recommended or the alternate eluent.
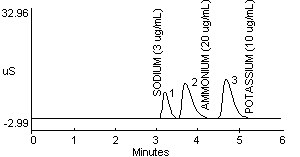
| (Note: | The MEA used for this study contained trace contaminants as shown by peaks 1 and 3 in Figure 1f. These peaks probably represent trace amounts of sodium and potassium ions, respectively.) |
4.2.3 Diethanolamine (DEA) also produces a response; however, this response is only noticeable at very large concentrations. A concentration of 10 µg/mL DEA did not produce a measurable peak.
4.2.4 Propanolamine and isopropanolamine elute at approximately the same time and with a similar response as MEA.
4.2.5 If necessary, the presence of ammonia, methyl- or dimethylamine, MEA, isopropanolamine or propanolamine can be confirmed using mobile phase ion chromatography (8.6).
4.3 Contaminant cations, such as Na+ and K+, do not interfere when using the conditions and instrumentation specified. When using the conditions described in Section 6, peak retention times of individual 10 µg/mL solutions of various analytes were:
| Analyte urea methanol diisopropanolamine triisopropanolamine diethanolamine (10 µg/mL) triethanolamine sodium monoethanolamine isopropanolamine ammonium propanolamine diethanolamine (1,000 µg/mL) methylamine dimethylamine ethylamine diethylamine potassium |
Retention Time (min) no response no response no response no response no response no response 3.28 3.67 3.68 3.70 3.77 3.80 4.08 4.17 4.35 4.77 4.83 |
| Note: | The listing above is for information only. The majority of these analytes will most likely not be present when sampling for ammonia. Retention times may vary slightly. |
4.4 Interferences may be minimized by changing the eluent, eluent concentration or pump flow rate.
4.5 Complete separation and quantitation of low molecular weight alkyl amines as well as the alcoholic amines can be achieved using mobile phase ion chromatography (8.6) or alternate sampling and analytical methods (8.12, 8.13).
4.6 Alternate ISE or calorimetric methods can also be used (8.1, 8.2); however, interferences are a significant problem for both methods.
4.7 Ammonium salts present as dust would interfere; however, this material should be collected in the glass wool plug preceding the collecting medium. A prefilter consisting of a mixed-cellulose ester filter in a polystyrene cassette can also be used if a large amount of particulate is present in the atmosphere. Preliminary tests comparing sampling tubes with and without a prefilter did not indicate a significant difference in recoveries; therefore, ammonia did not react with the prefilter components. Tests were conducted using a dynamic test atmosphere of 184 ppm NH3 at 50% RH and 25°C.
- Sampling
5.1 Equipment - Ammonia Sampling
5.1.1 Personal sampling pumps capable of sampling within ±5% of the recommended flow rate of 0.1 L/min.
5.1.2 Carbon bead, 20/30 mesh (Kureha Chemical Industry Co., 420 Lexington Ave., Suite 1742, NY, 10170, phone no. 212-867-7040).
5.1.3 Sampling tubes which contain an adsorbing section consisting of carbon beads treated with H2SO4. Tubes are commercially available, but may also be easily prepared (Caution: Sulfuric acid can cause severe burns. Wear protective gloves, labcoat and eyewear when using H2SO4).
- The commercially available tube consists of two sections; a 500-mg carbon bead front and a 250-mg backup section (ORBO-77 Tubes, cat. no. 582-12, Supelco Inc., Bellefonte, PA or SKC cat. no. 226-29, SKC, Eighty Four, PA).
- Ammonia collection tubes may be prepared according to the method of Bishop, Belkin and Gaffney (8.3). The following is a variation of this method: Thirty-one sampling tubes can be prepared using 23 g of carbon beads. The beads are placed in a beaker, rinsed five times with 0.01 N H2SO4 and then five times with DI H2O . Sufficient concentrated H2SO4 (1.2 g of acid for 23 g of beads) is added so the final product, when dried, will consist of 5% acid by weight. Enough DI H2O to just cover the beads is also added and the con ents are mixed. The product is dried at 110°C overnight in a drying oven. The beads are mixed and then packed into glass tubes, 10 cm × 8 mm o.d. × 6 mm id. The front absorbing section contains 500 mg and the backup section 250 mg of carbon beads. Each section is held in place by glass wool plugs. The tubes are capped with plastic end caps or are fire sealed.
5.1.4 A stopwatch and bubble tube or meter are used to calibrate the pumps. A blank sampling tube or device is placed in-line during flow rate calibration.
5.1.5 Various lengths of flexible tubing are used to connect the sampling tubes to the pumps.
5.1.6 Mixed-cellulose ester filters and polystyrene cassettes can be used as prefilters if particulate are a potential problem. See Section 5.3 for further details.
5.2 Sampling Procedure - Ammonia
5.2.1 Calibrate the sampling pumps to the recommended flow rate of 0.1 L/min for TWA determinations or to 0.5 L/min for STEL measurements.
5.2.2 Connect the sampling tube to the pump such that air enters the larger (500 mg) section first.
5.2.3 Place the sampling tube in the breathing zone of the employee.
5.2.4 Sample with the pre-calibrated pump at the listed flow rate and sampling time. The recommended sampling time is 4-h for TWA assessments, giving a total air volume of about 24-L. For STEL determinations, sample for 15 min.
5.2.5 Prepare one sampling tube as a blank sample. Treat this tube the same as the samples except that no air is drawn through it.
5.2.6 Place plastic end caps on each tube after sampling. Attach an OSHA seal around each tube to secure the end caps. Send the samples along with a blank sample to the laboratory with the OSHA 91A paperwork requesting ammonia analysis.
5.2.7 Bulks can also be submitted for analysis. Ship bulk samples separately from air samples. They should be accompanied by Material Safety Data Sheets if available. Check current shipping restrictions and ship laboratory by the appropriate method.
5.3 Sampling for Ammonium Chloride or Ammonium Sulfamate
The following equipment is used:
- Mixed-cellulose ester (MCE) filters (0.8 µm pore size), cellulose
backup pads, and cassettes,
37-mm diameter (part no. MAWP 037 AO, Millipore Corp., Bedford, MA).
- Gel bands (Omega Specialty Instrument Co., Chelmsford, MA) for sealing cassettes.
- Calibrated sampling pumps - 0.1 to 2 L/min flow rate.
Connect the MCE filter/cassette assembly to a calibrated sampling pump and collect samples at a flow rate of about 2 L/min.
| Note: | If the filters are to be used as prefilters, attach the cassette to the CISA sampling tube with a minimum amount of tubing, and attach the free end of the CISA tube to the sampling pump. Sample at a flow rate of 0.1 L/min if a prefilter is used. |
Sample for at least 15 min for STEL measurements and up to 8 h for TWA determinations. After sampling, seal and submit the samples to the laboratory. Request analysis for ammonium sulfamate or ammonium chloride.
6.1 Precautions
6.1.1 Refer to instrument and standard operating procedure (SOP) manuals (8.14) for proper operation.6.1.2 Observe laboratory safety regulations and practices. Caution: Sulfuric or hydrochloric acid can cause severe burns. Wear protective gloves, labcoat and eyewear when using these acids.
6.2 Equipment
6.2.1 Ion chromatography (Model 2010i, Dionex, Sunnyvale, CA) equipped with a conductivity detector.
6.2.2 Automatic sampler (Model AS-1, Dionex) and sample vials (0.5 mL).
6.2.3 Data processing system (AutoIon 400 System, Dionex).
6.2.4 Printer.
6.2.5 Cation separator column (Model HPIC-CS3, Dionex).
6.2.6 Cation guard column (Model HPIC-CG3, Dionex).
6.2.7 Cation micromembrane suppressor (Model CMMS-1 suppressor, Dionex).
6.2.8 Disposable syringes (1 mL) and prefilters.
| (Note: | Some prefilters are not cation- or anion-free. Tests should be done with blank solutions first to determine suitability for the analyte being determined). |
6.2.9 Polyethylene scintillation vials (20 mL) with polyethylene cap liners (Part No. 58515, Kimble, Toledo, OH).
6.2.10 Miscellaneous volumetric glassware: Beakers, graduated cylinders, beakers, and volumetric flasks (0.25 to 4 L).
6.2.11 Analytical balance (0.01 mg).
6.3 Reagents - All chemicals should be reagent grade or better
6.3.1 Deionized water (DI H2O) with a specific conductance of less than 10 microsiemens.
6.3.2 Hydrochloric acid (HCl) solution (1 N):
Dilute 166 mL of concentrated HCl to 2.0 L with DI H2O.
6.3.3 Strong eluent (48 mM HCl, 4 mM DAP-HCl, 4 mM L-histidine -HCl): Weigh 0.560 g 2,3-diaminopropionic acid monohydrochloride (DAP-HCl) and 0.840 g L-histidine monohydrochloride monohydrate and then place in a 1-L volumetric flask. Add 48 mL of 1 N HCl. Dilute to volume with DI H2O. Mix thoroughly. Prepare monthly.
6.3.4 Weak eluent (12 mM HCl, 0.25 mM DAP-HCl, 0.25 mM L-histidine-HCl):
| Note: | Prepare a new solution for each analysis. Aged solutions of weak eluent tend to lose buffering capacity. Chromatographic dips in the vicinity of the ammonium peak have been noted using aged eluent and may lead to erroneous results. These dips only occur with samples (which contain a small amount of sulfuric acid) and do not occur with standards (prepared with DI H2O). |
Dilute 252 mL of strong eluent and 36 IRL of 1 N HCl to 4.0 L with DI H2O. Mix thoroughly.
6.3.5 Alternate eluent (12 mM HCl): This eluent is only used if potentially resolvable interferences are present (See Section 4.2.1 for further information). Dilute 48 mL of 1 N HCl to 4.0 L with DI H2O. Prepare a new solution for each analysis.
6.3.6 Regeneration solution [0.04 N tetramethylammonium hydroxide (CH3)4NOH (TMAOH)] (Note: The purity of the reagent must be considered when preparing the 0.04 N TMAOH solution.): Commercially prepared solutions of 25% TMAOH can be used (25% TMAOH, cat. no. 33,163-5, Aldrich Chemical Co., Milwaukee, WI). Dilute 57.4 mL of 25% TMAOH to 4 L with DI H2O. An alternative preparation is to dissolve 29.00 g of tetramethylammonium hydroxide pentahydrate [(CH3)4NOH·5H2O] in 4.0 L of DI H2O.
6.3.7 The eluent used with CSRS suppressor, IonPac CS12 column, and CG12 guard column is 20 mM methane sulfonic acid (CH3SO3H) solution. Dilute 5.2 mL methane sulfonic acid to 2.0 L with DI H2O (Methane sulfonic acid, cat. no. M860-6, Aldrich Chemical Co., Milwaukee, WI).6.3.8 Sulfuric acid solution (0.1 N): Dilute 5.6 mL of concentrated H2SO4 to 2.0 L with DI H2O.
6.3.9 Ammonia stock standard (1,000 µg/mL ammonia): Dissolve 3.141 g of ammonium chloride in 0.1 N H2SO4 and dilute to the mark in a 1-L volumetric flask. Prepare every month.
6.3.10 Ammonia standard (100 µg/mL). Dilute 50 mL of the 1,000 µg/mL ammonia stock standard to 500 mL with DI H2O. Prepare weekly.
6.3.11 Ammonia standard (10 µg/mL). Dilute 50 mL of the 100 µg/mL ammonia stock standard to 500 mL with DI H2O. Prepare weekly.
6.4 Working Standard Preparation
6.4.1 Ammonia working standards may be prepared weekly in the ranges specified:
| Working STD | Standard | Aliquot | Final Vol. |
| (µg/mL) |
Solution (µg/mL) |
(mL) |
(mL) |
| 1 | 10 | 10 | 100 |
| 2 | 10 | 20 | 100 |
| 5 | 100 | 5 | 100 |
| 10 | 10 | * | * |
| 15 | 100 | 30 | 200 |
| 20 | 100 | 20 | 100 |
| * Already prepared in Section 6.3 | |||
6.4.2 Pipette appropriate aliquots from standard solutions prepared in Section 6.3 into volumetric flasks of the final volumes specified. Dilute to volume with DI H2O.
6.4.3 Pipette a 0.5- to 0.6-mL portion of each standard solution into separate automatic sampler vials. Place a 0.5-mL filter cap into each vial. The large exposed filter portion of the cap should face the standard solution. Also prepare a reagent blank from the DI H2O used for standard preparation.
6.5 Sample Preparation - CISA Samples
| Note: | For the CISA samples, always use a final solution volume >25 mL. |
6.5.1 Carefully remove and discard the glass wool plugs from the sample tubes, making sure that no sorbent is lost in the process. Transfer each sorbent section into individual polyethylene vials.
6.5.2 Add 10 mL of DI H2O to each vial, cover vials with polyethylene lined caps and then shake vigorously for about 30 s. Allow the solutions to settle for at least 1 h.
6.5.3 Quantitatively transfer each front section desorption solution to individual 25- or 50-mL volumetric flasks.
6.5.4 Rinse the beads in the vial with additional portions of DI H2O and also transfer this rinse to the flask. Take care so the beads are not transferred to the flask.
6.5.5 Dilute to volume with DI H2O. Also transfer each backup section resorption solution to individual 25- or 50-mL volumetric flasks and dilute to volume.
6.5.6 An alternate method of resorption and dilution is: Place the beads into 25- or 50-mL volumetric flasks. Measure the appropriate amount of DI H2O using a pipette or graduated cylinder and add this to the carbon beads.
6.5.7 If the sample solutions contain particulate, remove the particles using a prefilter and syringe. Fill the 0.5-mL automatic sampler vials with sample solutions and push a filtercap into each vial.
6.5.8 Load the automatic sampler with labeled samples, standards and blanks.
6.6 Sample Preparation - Ammonium Chloride Fume or Ammonium Sulfamate
6.6.1 Open the filter cassette, carefully remove the sample filter with forceps, and place in a scintillation vial. If the cassette contains loose dust, carefully rinse the dust into the vial with DI H2O. If necessary, wipe out the dust with a clean MCE filter and place this filter in the vial. If the backup pad appears to be discolored, it may be due to leakage of air around the filter during sampling. In these cases, the pad should also be prepared and analyzed. Place the backup pad in a separate vial. Also prepare a blank backup pad.
6.6.2 Add 10 mL of DI H2O to each scintillation vial. Allow to sit for at least 1 h with occasional agitation of the solution and filter. Proceed with the analysis as described in Sections 6.5.7-6.5.8 and 6.7
6.7 Analytical Procedure
6.7.1 Set up the ion chromatography in accordance with the SOP (8.14) or instrument manuals.
Typical operating conditions for a Dionex 2010i with an automatic sampler are listed below:
| Ion chromatograph | |
| Eluent (Section 6.3.4): | DAP-histidine-HCl |
| Eluent conductivity: | approximately 7 microsiemens |
| Regenerant flow: | 2 to 3 mL/min (0.04 N TMAOH) |
| Sample injection loop: | 50 µL |
| Pump | |
| Pump pressure: | approximately 550 psi |
| Flow rate: | 1 mL/min |
| Chromatogram | |
| Run time: | 5 min |
| Average peak retention time: | 3.7 to 3.9 min |
| Note: | If the alternate eluent is used, allow a longer period of time for the ion chromatography to equilibrate (2 to 3 h). The retention time of the ammonium ion will be much longer with the alternate eluent. |
6.7.2 If an ion chromatography is not available, the sample solutions may be acidified with H2SO4 to 0.1 N and analyzed with an ammonia ISE as described in Method No. ID-164 (8.1).
- Calculations
7.1 After the analysis is completed, peak areas and heights can be retrieved using a variety of methods or programs (8.14). Hard copies of chromatograms, which list peak heights and areas, can be obtained from a printer. An example chromatogram containing 3 µg/mL sodium, 20 µg/mL ammonium, and 10 µg/mL potassium ions is shown in Figure 2.
7.2 prepare a concentration-response curve by plotting the concentration of the standards in µg/mL (or µg/sample if the same solution volumes are used for samples and standards) versus peak areas or peak heights. Blank correct each sample section (sample and blank solution volumes should be the same). Add the backup section results to the front section results for each tube.
7.3 The concentration of ammonia in each air sample is expressed in ppm. The equation is:
| ppm NH3 = | molar volume × µg/mL NH3
× solution volume (mL)
formula weight × air volume (L) |
| Where: | ||
| Molar Volume | = | 24.46 (25°C and 760 mm Hg) |
| Formula Weight (NH3) | = | 17.03 |
| µg/mL NH3 | = | Blank corrected value from Section 7.2 |
7.4 For ammonium chloride fume or ammonium sulfamate:
| mg/m3 analyte = | µg/mL NH3 × solution
volume (mL) × GF
air volume |
| Where: | ||||||||
| µg/mL NH3 | = | Blank corrected value from Curve | ||||||
| GF | = | Gravimetric Factor:
|
7.5 Report CISA results to the industrial hygienist as ppm ammonia. Report ammonium chloride or ammonium sulfamate results as mg/m3. Ammonium chloride or sulfamate results are based on the analysis of the ammonium ion; other ammonium salts present in the air during sampling may be a positive interference.
- References
8.1 Occupational Safety and Health Administration Analytical Laboratory: OSHA Analytical Methods Manual (OSHA-SLCAL Method No. ID-164). Cincinnati, OH: American Conference of Governmental Industrial Hygienists (Pub. No. ISBN: 0-936712-66-X), 1985.
8.2 Occupational Safety and Health Administration Analytical Laboratory: OSHA Manual of Analytical Methods edited by R.G. Adler (OSHA-SLCAL Method No. VI-1). Salt Lake City, UT. 1977.
8.3 Bishop, R.W., F. Belkin and R. Gaffney: Evaluation of a New Ammonia Sampling and Analytical Procedure. Am. Ind. Hyg. Assoc. J. 47: 135-137 (1986).
8.4 National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 2nd ed., Vol. 1 (HEW/NIOSH Pub. No. 77-157-A). Cincinnati, OH: National Institute for Occupational Safety and Health, 1977.
8.5 Occupational Safety and Health Administration Analytical Laboratory: Ammonia Detector Tubes (PE-7). Salt Lake City, UT. 1987.
8.6 Dionex Corp.: Basic Ion Chromatography. Sunnyvale, CA: Dionex Corp., 1983.
8.7 National Institute for Occupational Safety and Health: Criteria for a Recommended Standard . ..Occupational Exposure to Ammonia) (HEW/NIOSH Pub. No. 74-136). Cincinnati, OH: National Institute for Occupational Safety and Health, 1974.
8.8 Windholz, M., S. Budavari, R.F. Blumetti, and E.S. Otterbein, ed.: The Merck Index. 10th ed. Rahway, NJ: Merck & Co., 1983. p. 498.
8.9 Proctor, N.H. and J.P. Hughes: Chemical Hazards of the Workplace. Philadelphia, PA: J.B. Lippincott Company, 1978. pp. 101-102.
8.10 Frank, R.: Acute and Chronic Respiratory Effects of Exposure to Inhaled Toxic Agents. In Occupational Respiratory Diseases, edited by J.A. Merchant. Washington, D.C.: U.S. Government Printing office, 1986. pp. 573-576.
8.11 Occupational Safety and Health Administration Technical Center: Ammonia Backup Data Report (ID-188). Salt Lake City, UT, Revised 1991.
8.12 Occupational Safety and Health Administration Analytical Laboratory: OSHA Analytical Methods Manual (OSHA-SLCAL Method Nos. 34, 36, 40, 41). Cincinnati, OH: American Conference of Governmental Industrial Hygienists (ACGIH Publ. No. ISBN: 0-936712-66-X), 1985.
8.13 Occupational Safety and Health Administration Analytical Laboratory: Ethanolamine and Diethanolamine (OSHA-SLCAL Stopgap Method). Salt Lake City, UT. 1987 (unpublished).
8.14 Occupational Safety and Health Administration Technical Center: Standard Operating Procedure-Ion Chromatography. Salt Lake City, UT. In progress (unpublished).
All identified species are 10 µg/mL
| PEAK NUM |
RET TIME |
CONC in ppm | AREA |
HEIGHT |
|
| ||||
| 1 2 3 |
3.22 3.73 4.70 |
8.906e+004 1.775e+005 1.940e+005 |
7972 10273 11514 | |