| Method Number: | ID-143 |
| Matrix: | Air |
| OSHA Permissible Exposure
Limits Zinc Oxide Fume (Final Rule Limit): |
5 mg/m3 Time Weighted Average (TWA) 10 mg/m3 Short-Term Exposure Limit (STEL) |
| Zinc Oxide Dust (Final Rule
Limit) Respirable Fraction: Total Dust: |
5 mg/m3 TWA 10 mg/m3 TWA |
| Zinc Oxide Fume (Transitional Limit): | 5 mg/m3 TWA |
| Zinc Oxide Dust (Transitional
Limit) Respirable Fraction: Total Dust: |
5 mg/m3 TWA 15 mg/m3 TWA |
| Collection Device: | A tared, 5-µm, 37-mm polyvinyl chloride filter is used with a personal sampling pump |
| Recommended Sampling Rate: | 2 L/min |
| Recommended Air Volume: | 30 L (STEL), 960 L (TWA) |
| Analytical Procedure: | A suspension of the sample particulate in
tetrahydrofuran (THF) is created by dissolving the sample filter in
THF and then sonicating. The sample particulate is then deposited
onto a silver membrane filter and analyzed by |
| Detection
Limits Qualitative: |
30 µg zinc oxide |
| Quantitative: | 50 µg zinc oxide |
| Precision and
Accuracy Validation Range: |
2.5 to 10 mg/m3 (200-L air sample) |
| CV1 | 0.05 |
| Method Classification: | Validated Method |
| Date (Date Revised): | 1985 (March, 1989) |
descriptive use only and do not constitute endorsements by USDOL-OSHA.
Similar products from other sources can be substituted.
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the collection of airborne zinc oxide (ZnO) in
the breathing zone of personnel and the subsequent analysis by
- a high-volume filter sample
- a representative settled dust (rafter) sample
- a sample of the bulk material in the workplace which is considered to be ZnO.
- Prepare three suspensions of ZnO standard in
2-propanol by weighing 10, 50, and 500 mg of the ZnO to the nearest 0.01 mg. Quantitatively transfer each to a 1-L volumetric flask using2-propanol , and bring each to half volume. - Disperse the ZnO in the
2-propanol by using an ultrasonic bath for 10 min. Remove from the bath and allow to cool to room temperature for 15 min. Dilute each flask to the mark with2-propanol . - Prepare a series of working standards on silver membranes from
10-, 50-, and
500-µg/mL stock ZnO suspensions by pipetting appropriate aliquots using the procedure outlined in steps 3 through 5. Prepare three sets of the standards listed below. These will be used to construct the calibration curve.Stock Standards
(µg/mL)
Aliquot
(mL)
Working Standards
(µg)
500 2, 4, 6 1,000, 2,000, 3,000 50 2, 5, 10 100, 250, 500 10 3, 5 30, 50 - Assemble the filtering apparatus and liquid nitrogen cold
trap. Connect the cold trap to the filtering apparatus to collect
the waste suspending media (
2-propanol or THF). The waste vapors should not enter the vacuum pump. - Center a silver membrane on a fritted-glass base of the filtering apparatus. Also center the glass chimney on top of the base and secure it with a clamp.
- Add a stirring bar to the stock standard suspension and
withdraw aliquots using the following technique:
- Place the flask on a magnetic stirrer and turn the stirrer
on until a uniform suspension is achieved.
- Turn off the stirrer, remove and invert the flask, allowing
the stirring bar to slide into the neck.
- Shake the flask vigorously to ensure an even suspension.
- Cautiously revert the flask and return it to the stirrer.
- Turn on the stirrer and immediately withdraw an aliquot from the center of the stirred solution using an appropriate volumetric pipette (care should be taken not to withdraw solution above the volume mark of the pipette).
- Place the flask on a magnetic stirrer and turn the stirrer
on until a uniform suspension is achieved.
- With the vacuum off, place 2 mL of
2-propanol in the chimney of the previously assembled vacuum filtering apparatus. Transfer the pipetted aliquot to the chimney. After the transfer, bring the total volume in the chimney to 20 mL with2-propanol . - Apply vacuum to the filtering apparatus, drawing the
2-propanol through it. This should result in a thin, even layered deposition of ZnO onto the silver membrane. Do not rinse the chimney after the ZnO has been deposited on the membrane. Rinsing can disturb the thin layer deposition just created. Vacuum should be applied for sufficient time to dry the membrane. - Carefully disassemble the chimney and clamp. Remove the silver membrane from the fritted-glass base using forceps. Place 2 drops of 1.5% parlodion solution on a glass slide. Fix the standard to the membrane by placing the bottom side of the membrane in the parlodion solution. By capillary action, the membrane draws the parlodion solution to the ZnO surface. Place a Teflon sheet on top of an explosion-proof hot plate which is set at the lowest setting. Then place the membrane on top of this heated Teflon sheet. When dry, place the fixed ZnO standard in a labeled Petri dish.
- Inspect the deposition for uniformity; clumping indicates that insufficient sonication was used. The standard will have to be remade if a significant amount of clumping occurs.
- Bulk samples approximating respirable particle size:
Weigh an aliquot of 1 to 2 mg on a PVC filter, and place in a round bottom centrifuge tube.
- Non-respirable bulks:
Grind the sample to a fine powder using either a mortar and pestle or a freezer mill. Then size the sample, using a 325-mesh sieve or sonic sifter. This results in a sample particle size of less than 45 µm. An aliquot of the sized sample is weighed on a PVC filter and placed in a round bottom centrifuge tube. - 2q limits for each analytical line
- 2q scanning increment (0.02° 2q)
- Integration and background counting times (1 s)
- Calibration using the secondary silver line (44.33° 2q)
- Present 2q location of the
X-ray instrument - Perform a silver line angle calibration (44.33° 2q) before each standard or sample is analyzed.
- Scan the standard or sample over the selected 2q range in 0.02-degree increments (1 s each).
- A standard is analyzed after every fourth or fifth sample to assure correct instrumental operation.
- Releasing the sample from the silver membrane
- Dividing the sample into aliquots
- Depositing each aliquot onto a membrane
- 1.1. History
- 1.1.1. There have been several methods used to analyze for ZnO.
These include atomic absorption (AA), colorimetry, gravimetry,
titrametry, and XRD. The XRD determination of three or more lines is
considered the preferred method, because ZnO can be distinguished
from other compounds of zinc (Zn) or metallic Zn. Also, the
diffraction method is less tedious than most alternative methods.
1.1.2. The previous analytical method (8.1.) used by the OSHA Salt Lake City Technical Center (OSHA-SLTC) was an AA method. Zinc oxide was collected on a cellulose membrane filter, digested, and analyzed according to reference 8.1. The AA method was not specific for ZnO since only Zn is analyzed.
1.2. Principle
A sample is collected by drawing air at approximately 2 L/min
through a pre-weighed polyvinyl chloride (PVC) filter. The filter is
post-weighed before submission to the lab for analysis. At the
laboratory, the filter is dissolved in tetrahydrofuran (THF), the
sample particulate is suspended in the THF, and then deposited on a
silver membrane. The membrane is analyzed by XRD. The
- nl = 2d sin q
Where:
| n | = | order of diffracted beam |
| l | = | wavelength of |
| d | = | distance between diffracting planes (in angstroms) |
| q | = | angle between incident |
| Note: | Most |
1.3. Advantages and Disadvantages
- 1.3.1. This method is specific for ZnO; XRD can distinguish
crystal structure based on the Bragg equation. The method does not
distinguish between dust or fume forms of ZnO.
1.3.2. Zinc oxide can be identified and quantified on three or more different angles of diffraction.
1.3.3. Interferences can be minimized by using alternate angles
of diffraction,
1.3.4. This method is accurate and offers better sensitivity compared to previous methods of analysis.
1.3.5. Sample preparation is simple and analysis can be computer-controlled and automated. Also, sample preparation and analysis involves a non-destructive technique. Samples can be reanalyzed at a later date.
1.3.6. A disadvantage is the high cost of instrumentation, especially if equipped with computer hardware.
1.3.7. Another disadvantage is the sophisticated maintenance required for the instrumentation.
1.4. Physical and Chemical Properties of ZnO (8.2.)
| CAS No.: | 1314-13-2 |
| Chemical Symbol: | ZnO |
| Synonyms: | china white, zinc white, zincite |
| Formula Weight: | 81.37 |
| Crystalline Form: | hexagonal |
| Specific Gravity: | 5.606 |
| Melting Point: | 1975°C |
| Solubility*: | 0.00016 g/100 mL water at 29°C; soluble in mineral acids, dilute acetic acid and ammonium chloride |
| Color: | white |
*Tests conducted at the OSHA lab indicated the solubility of ZnO
in suspending media such as
1.5. Uses
Occupations with potential exposures to Zn and Zn compounds are listed (8.3.):
| Alloy and electric fuse makers Printing plate makers Roofing makers Metal cutters Shipyard workers Arc welders Electric metallizers, galvanizes and electroplaters Zinc, brass and bronze foundry workers Metal sprayers Braziers Paint manufacturers Smelters Junk metal refiners |
2. Analytical Working Range
- 2.1. Qualitative detection limits when using typical instrumental
conditions are:
| Diffraction Peak |
Peak ° 2q |
Integration Time (sec) |
Detection Limit (µg) |
| Primary (1') | 36.30 | 1 | 30 |
| Tertiary (3') | 34.46 | 1 | 30 |
| Quaternary (4') | 56.62 | 1 | 30 |
| Quinternary (5') | 62.88 | 1 | 30 |
| Note: | 2q peaks are more informative than absolute. Peak values may vary slightly. |
2.2. The quantitative detection limit for the analytical method was 50 µg. The determination was performed using the primary or most sensitive ZnO diffraction line.
2.3. The range for samples containing mixtures and impurities is
dependent upon the amount of interfering substances and
3. Method Performance
- 3.1. The overall coefficient of variation (CV) for ZnO fume was
reported to be 0.088 (8.4.).
3.2. The analytical method was tested at the OSHA lab in the range of 2.5 to 10 mg/m3. The CV for the analytical method was 0.05. This value was determined from known samples in the range of 500 to 2,000 µg (N = 18) for the primary ZnO diffraction line. The mean recovery was 99.98% (8.5.).
4. Interferences
According to data listed in a computer search of the Joint Committee on Powder Diffraction Studies (JCPDS) powder diffraction file (8.9.), several compounds have diffraction peaks that can potentially interfere with ZnO. Many of these compounds are listed in Appendix A.
- 4.1. The secondary ZnO line is normally not scanned. The
possibility of silver chloride formation on the surface of silver
membranes used in sample analyses has limited the use of this line.
Silver chloride has a diffraction peak in close proximity to the
secondary ZnO line.
4.2. Some elements (iron, in particular) can cause appreciable
4.3. If a severe interference is present on the primary ZnO line, analytical results are reported using another diffraction line.
4.4. The presence of interference can be verified and usually identified by XRD analysis. Interfering peaks are usually resolved at the OSHA-SLTC using custom in-house software (8.7.). Similar software developed by the instrument manufacturers or in-house can also be used.
4.5. Wide-angle scans are also performed to help identify interferences or assist in identification of ZnO. These scans are usually performed with line profile libraries where the diffraction lines of the sample are compared to lines of known compounds contained in the libraries.
5. Sampling
- 5.1. Sampling Equipment
- 5.1.1. Sample assembly:
- Filter holder consisting of a two- or three-piece cassette,
37-mm diameter.
Backup pad, 37-mm, cellulose.
Low ash PVC membrane filter, 37-mm, 5-µm pore size [part no. 625413, Mine Safety Appliances (MSA), Pittsburgh, PA or cat. no. P-503700, Omega Specialty Instrument Co., Chelmsford, MA].
Note: During preparation for analysis, the sample is dissolved in tetrahydrofuran (THF). Certain acrylic copolymers added to PVC filters are insoluble in THF. If the membrane filter composition is unknown, a laboratory test should be conducted with THF to determine suitability before use.
5.1.2. For respirable samples only, a cyclone is also used. Cyclone: Nylon, 10-mm (BDX-99R, part no. 7010048-1, Sensidyne Inc., Largo, FL, or part no. 456243, MSA, Pittsburgh, PA).
5.1.3. Pump calibration system: Stop watch and bubble tube or electronic meter.
5.1.4. Sampling pump: For fume or nonrespirable dust samples,
calibrate the personal sampling pump to approximately 2 L/min. Each
pump must be calibrated with a representative sampler
5.1.5. Assorted flexible tubing.
5.1.6. Analytical balance (0.01 mg).
5.2. Sampling Procedure
- 5.2.1. Desiccate and then weigh the PVC filter before sampling.
5.2.2. Place the pre-weighed PVC filter and a cellulose backup pad in a two- or three-piece cassette.
5.2.3. Attach the cassette to a calibrated personal sampling pump using flexible tubing. For respirable samples, attach a cyclone to the cassette.
5.2.4. Place the sampling assembly in the breathing zone of the worker or sampling area and place the pump in an appropriate position. For fume or nonrespirable dust samples, take up to 960 L of air through the filter cassette at approximately 2 L/min. For respirable samples, take up to 816 L at approximately 1.7 L/min. Do not allow the cyclone to be inverted during or after sampling.
5.2.5. Check the pump and sampling assembly periodically to verify performance and to assure that the filter is not overloaded. If the filter becomes overloaded during the sampling interval, replace it with another filter.
5.2.6. Terminate sampling at the predetermined time and record the pump flow rate and collection time. Remove the filter, being careful not to lose any particulate. Desiccate and then weigh the filter. Replace the filter, and then firmly seal the cassette with plastic plugs in both the inlet and outlet ports. Calculate the net weight gain and the weight/air volume ratio of the filter. If this ratio is less than the Permissible Exposure Limit, do not submit the sample for ZnO analysis. Respirable dust samples are also not submitted to the lab; these samples are gravimetrically compared to the PEL of 5 mg/m3 for respirable particulate.
5.2.7. Record on the OSHA 91 form all pertinent sample data and any potential interferences. When other compounds are known or suspected to be present in the air, such information, including their suspected identities, should be transmitted with the samples.
5.2.8. With each batch of up to 20 samples, submit an appropriate blank filter for analysis.
5.2.9. Seal the filter cassette and identify it with an OSHA Form 21. Mail samples to the laboratory in a suitable container designed to prevent damage.
5.3. Bulk samples
In order of laboratory preference, bulk samples may be one of following:
The type of bulk sample should be stated on the OSHA 91 and cross-referenced to the appropriate air sample(s).
6. Analysis
- 6.1. Safety Precautions
- 6.1.1. Tetrahydrofuran (THF) has a low flash point, -14°C (6°F),
and is extremely flammable. Always use THF in a hood. THF is an
ether which can form explosive peroxides upon exposure to air;
therefore, it should be stored in closed containers. Always use
latex gloves, a labcoat, and safety glasses when handling THF.
6.1.2. Parlodion and isopentyl acetate are flammable.
6.1.3. Always use a hood when working with dry ZnO.
6.1.4. Most
6.1.5. A bench top warning light (yellow) is recommended. When
lit, the warning light indicates the
6.1.6. Most
6.1.7. An additional Geiger type alarm monitor to measure the
general work area is recommended. The monitor can be interfaced to
the
6.1.8. Avoid inserting fingers into the sample compartment. Use forceps to change samples.
6.1.9. Radiation monitors are worn by all
6.2. Analytical Equipment
- 6.2.1.
- Automated Powder Diffractometer (APD).
Long, fine-focus copper target
Scintillation counter detector.
Recirculating cooling system for the
6.2.2. Computer system consisting of:
- Hardware and software for data reduction and graphic
presentations.
Interface: Between the computer and the automated powder diffractometer an optical isolator and a mechanically operated switch have been used at
Line profile library (JCPDS-International Center for Diffraction Data Powder Diffraction File, JCPDS, Swarthmore, PA).
6.2.3. Standard and sample preparation:
- Centrifuge tubes: Round bottom 40-mL (Pyrex 8260).
Drying oven.
Explosion-proof hot plate (Model HP-11515B, Sybron/Thermolyne, Dubuque, IA).
Filtration apparatus, 25 mm, (Filter Holder Hydrosol Manifold, cat. no. XX25 047 00, filtering clamps, cat. no. XX10 025 03, fritted glass bases with stoppers, cat. no. XX10 025 02, and glass funnels, cat. no. XX10 025 11, Millipore Corp., Bedford, MA).
Forceps.
Latex gloves (Cat. no. 8852, American Pharmaseal Lab., Glendale, CA).
Liquid nitrogen cold-trap system for suspending media collection (dewar, polypropylene vacuum flask, liquid nitrogen, etc.).
Micro-analytical balance (0.01 mg).
Plastic petri dishes (Product no. 7242, Gelman Sciences, Ann Arbor, MI).
Silver membrane filters: Diameter 25-mm, 0.45-µm pore size (Cat. no. FM25-0.45, Osmonics, Inc. , Minnetonka, MN).
Teflon sheet, 0.3 to 1 mm thick.
Vacuum system.
Volumetric pipettes, eyedropper, volumetric flasks and graduated cylinders.
Ultrasonic bath.
6.2.4. Bulk sample preparation for membrane deposition:
- Freezer mill (Model no. 6700, Spex Industries, Edison,
NJ).
Mortar and pestle.
Sieve or sonic sifter: Sieve, 325 mesh (or Model ATML3P Sonic Sifter with 325 mesh sieve, ATM Corporation, Milwaukee, WI).
6.3. Reagents - All chemicals should be reagent grade or better.
- 6.3.1. Tetrahydrofuran (THF).
6.3.2. Parlodion (Pyroxylin).
6.3.3. Isopentyl (Isoamyl) acetate.
6.3.4. Parlodion in isopentyl acetate, 1.5% (w/v): Dissolve 1.5 g of parlodion in isopentyl acetate and dilute to 100 mL with isopentyl acetate.
6.3.5. Zinc oxide standard, < 325 mesh.
6.3.6.
6.4. Standard Preparation
- 6.4.1. Preparation of ZnO stock standards:
Dry the ZnO standard for 2 h at 110°C. This material is used for stock and working standards.
6.4.2. Preparation of ZnO working standards:
6.5. Sample Preparation
- 6.5.1. When sample weights are greater than 3 mg, aliquots are
taken to achieve depositions within the working range.
6.5.2. Examine the filter and backup pad to determine if any breakthrough to the backup pad has occurred. If there is significant breakthrough, the sample is either not analyzed or results are reported with a disclaimer (see Section 7.2.3. for reporting results).
6.5.3. Full-shift samples having calculated weight/volume ratios less than the PEL are normally not analyzed.
6.5.4. Filters:
Carefully transfer the air sample (PVC filter) from the cassette to a round-bottom 40-mL centrifuge tube. Add 10 mL THF to dissolve the filter and suspend the sample. Sonicate the sample suspension for 5 to 10 min. Quantitatively transfer the suspension with rinses of THF to a glass chimney of the vacuum filtering apparatus (described in Section 6.4.2., step 2). The total volume in the chimney should not exceed 20 mL. Apply a vacuum to achieve a thin, even deposition of sample on the membrane. Do not rinse the chimney after the vacuum has been applied. Remove the membrane and fix the deposition to the membrane in the same manner as for a standard (Section 6.4.2., step 7).
6.5.5. Bulks:
To prevent the possibility of contamination, a separate filtering apparatus should be used for bulk preparation.
Add THF and deposit the weighed sample onto a silver membrane in the same fashion as an air sample. Care must be taken when transferring the membrane before fixing. Fix the sample as described in Section 6.4.2., step 7.
6.6. Analytical Procedure
Refer to the Standard Operating Procedure (8.8.) or instrument manuals for system startup and initialization procedures.
- 6.6.1. The
6.6.2. Enter the following information into the controlling unit:
Normal analytical parameters are:
| Zinc Oxide |
Scanning Range |
Peak Location |
Peak Range |
| 1' | 35.50 to 36.80 | 36.30 | 36.25 to 36.35 |
| 3' | 33.80 to 35.10 | 34.46 | 34.41 to 34.51 |
| 4' | 55.90 to 57.20 | 56.62 | 56.57 to 56.67 |
| 5' | 62.10 to 63.40 | 62.88 | 62.83 to 62.93 |
| Note: | The peak locations listed are more informational than absolute. The actual peak is dependent on instrument and sample conditions and may vary slightly. |
6.6.3. Confirm the presence of ZnO by analyzing at least three of the lines listed above. The secondary ZnO line is not listed because the primary diffraction line for silver chloride is in the general proximity and could cause a positive interference.
6.6.4. A custom analytical computer program is used by OSHA-SLTC
to analyze samples by XRD. Directions for use and documentation of
this system can be found in the
6.6.5. Instrument considerations:
For each sample or standard, the peak location of the secondary
silver diffraction calibration line is used as an initial reference
point. If the silver line intensity of a sample is much less than a
standard, self-absorption of
7. Calculations
Prepare a linear regression concentration-response curve by plotting the concentration of the standards in µg versus counts. Blank correct the samples and then calculate the exposure as:
| mg/m3 ZnO = | µg found
air volume (L) |
| Note: | If aliquots of a sample were taken and analyzed, calculations are adjusted according to the size and number of aliquots. Results from each aliquot are combined if the sample was split into a series of aliquots. If only one aliquot was taken, the result (Analyte mg/m3) is multiplied by: |
| total suspension volume
aliquot volume taken |
- 7.1. Zinc oxide PEL
The PELs for ZnO (8.9.) are listed on the cover page of this document.
7.2. Reporting Results
- 7.2.1. Air sample results are reported to the industrial
hygienist as mg/m3 ZnO. When peak limits
are within acceptable ranges and ZnO amounts are in agreement on at
least two, preferably three lines, the reported ZnO value is taken
from the most sensitive, interference-free (or interference
resolved) line. If interferences are present, the analyst should use
available reintegration programs to resolve the interferences or
report results on a less sensitive line.
7.2.2. For bulk samples, the results are reported as approximate % ZnO.
7.2.3. Particulate present on the backup pad constitutes some sample loss. Occasionally this may be seen and can be due to a poor cassette seal on the filter, improper positioning of the filter, or poor quality control of the filter and/or cassette. A note indicating that some of the sampled material was found on the backup pad and the reported value may be lower than actual is relayed to the compliance officer.
8. References
- 8.1. Occupational Safety and Health
Administration Analytical Laboratory: OSHA Manual of Analytical
Methods (OSHA-SLCAL Method No. I-1). Salt Lake City, UT. 1977.
8.2. Weast, R.C., ed.: Handbook of Chemistry and Physics, 57th ed., Boca Raton, FL: Chemical Rubber Company Press, 1976, pp B176 - B177.
8.3. National Institute for Occupational Safety and Health: Criteria for a Recommended Standard ... Occupational Exposure to Zinc Oxide (DHEW/NIOSH Publ. No. 76-104), Cincinnati, OH, 1975, p 79.
8.4. National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 3rd ed. (Method 7502) edited by P.M. Eller (DHHS/NIOSH Pub. 84-100), Washington, D.C.: Government Printing Office, 1984.
8.5. Occupational Safety and Health Administration Analytical Laboratory: OSHA Laboratory Quality Control Division Data by R.G. Adler. Salt Lake City, UT. 1981 (unpublished).
8.6. Joint Committee on Powder Diffraction Standards (JCPDS): Powder Diffraction File 1988, Swarthmore, PA: International Center for Diffraction Data, 1988.
8.7. Occupational Safety and Health
Administration Analytical Laboratory:
8.8. Occupational Safety and Health Administration Analytical Laboratory: Standard Operating Procedure--X-Ray Diffraction. Salt Lake City, UT. In progress (unpublished).
8.9. "Air Contaminants; Final Rule": Federal Register 54:12 (19 Jan. 1989). pp. 2521-2523.
| Primary ZnO Line | |
| Interferent Name, Formula |
PDF No. * |
| Aluminum Chloride, AlCl3 | 22-10, 1-1133 |
| Ammonium Zinc Chloride, (NH4)3ZnCl5 | 30-69 |
| Ammonium Zinc Sulfate, (NH4)2Zn(SO4)2·6H2O | 35-767 |
| Calcium Aluminum Silicate, CaAl2SiO6 | 31-249 |
| Chaoite, C | 22-1069 |
| Ferric Oxide, e-Fe2O3, Fe2O3 | 16-653, 25-1402 |
| Ferrous Silicate, Fe2SiO4 | 12-284, 29-720, 34-178 |
| Lead Carbonate, PbCO3 | 5-417 |
| Magnesium Silicate, Mg2SiO4 | 13-230, 34-189, 34-556 |
| Manganese Oxide, MnO | 4-326 |
| Nickel Chloride, NiCl2 | 22-765, 1-1134 |
| Tridymite, SiO2 | 14-260, 18-1170 |
| Silver Chloride, AgCl | 22-1326 |
| Talc, Mg3Si4O10(OH)2 | 19-770 |
| Titanium Dioxide, TiO2 | 21-1236, 35-88, 29-1360, 21-1276, 23-1446 |
| Zinc, Zn | 4-831 |
| Secondary ZnO Line** | |
| Interferent Name, Formula |
PDF No.* |
| Silver Chloride, AgCl | 22-1326, 31-1238 |
| Tertiary ZnO Line** | |
| Interferent Name, Formula |
PDF No.* |
| Ammonium Zinc Chloride, (NH4)2ZnCl4 | 2-155 |
| Ammonium Zinc Chloride, (NH4)3ZnCl5 | 30-69 |
| Ferrous Silicate, FeSiO4 | 34-178 |
| Magnesium Silicate, Mg2SiO4 | 34-556 |
| Manganese Oxide, MnO | 7-730 |
| Manganese Sulfide, MnS | 6-518 |
| Montmorillonite, 18A or 21A | 12-219, 29-1499 |
| Potassium Aluminum Silicate, KAlSiO4 | 31-965, 33-989, 33-988, 11-313 |
| Talc, Mg3Si4O10(OH)2 | 19-770, 13-558, 29-1493 |
| Tridymite, SiO2 | 14-260 |
| Quarternary ZnO Line** | |
| Interferent Name, Formula |
PDF No.* |
| Ferric Oxide, g-Fe2O3 | 24-81 |
| Ferrous Silicate, Fe2SiO4 | 34-178 |
| Magnesium Silicate, Mg2SiO4 | 34-189, 34-556 |
| Manganese Oxide, MnO | 4-326 |
| Silicon Dioxide, SiO2 | 34-717 |
| Titanium Dioxide, TiO2 | 29-1360, 21-1276 |
| Quinternary ZnO Line** | |
| Interferent Name, Formula |
PDF No.* |
| Aluminum Chloride, AlCl3 | 1-1133 |
| Ferric Oxide, e-Fe2O3, Fe2O3, g-Fe2O3, Fe2O3 | 16-653, 33-664, 24-81, 25-1402 |
| Magnesium Silicate, Mg2SiO4 | 34-189 |
| Titanium Dioxide, TiO2 | 21-1236 |
*PDF No. = JCPDS Powder Diffraction File Number
**Only the most significant interference is listed for the secondary ZnO line.
| Note: | The majority of the analytes listed above will most likely not be present when sampling industrial operations which produce ZnO exposures. This list is presented as line-matches found in literature and not as definitive interferences. Some of these interferences may occur only when a large amount of interferent is present or at a temperature other than normal laboratory conditions. A substance is listed as a potential interference if one or more sensitive lines of that substance has a peak within ±0.65° 2q of the ZnO line. |
A custom OSHA-SLTC program reads and stores diffraction count data and evaluates or presents output data in the following ways:
- Uses a symmetric five-point digital filter (weights = 0.6, 0.8, 1.0, 0.8, 0.6) to smooth the spectral count data.
- Identifies peaks by maximum counts.
- Determines upper and lower 2q integration limits.
- Chooses the integration method (either valley-to-valley or perpendicular drop) by observation of background and signal counts.
- Integrates the peak by summing counts over the selected integration range.
- Calculates the concentration of analyte in total µg/m3.
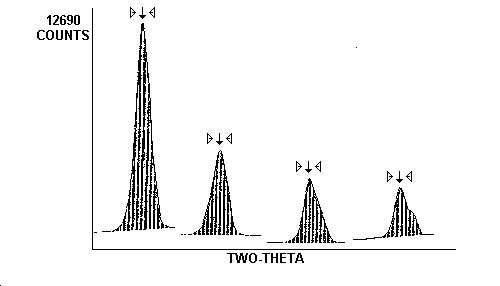
- Generates a hard copy report. An example of a report used at OSHA-SLTC is shown in Figure 1.
This program also allows the analyst to modify the computer selection of integration limits and method for each diffraction peak. A graphic representation of the scan can be displayed and an example of a 500 µg ZnO standard is shown in Figure 1. Areas of integration are shaded and outlying areas are non-shaded. The abbreviation "NORM CNTS" contained within the Figure stands for normalized counts (total counts/counting time). Other in-house or commercially available software programs can be used to minimize interferences and clarify results.
500 ZNO
| 500ZNO | ||||
| AIR VOL. | 1.00 | L | -STANDARD- | |
| SAMPLE WT. | 500. | UG | ||
AG CAL. 19484 COUNTS AT 44.28 DEG.
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||