| Method Number: | ID-142 | ||
| Matrix: | Air | ||
| OSHA Permissible Exposure
Limits Respirable Dust Containing Quartz: |
| ||
| Respirable Dust
Containing Cristobalite: |
Use 1/2 the value calculated from the mass formula for quartz. | ||
| Collection Device: | A | ||
| Recommended Sampling Rate: | 1.7 L/min | ||
| Recommended Air Volume: | 816 L | ||
| Analytical Procedure: | A suspension of the sample particulate in
tetrahydrofuran (THF) is created by dissolving the sample filter in
THF and then sonicating. The sample particulate is then deposited
onto a silver membrane filter and analyzed by | ||
| Detection
Limits: Qualitative: |
5 µg quartz 10 µg cristobalite | ||
| Quantitative: | 10 µg quartz 30 µg cristobalite | ||
| Precision and
Accuracy Validation Range |
Quartz 50 to 160 µg quartz per sample | ||
| CV1 | 0.106 | ||
| Bias | +5.2% | ||
| Overall Analytical Error | ±26% | ||
| Method Classification: | Validated Method | ||
| Date (Date Revised): | 1981 (December, 1996) | ||
Mention of commercial manufacturers and products in this method are for
descriptive use only and does not constitute endorsements by
Similar
OSHA Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the collection of airborne respirable a-quartz and/or cristobalite in the breathing zone of
personnel and the subsequent analysis by
- High-volume respirable filter sample (preferably > 1.0 g). Contact the SLTC for information on this.
- High-volume filter sample - nonrespirable (preferably > 1.0 grams)
- Representative settled dust (i.e., rafter sample (preferably > 1.0 grams)
- Sample of the bulk material in the workplace (preferably
10-20 grams). - Dry the sieved quartz (NIST SRM or equivalent material) or cristobalite for 2 h at 110°C. This material is used for stock and working standards.
- Prepare three standard suspensions in
2-propanol by first weighing 10, 50, and 200 mg of the quartz or cristobalite to the nearest 0.01 mg. Then quantitatively transfer each to individual1-L volumetric flasks using2-propanol , and bring each to half volume. - Disperse the analyte in the
2-propanol by using an ultrasonic bath for 10 min. Remove from the bath and allow the suspension to cool to room temperature. Dilute each flask to the mark with2-propanol . - Prepare a series of working standards on silver membranes from
the
10-, 50-, and200-µg/mL stock suspensions by pipetting appropriate aliquots using the procedure outlined in step 5 below. Prepare three sets of standards for the calibration curve as shown:Stock Standards Aliquot Working Standards (µg/mL)
(mL)
(µg)
200 5, 10, 15 1,000, 2,000, 3,000 50 2, 5, 10 100, 250, 500 10 1, 2, 5 10*, 20*, 50 * These standards are omitted for cristobalite analysis.
- Turn on the
explosion-resistant hotplate and set at the lowest setting. Place a Teflon sheet on top. - Assemble the filtering apparatus and liquid nitrogen cold
trap. Connect the cold trap to the filtering apparatus to collect
the waste suspending media (
2-propanol or THF). The waste vapors should not enter the vacuum pump. - Center a silver membrane on a
fritted-glass base of the filtering apparatus. Also center the glass chimney on top of the base and secure it with a clamp. - Add a stirring bar to the stock standard suspension and
withdraw aliquots using the following technique:
- Place the flask on a magnetic stirrer and turn the stirrer on until a uniform suspension is achieved.
- Turn off the stirrer, remove and invert the flask, allowing the stirring bar to slide into the neck.
- Shake the flask vigorously to ensure an even suspension.
- Cautiously revert the flask and return it to the stirrer.
- Turn on the stirrer and immediately withdraw an aliquot from the center of the stirred solution using an appropriate volumetric pipette (care should be taken not to withdraw solution above the volume mark of the pipette).
- With the vacuum off, place about 2 mL of
2-propanol in the chimney of the previously assembled vacuum filtering apparatus. Transfer the pipetted aliquot to the chimney. After the transfer, bring the total volume in the chimney to 20 mL with2-propanol . - Apply vacuum to the filtering apparatus, drawing the
2-propanol through it. This should result in a thin, even layered deposition of the analyte onto the silver membrane. Do not rinse the chimney after the material has been deposited on the membrane. Rinsing can disturb the thin layer deposition just created. Vacuum should be applied for sufficient time to dry the membrane. - Carefully disassemble the chimney and clamp. Remove the silver
membrane from the
fritted-glass base using forceps. Place 2 drops of 1.5% parlodion solution on a glass slide. Fix the standard to the membrane by placing the bottom side of the membrane in the parlodion solution. By capillary action, the membrane draws the parlodion solution to the analyte surface. Then place the membrane on top of the heated Teflon sheet. When thoroughly dry, place the fixed standard in a labeled Petri dish. (If placed in the plastic petri dish before dry, the membrane may become affixed to the dish.) - Inspect the deposition for uniformity; clumping indicates that insufficient sonication was used. The standard will have to be remade if a significant amount of clumping occurs.
- Bulk samples approximating respirable particle size: Weigh an aliquot of 1 to 2 mg on a PVC filter, and place in a round bottom centrifuge tube.
- Non-respirable bulks: Grind the sample to a fine powder using
either a mortar and pestle or a freezer mill. Then size the
sample, using a
325-mesh sieve or sonic sifter. This results in a sample particle size of less than 45 µm. An aliquot of the sized sample is weighed on a PVC filter and then placed in a round bottom centrifuge tube. - 2q limits for each analytical line
- 2q scanning increment (0.02° 2q)
- Integration and background counting times
- Diffraction angle calibration using the secondary silver line
(44.33° 2q)
- Present 2q location of the
X-ray instrument - The calculated exposure from the primary line analysis is less than 0.75 × PEL and the air volume is greater than 500 L.
- The calculated exposure is less than 0.5 × PEL and the air volume is less than 500 L.
- The most common transform used in
X-ray work is the square root which weights count data more equitably if Poisson counting error is the primary source of error in the measurement. The error is usually larger across the calibration range than can be accounted for by Poisson counting error alone. The additional errors arise from errors associated with taking aliquots of a suspension, particle statistics, and geometric factors in sample presentation. The square root transform of the µg data helps to spread the data so that it is not as tight at the low end. This helps prevent a polynomial fit from exhibitingnon-monotonic behavior in the calibration range. - The logarithmic transform converts the approximate geometric
series of µg data along the abscissa into more
equally-spaced data making a polynomial fit well conditioned and further preventingnon-monotonic behavior. The logarithmic transform also treats the relative error in the ordinate more equitably across the calibration range. A small offset is added to each value (1 count and 1 µg) prior to the logarithmic transform so that zero counts and zero µg can be included in the calibration. The offset is removed when the inverse transform is taken. - The fourth root transform in approximately intermediate in effect between the square root transform and the logarithmic transform (which is generally comparable in effect to a sixth root).
- The lower region is usually expected to show reduced
sensitivity due to the combined effects of:
- Penetration of some of the analyte into the pores of the silver membrane which shields the analyte partially from X rays.
- Selection of narrower integration limits for less intense peaks. Wider limits would increase sensitivity at the expense of decreased precision.
- The middle linear region is centered about 250 µg.
- The upper region shows gradually decreasing sensitivity up to
3 mg due to sample
self-absorption of X rays. - Perform a silver line calibration (44.33° 2q) before each standard or sample is analyzed.
- Scan the standard or sample over the selected 2q range in 0.02° increments.
- A standard is analyzed at the start and end of a run of samples and after every fourth or fifth sample analysis to assure correct instrumental operation. A new run is defined here as beginning after any significant change in analytical condition (following power interruptions, when spikes are noted, after changing to a different set of analyte peaks, etc.).
- Releasing the sample from the silver membrane
- Dividing the sample into aliquots and depositing each aliquot
onto a silver membrane and
re-analyzing or performing theacid-wash procedure mentioned in Section 6.6.9. - Place each sample filter (silver or PVC) into an individual
125- or250-mL Phillips beaker. Add 5 mL of nitric acid (HNO3). If the sample is on a PVC filter, also add 2 mL perchloric acid (HClO4) (see Section 6.1.4. - do not add HClO4 to silver filters). - Place beakers on a hotplate and digest filters until approximately 1 mL remains.
- Slowly add 25 mL of phosphoric acid
(H3PO4) to
each beaker (if cristobalite is being analyzed, add 25 mL 1:1
H3PO4:H2O
instead). Place a
bent-stem glass funnel in each beaker. Then place each beaker on a rotating hotplate, allow the solution to boil, and then rotate and gently boil for 8 min. - Remove from the hotplate, and continue to swirl until the solution reaches room temperature.
- Add 75 to 100 mL hot DI H2O while vigorously swirling each beaker.
- Wash down the sides of each beaker with 10 mL fluoboric acid (HBF4) and again vigorously swirl.
- Let each solution stand about 1 h. Filter each sample onto a PVC filter (0.6 µm) using the filtration apparatus mentioned in Section 6.2.5. Rinse the sides of the beaker with DI H2O and add the solution to the funnel assembly. Apply a vacuum.
- If excessive silver chloride or gel formation is noted on the PVC filter, add approximately 5 mL of ammonium hydroxide (aqueous ammonia) to the funnel assembly and apply a vacuum.
- Dry the PVC filter on a warm hot plate. Transfer the filter to
a
40-mL centrifuge tube and proceed as in Section 6.5.3. - % analyte
- mg respirable dust containing analyte
- mg respirable dust containing analyte/m3
- less than or equal (<) X% analyte
- < or X mg analyte/m3
- mg respirable dust
- 1.1. History
- 1.1.1. There have been several methods used to analyze quartz.
These include atomic absorption, colorimetry, gravimetry,
microscopy, infrared spectroscopy, and XRD. The preferred method is
considered to be XRD, because it can distinguish and quantitate the
different polymorphs of free silica in a widest range of industrial
dust matrices.
1.1.2. The previous method (8.1.)
used by the OSHA Salt Lake City Technical Center
1.2. Principle
A respirable sample is collected by drawing air at approximately
1.7 liter per minute (L/min) through a
| Aerodynamic
diameter (unit density sphere) |
Percent
passing selector |
|
|
|
| 2 µm | 90 |
| 2.5 | 75 |
| 3.5 | 50 |
| 5.0 | 25 |
| 10 | 0 |
For efficient communication to distinguish the various competing models for respiratory retention, it is common to refer to only the 50% cumulative cut point in terms of the equivalent spherical aerodynamic diameter (D50). The respiratory model in the Federal Register refers to the old ACGIH definition (D50 = 3.5 µm) which differs from both the more recent ACGIH recommended model (D50 = 4 µm) and the BMRC model (D50 = 5 µm). The mathematical function of each model differs the others. Adjusting the flow rate of any other sampler design until a 50% cut is achieved at 3.5 µm aerodynamic diameter may not achieve comparable aerodynamic diameters to those specified at the 0, 25, 75, and 90% cut points. ( For a review of the various respiratory models see 8.6.)
At the laboratory, the filter is dissolved in tetrahydrofuran
(THF), the sample particulate is suspended in THF, and then deposited
on a silver membrane. The membrane is scanned by XRD giving a series
of diffraction peaks (lines) occurring at different angles relative to
the sample and
- nl = 2d sinq
| Where: | ||
| n | = | order of diffracted beam |
| l | = | wavelength of |
| d | = | distance between diffracting planes (in angstroms) |
| q | = | angle between incident X rays and the diffracting planes (in degrees) |
Note: Most
1.3. Advantages and Disadvantages
- 1.3.1. This method is specific for quartz and cristobalite; XRD
can distinguish crystal structure.
1.3.2. Sample preparation is simple and analysis can be
1.3.3. Quartz and cristobalite can be identified and quantified on three or more different angles of diffraction.
1.3.4. Interferences can be minimized by using alternate angles
of diffraction,
1.3.5. This method is more accurate and offers better sensitivity than previous methods of analysis.
1.3.6. The method is not an elemental analysis. It is highly
specific; the location of diffraction peaks requires both elemental
and structural parameters to be "just right". This is true for both
analytes and interferences. Substitution of one element for
another in a given structure results in a different diffraction line
pattern. Substitution of one structure for another likewise results
in a different pattern. For example, zircon
(ZrSiO4) has a different pattern from
zirconia (ZrO2) and indeed from any other
compound containing zirconium (Zr). The diffraction pattern is
therefore also independent of whether a mineralogist's chemical
shorthand convention is used to the describe the compound on
the MSDS. For example, on an MSDS the compound zircon may be
shown in mineralogist's chemical shorthand as consisting of 32.8%
SiO2 and 67.2%
ZrO2. The diffraction pattern of zircon
however bears no relation
1.3.7. A disadvantage is the high cost of instrumentation and maintenance.
1.3.8. Another disadvantage is the requirement of a known
particle size distribution and a sample weight compatible with a
1.4. Physical and Chemical Properties (8.7.)
| CAS No. | 14808-60-7 |
| Chemical Symbol: | SiO2 |
| Synonyms: | free crystalline silica, silicon dioxide, silica flour |
| Formula Weight: | 60.08 |
| Crystalline Form: | hexagonal habit |
| Specific Gravity: | 2.635-2.660 |
| Melting Point: | 1610°C |
| Boiling Point: | 2230°C (decomposes) |
| Solubility: | soluble in hydrofluoric acid |
| Color: | colorless in pure form |
| CAS No. | 14464-46-1 |
| Chemical Symbol: | SiO2 |
| Synonyms: | calcined diatomite |
| Formula Weight: | 60.08 |
| Crystalline Form: | cubic or tetragonal |
| Specific Gravity: | 2.32 |
| Melting Point: | 1723°C |
| Boiling Point: | 2230°C (decomposes) |
| Solubility: | soluble in hydrofluoric acid |
| Color: | colorless in pure form |
1.5. Uses and Occupational Exposures
Quartz is primarily used as an abrasive (sandblasting, cleaning, etc.) in the production of stone, clay and glass products, and foundry molds. The most serious exposures result from quartz in the form of respirable dust produced by grinding, blasting, and mixing operations. Occupations having a high potential for exposure to quartz are listed (8.8.):
Coal mining
Non-metallic minerals (except fuels)
Stone, clay, and glass products
Foundries
Agriculture
Chemical production
Concrete work
Sandblasting
Cristobalite is used in the manufacture of insulation, filters, and refractory materials (8.8.). In nature, cristobalite usually occurs together with tridymite. Both cristobalite and tridymite can be found naturally occurring in volcanic rock or can be synthetically produced by heating amorphous or crystalline silica. Under ideal conditions tridymite forms at temperatures above 870°C and cristobalite above 1470°C, however, between these temperatures (particularly in the absence of alkali or alkaline earth impurities) a disordered form of cristobalite may often form instead of tridymite (8.9.). Occupational exposure to cristobalite can occur during manufacture of stone, clay, glass, and other ceramic products. Other sources of exposure to cristobalite can occur in diatomaceous earth operations, or high temperature operations such as foundries.
2. Analytical Working Range
- 2.1. Qualitative detection limits for quartz are:
| Diffraction Peak |
Peak ° 2q |
Integration Time(s) |
Detection Limit (µg) |
| Primary | 26.66 | 1 | 5 |
| Secondary | 20.88 | 25 | 5 |
| Tertiary | 50.18 | 25 | 5 |
| Note: | Peak 2q values are dependent on instrumental characteristics and may vary slightly. The terms "peak" and "line are used interchangeably in this method. |
The detection limits listed above were determined by both
parametric (t-test) and
2.2. The quantitative detection limit for quartz using the primary
diffraction line is 10 µg. The coefficient of variation of
2.3. The qualitative and quantitative detection limit for cristobalite are 10 and 30 µg, respectively.
2.4. The analytical range is dependent upon the amount of
interfering substances and
3. Method Performance
- 3.1. Typical quality control data over the years:
| Range |
80 to 200 µg
quartz |
50 to 160 µg
quartz |
50 to 210 µg
quartz |
| CV1 | 0.136 | 0.106 | 0.110 |
| Mean Recovery | 95.6% | 105% | 96.0% |
| n | n = 60 | 300 | 100 |
| Time Period | April 1981 to June 1981 (8.12.) |
December 1986 to September 1988 (8.13.) |
February 1996 to November 1996 (8.14.) |
Applicability of the Gaussian (normal) statistical model was tested using order statistics for the 1,000 quartz QC results (less 6 outliers) for the period from May 1987 to March 1995 and was found to be appropriate (8.15.)
3.2. Limited data is available concerning precision and accuracy for the analysis of cristobalite; however, the sample preparation and analytical technique are the same as quartz. Analytical error should be comparable to the quartz analysis.
4. Interferences
According to data listed in a computer search of the Joint Committee
on Powder Diffraction Studies (JCPDS) powder diffraction file (8.16.),
several compounds have diffraction peaks that may interfere with a-quartz or cristobalite. Such positive interferences
add to the intensities of only those specific quartz diffraction peaks
affected. Many of these compounds are listed in Appendix
A. The majority of the interferences listed will most likely not be
present together when sampling industrial operations which produce
quartz or cristobalite exposures. This list is presented as
- 4.1. Some elements (iron, in particular) can cause appreciable
4.2. If severe interferences are present on the primary analytical peak, results are reported using the secondary or tertiary diffraction peak. The decrease in sensitivity and precision expected when using a less sensitive line than the primary can be compensated, to a limited extent, by increasing the counting time.
4.3. The presence of interference can be verified and usually
identified by XRD analysis. Interfering peaks are usually resolved at
the
4.4.
5. Sampling
- 5.1. Sampling Equipment
- 5.1.1. Sampler assembly (if weighings are performed in the
field):
Filter holder consisting of a
Backup pad,
Low ash homopolymeric PVC membrane filter,
Note: During preparation for analysis, the sample filter is dissolved in tetrahydrofuran (THF). Certain acrylic copolymers added to PVC filters are insoluble in THF. If the membrane filter composition is unknown, a laboratory test should be conducted with THF to determine suitability before use.
5.1.2. Sampler assembly (if preweighed filter cassettes are
used): Cassette, Aerosol,
5.1.3. Cyclone: Nylon,
5.1.4. Plastic coupler for preweighed filter cassette sampling used with Cyclone Assembly: (MSA part number 457391, MSA, Pittsburgh, PA., or CTC OPMIS Filter Holding Coupler, #FES0000154).
5.1.5. Pump calibration system: Stop watch and bubble tube or electronic meter.
5.1.6. Sampling pump: Calibrate the personal sampling pump to
approximately 1.7 L/min. Each pump must be calibrated with a
representative sampler (cyclone, filter, etc.)
5.1.7. Assorted flexible tubing.
5.1.8. High volume sampling pump with cyclone (optional - for bulk sample collection).
5.1.9. Calibrated analytical balance (0.01 mg).
5.1.10. Desiccant (Drierite or similar material) and desiccating chamber if not using preweighed filters.
The last two items are not needed if the samples are weighed by the laboratory.
5.2. Sampling Procedure
[Compliance safety and health officers (CSHOs) should refer to the OSHA Technical manual (8.19.) for pump calibration information when sampling with cyclones.]
Samples are
- 5.2.1. Desiccate and then weigh the PVC filter before sampling.
5.2.2. Place the PVC filter and a cellulose backup pad in a
5.2.3. Attach the cassette, which is preceded by a
5.2.4. Place the sampling assembly in the breathing zone of the worker or sampling area and place the pump in an appropriate position. Take from 408 to 816 L of air through the cassette at approximately 1.7 L/min. Do not allow the cyclone to be inverted during or after sampling. If confirmation for cristobalite is necessary, take full shift samples if possible.
5.2.5. Check the pump and sampling assembly periodically to verify performance and to monitor particulate loading on the sample filter. If the filter becomes overloaded (>3 mg) during the sampling interval, replace it with another filter.
5.2.6. Terminate sampling at the predetermined time and record the pump flow rate and collection time. Carefully remove the filter, desiccate, and then weigh to determine the net weight gain. Carefully replace the filter and firmly seal the cassette by placing plastic plugs in both the inlet and outlet ports.
5.2.7. Record on the OSHA 91 form all pertinent sample data. When other compounds are known or suspected to be present in the air, such information, including their suspected identities, should be transmitted with the samples. Also indicate whether the requested analysis is for quartz, cristobalite or both. Operations where the material has been heated to high temperatures generally should be analyzed for both.
5.2.8. Identify and submit an appropriate blank filter from each lot of filters used.
5.2.9. Seal each filter cassette and identify it with an OSHA Form 21. Mail samples to the laboratory in a suitable container designed to prevent damage.
For samples weighed at the laboratory:
5.2.1a. Obtain the preweighed sampling cassettes from the laboratory.
5.2.2a. Attach the cassette, which is preceded by a
5.2.3a. Place the sampling assembly in the breathing zone of the worker or sampling area and place the pump in an appropriate position. Take from 408 to 816 L of air through the cassette at approximately 1.7 L/min. Do not allow the cyclone to be inverted during or after sampling. If confirmation for cristobalite is necessary, take full shift samples if possible.
5.2.4a. Check the pump and sampling assembly periodically to verify performance and to monitor particulate loading on the sample filter. If the filter becomes overloaded (>3 mg) during the sampling interval, replace it with another filter.
5.2.5a. Terminate sampling at the predetermined time and record the pump flow rate and collection time. Seal the cassette by replacing the plastic plugs in both the inlet and outlet ports.
5.2.6a. Record on the OSHA 91 form all pertinent sample data. When other compounds are known or suspected to be present in the air, such information, including their suspected identities, should be transmitted with the samples. Also indicate whether the requested analysis is for quartz, cristobalite or both. Operations where the material has been heated to high temperatures generally should be analyzed for both.
5.2.7a. Identify and submit an appropriate blank filter from each lot of filters used.
5.2.8a. Seal each filter cassette and identify it with an OSHA Form 21. Mail samples to the laboratory in a suitable container designed to prevent damage.
5.3. Bulk Samples
In order of laboratory preference, bulk samples may be one of the following:
Although bulks of type 1) and 2) are the most preferred, it is
recognized that 3) and 4) are often the most practical to collect. The
type of bulk sample should be stated on the OSHA 91 and
6. Analysis
Samples submitted on
- 6.1. Safety Precautions
- 6.1.1. Tetrahydrofuran (THF) has a low flash point, -14°C (6°F),
and is extremely flammable. Always use THF in a hood. THF is an
ether which can form explosive peroxides upon exposure to air;
therefore, it should be stored in closed containers. Always use
latex gloves, a labcoat, and safety glasses when handling THF. Use
appropriate measures (conductive mats, wrist straps, etc.) as needed
to reduce static electrical charge: electrical sparks can ignite THF
vapors.
6.1.2. Parlodion and isopentyl acetate are flammable.
6.1.3. Always use a hood when grinding bulk materials or when working with dry quartz or cristobalite.
6.1.4. If sample
6.1.5. Most
6.1.6. A bench top warning light (yellow) is recommended. When
lit, the warning light indicates the
6.1.7. Most
6.1.8. If the
6.1.9. Avoid inserting fingers into the sample compartment. Use forceps to change samples.
6.1.10. Radiation monitors are worn by all
6.2. Analytical Equipment
- 6.2.1.
- Automated Powder Diffractometer (APD).
Long,
Scintillation counter detector.
Recirculating cooling system for the
6.2.2. Computer system consisting of:
- Hardware and software for data reduction and graphic
presentations.
Microprocessor Interface: Between the computer and the goniometer.
Line profile library (JCPDS-International Center for Diffraction Data Powder Diffraction File, JCPDS, Swarthmore, PA).
6.2.3. Standard and sample preparation:
- Centrifuge tubes: Round bottom
Drying oven.
Explosion-resistant hot plate (Model
Filtration apparatus, 25 mm (Filter Holder Hydrosol Manifold, cat. no. XX25 047 00, filtering clamps, cat. no. XX10 025 03, fritted glass bases with stoppers, cat. no. XX10 025 02, and glass funnels, cat. no. XX10 025 11, Millipore Corp., Bedford, MA).
Forceps.
Latex or other
Liquid nitrogen
Micro-analytical balance (0.01 mg) for preparing bulks.
Plastic petri dishes (Product no. 7242, Gelman Sciences, Ann Arbor, MI).
Silver membrane filters: Diameter
Teflon sheet, 0.3 to 1 mm thick.
Vacuum system.
Volumetric pipettes, eyedropper, volumetric flasks and graduated cylinders.
Ultrasonic bath.
6.2.4. Bulk sample preparation for membrane deposition:
- Freezer mill (Model no. 6700, Spex Industries, Edison, NJ) for
plastic or other bulks that cannot be ground by a mortar and
pestle.
Mortar and pestle.
Sieve or sonic sifter: Sieve, 325 mesh, (or Model ATML3P Sonic Sifter with 325 mesh sieve, ATM Corporation, Milwaukee, WI).
6.2.5.
- All glass filtering apparatus,
Phillips beakers,
Rotating
Funnels, glass,
PVC
6.3. Reagents
All chemicals should be reagent grade or better.
- 6.3.1. Tetrahydrofuran (THF).
6.3.2. Parlodion (Pyroxylin).
6.3.3. Isopentyl (Isoamyl) acetate.
6.3.4. Parlodion in isopentyl acetate, 1.5% (w/v): Dissolve 1.5 g of parlodion in isopentyl acetate and dilute to 100 mL with isopentyl acetate.
6.3.5. Respirable quartz (5 µm):
The National Institute of Standards and Technology (NIST) manufactures the standard reference material (SRM) used in the analysis of respirable quartz. NIST SRM 1878 consists of quartz in a distribution of particle sizes with a mass mean equivalent spherical diameter of 1.62 µm that is intended to be representative of respirable particles sampled as per the respiratory model published in the Federal Register (8.20.). Because XRD is generally less sensitive to the smaller crystalline particles in aerosols (8.21. and 8.22.) different consensus certified quartz reference materials may be required if a new respiratory model is adopted where more of the mass may be represented by larger particles. SRM 1878 should be sieved prior to use in order to remove any particles larger than 10 µm (8.22.).
National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 1878 or 1878a.
Alternative respirable quartz material (can be used if compared to one of the reference materials listed above - use appropriate gravimetric factors):
Min-U-Sil 5 Quartz (Pennsylvania Glass Sand Co., Berkley Springs, WV).
6.3.6. Respirable cristobalite:
NIST SRM 1879, 1879a.
6.3.7.
6.3.8. If
6.4. Standard Preparation
The sample preparation and analytical procedure listed below are modifications of techniques found in references 8.23.-8.25.
- 6.4.1. Preparation of separate quartz or cristobalite stock
standards:
6.4.2. Preparation of quartz or cristobalite working standards:
6.5. Sample Preparation
- 6.5.1. When sample weights are greater than 3 mg, aliquots are
taken to achieve depositions within the working range.
6.5.2. Examine the filter and backup pad to determine if any breakthrough to the backup pad has occurred. If there is significant breakthrough, the sample is either not analyzed or results are reported with a disclaimer (see Section 7.5.3. for reporting results).
6.5.3. Carefully transfer the respirable air
sample (PVC filter) from the cassette to a
6.5.4. Samples collected without a cyclone or at a flow rate
outside of the range of 1.5 to 2.0 L/min should be considered as
6.5.5. To prevent the possibility of contamination, a separate filtering apparatus should be used for bulk preparation.
6.5.6. Add THF and deposit the weighed sample onto a silver membrane in the same fashion as an air sample. Care must be taken when transferring the membrane before fixing. Fix the sample as described in Section 6.4.2., step 8.
6.6. Analytical Procedure
Refer to the Standard Operating Procedure (8.26.) or instrument manuals for system startup and initialization procedures.
- 6.6.1. Set the
6.6.2. Enter the following information into the controlling unit:
Normal analytical parameters are:
| Quartz | Scanning Range | Peak Location | Peak Range | D Space |
| Primary | 25.90 to 27.20 | 26.66 | 26.61 to 26.71 | 3.341 |
| Secondary | 20.06 to 21.40 | 20.88 | 20.83 to 20.93 | 4.251 |
| Tertiary | 49.40 to 50.7 | 50.18 | 50.13 to 50.23 | 1.817 |
| Quaternary | 59.40 to 60.70 | 60.00 | 59.95 to 60.10 | 1.541 |
| Cristobalite | ||||
| Primary | 21.20 to 22.50 | 22.00 | 21.95 to 22.05 | 4.046 |
| Secondary | 35.50 to 36.80 | 36.07 | 36.02 to 36.12 | 2.487 |
| Tertiary | 30.76 to 32.06 | 31.42 | 31.37 to 31.47 | 2.845 |
| Note: | The peak locations and d spaces listed are more informational than absolute. Peak locations are dependent on instrument and sample conditions and may vary slightly. |
6.6.3. Confirm the presence of quartz or cristobalite by analyzing at least three of the lines listed above for each compound (Note: The analytical determination can end after scanning only the primary line when one of the following conditions is met:
6.6.4. A
The
The analyst reviews the results of calibrations using the
untransformed and transformed data and selects the best overall fit
of the data. The corresponding transform and coefficient data are
programmed into the computer interface. Calibration checks are
performed during the analysis. If the curve data is not current, or
analytical conditions (viewing height of sample,
6.6.5. Custom analytical computer programs are used by
6.6.6. Instrument considerations:
For each sample or standard, the peak location of the secondary
silver diffraction calibration line is used as an initial reference
point. If the silver line intensity of a sample is much less than a
standard (<40% is a suggested guideline), significant
6.6.7. The normal counting time for the primary quartz line is 1
s for each 0.02° increment. To achieve maximum sensitivity it is
recommended to change counting times for the secondary and tertiary
lines. These changes are dependent on the quantity of quartz found
at the primary line. For a primary integrated peak sensitivity of
100 counts/µg (using a
| Microgram level: | >125 | >50<125 | >25<50 | <25 |
| Counting time: | 1 s | 5 s | 10 s | 25 s |
For cristobalite, 0.02° increments and the following counting times are used:
| Microgram level: | >125 | <125 |
| Counting time: | 1 s | 5 s |
Higher counting times are needed for less sensitive instrumentation.
6.6.8. If interferences are present in any of the primary, secondary, or tertiary lines, the analyst should evaluate different software approaches for peak area integration, an alternate analytical line, or chemical treatment to resolve the interferences. The most sensitive alternate line for quartz is listed in Section 6.6.2. This line (peak at 60.00° 2q) may not be quantitative below 50 µg quartz. For cristobalite, the alternate lines are not very sensitive or are interference-prone.
6.6.9. If major, unresolvable interferences
are present, the analyst should attempt to alleviate them by using
the
7. Calculations
As previously mentioned in Section 6.6.4.,
each
- 7.1. The PELs for quartz and cristobalite are listed on the cover
page of this method (8.5.).
7.2. The calculation to determine the PEL for the reportable result is:
| % Respirable Analyte = | weight of analyte (µg) × 100
total air sample weight (µg) |
The total air sample weight (in µg) is the net filter weight gain as determined by the industrial hygienist or by the laboratory. The weight of analyte is determined from the integrated analyte peak intensity obtained by XRD using the calibrated response vs. mass curve for the analyte peak.
The corresponding PELs are then calculated:
| PEL for Respirable dust containing quartz PEL (mg/m3) = | 10 mg/m3
2 + Respirable Quartz |
| PEL for Respirable
dust containing cristobalite (mg/m3) |
= | Use 1/2 the value
calculated from the mass formula for quartz |
An air concentration/PEL ratio can be determined by:
| Ratio of exposure = | AIR CONC
PEL |
Where:
| AIR CONC = | total sample weight (µg)
total air volume (L) |
Other factors may have to be considered before arriving at a final exposure value. For example, the TWA calculation may require combining two or more sample results and adjust to an 8 h work day. Consult Silicosis SEP Appendix E (8.27.) or OSHA Technical Manual (8.19.) or for combining sample results.
| Note: | If aliquots of a sample were taken and analyzed,
calculations are adjusted according to the size and number of
aliquots. Results from each aliquot are combined if the sample
was split into a series of aliquots. If only one aliquot was
taken, the result (Analyte mg/m3) is
multiplied by:
|
7.3. If an overexposure appears to have occurred, any bulk sample(s) taken with the corresponding sample set should also be analyzed using a wide angle qualitative scan to confirm the presence of quartz or cristobalite. Bulk samples must be representative of the workplace being sampled for these scans to be meaningful. Comparisons with patterns in the powder diffraction pattern library should be made in order to match the bulk diffraction lines with quartz and/or cristobalite, and a positive identification of other compounds contained in the bulk should be attempted.
7.4. A graphic portrayal of each sample should be generated as a hard copy (for further information regarding the OSHA custom software and hard copies, see Appendix B).
7.5. Reporting Results
When peak limits are within acceptable ranges and analyte amounts
are in agreement on at least two, preferably three lines, the reported
value is taken from the most sensitive,
- 7.5.1. Respirable air sample results for individual samples are
reported to the industrial hygienist in various ways for the
analytes consisting of quartz and/or cristobalite:
Other laboratories may report results in various ways useful to the industrial hygienist such as mg analyte/m3 so as to address various consensus standards which differ from the current PEL.
7.5.2. If the results from the analysis on the primary line indicated that the sample exposure was less than the criteria listed in Section 6.6.3., the sample result X was not subsequently confirmed on alternate lines because it did not represent a significant fraction of the PEL. The result X represents an upper estimate of the amount of quartz potentially present and is reported as:
7.5.3. Particulate present on the backup pad constitutes some sample loss. Occasionally this may be seen and can be due to a poor cassette seal on the filter, improper positioning of the filter in the cassette, or poor quality control of the filter and/or cassette. A note indicating that some of the sampled material was found on the backup pad and the reported value may be lower than actual is relayed to the compliance officer if this type of contamination occurs.
7.5.4. For samples collected without a cyclone or at a flow rate different than recommended in Section 5.1.4., results are reported as approximate % or mg/m3.
7.5.5. For bulk or high volume samples, the results are reported as approximate % quartz or cristobalite:
| Approximate % Analyte = | weight of analyte found (µg) ×
100
total sample weight (µg) |
8. References
- 8.1. Occupational Safety and Health
Administration Analytical Laboratory: OSHA Manual of Analytical
Methods (OSHA-SLCAL Method No.
8.2. Talvitie, N.A.: Determination of Free
Silica: Gravimetric and Spectrophotometric Procedure Applicable to
8.3. National Institute for Occupational
Safety and Health: NIOSH Manual of Analytical Methods, 3rd
ed. (Method 7500) edited by P.M. Eller (DHHS/NIOSH Pub.
8.4. National Institute for Occupational
Safety and Health: NIOSH Manual of Analytical Methods, 2nd
ed. Vol. 5., P&CAM 259, U.S. Department of Health, Education, and
Welfare, Publ. (NIOSH)
8.5. Code of Federal Regulations, 29
CFR 1910.1000, Table
8.6. Groves, W.A., R.M.A. Hahne, S.P. Levine,
and M.A. Schork: A Field Comparison of Respirable Dust Samplers,
Am. Ind. Hyg. Assoc. J., 55:
8.7. Weast, R.C., ed.: Handbook of Chemistry and Physics, 67th ed., Boca Raton, FL: Chemical Rubber Company Press, 1986.
8.8. National Institute for Occupational
Safety and Health: Criteria for a Recommended Standard ...
Occupational Exposure to Crystalline Silica. (DHEW/NIOSH Publ. No.
8.9. Othmer, D.F. et al ed.: Kirk-Othmer Encyclopedia of Chemical Technology, 3rd Ed., New York: John Wiley & Sons, 1982. Vol. 20, pp. 756-757.
8.10. Dixon, W.J., and F.J. Massey:
Introduction to Statistical Analysis,
2nd ed., New York:
8.11. Occupational Safety and Health Administration Analytical Laboratory: OSHA Laboratory Quartz Detection Limit Study by P. Giles and R. Cee. Salt Lake City, UT. 1981 (unpublished).
8.12. Occupational Safety and Health Administration Analytical Laboratory: OSHA Laboratory Quality Control Division Data by R.G. Adler. Salt Lake City, UT. 1981 (unpublished).
8.13. Occupational Safety and Health Administration Salt Lake Technical Center: OSHA Laboratory Quality Control Division Data by B. Babcock. Salt Lake City, UT. 1988 (unpublished).
8.14. Occupational Safety and Health Administration Salt Lake Technical Center: OSHA Laboratory Quality Control Division Data by B. Babcock. Salt Lake City, UT. 1996 (unpublished).
8.15. Occupational Safety and Health Administration Salt Lake Technical Center: Rank Statistical Tests on OSHA Laboratory Quality Control Division Quartz and Lead Data by M. Rose. Salt Lake City, UT. 1996 (unpublished).
8.16. Joint Committee on Powder Diffraction Standards (JCPDS): Powder Diffraction File 1988, Swarthmore, PA: International Center for Diffraction Data, 1988.
8.17. Occupational Safety and Health
Administration Analytical Laboratory:
8.18. Ettinger, H.J. and G.W. Royer:
Calibration of a
8.19. Assistant Secretary for Occupational
Safety and Health, U.S. Department of Labor: OSHA Technical
Manual, OSHA Instruction CPL
8.20. National Bureau of Standards: Certificate of Analysis Standard Reference Material 1878, November 3, 1983.
8.21. Elton, N.J., P.D. Salt, and J.M.
Adams: Determination of quartz in kaolins by
8.22. Palassis, J.: Internal Report to
Director DPSE, NIOSH (August 8, 1988); presented as Particle Size
Effects on the Accuracy of Respirable Silica Analyses by
8.23. Bumsted, H.E.: Determination of
a-Quartz in the Respirable Portion of
Airborne Particulates by
8.24. U.S. Department of the Interior (Mining Enforcement and Safety Administration): The Determination of Free Silica in Airborne Dust Collected on Membrane Filters by J.W. Thatcher (Informational Report 1021). Washington, D.C., 1975.
8.25. Anderson, C.C.: Collaborative
Tests of Two Methods for Determining Free Silica in Airborne Dust,
DHHS (NIOSH) Publication No.
8.26. Occupational Safety and Health
Administration Technical Center: Standard Operating
8.27. Assistant Secretary for Occupational Safety and Health, U.S. Department of Labor: Special Emphasis Program (SEP) for SILICOSIS, Washington, DC, May 2, 1996.
| Primary Quartz Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 10-423 |
| Biotite, K(Fe,Mg)3AlSi3O10(OH)3 | 2-45 |
| Clinoferrosilite, FeSiO3 | 17-548 |
| Graphite, C | 23-064, |
| High albite, NaAlSi3O8 | 20-572 |
| Iron carbide, FeC | 3-411 |
| Lead chromate, PbCrO4 | 8-209, |
| Lead sulfate, PbSO4 | 36-1461 |
| Leucite, KAlSi2O6 | 31-967, 38-1423 |
| Microcline, KAlSi3O8 | 19-932, |
| Muscovite, | |
| KMgAlSi4O10(OH)2 | 21-993 |
| KAl2Si3AlO10(OH)2 | 7-25 |
| KAl2(Si3Al)O10(OH,F)2 | 6-263 |
| K(Al,V)2(Si,Al)4O10(OH)2 | 19-814 |
| (K,Na)Al2(Si,Al)4O10(OH)2 | 34-175 |
| (Ba,K)Al2(Si3AlO10)(OH)2 | 10-490 |
| (K,Ca,Na)(Al,Mg,Fe)2(Si,Al)4O10(OH)2 | 25-649 |
| (K,Na)(Al,Mg,Fe)2(Si3.1Al0.9)O10(OH)2 | 7-42 |
| Orthoclase, | |
| KAlSi3O8 | 31-966 |
| (K,Ba)(Si,Al)4O8 | 19-3 |
| (K,Ba,Na)(Si,Al)4O8 | 19-2 |
| Potassium hydroxide, KOH | 15-890 |
| Sanidine, | |
| (K,Na)AlSi3O8 | 19-1227 |
| KAlSi3O8 | 25-618 |
| Sillimanite, Al2SiO5 | 38-471 |
| Wollastonite, | |
| CaSiO3 | 27-1064, |
| (Ca,Fe)SiO3 | 27-1056 |
| Zircon, ZrSiO4 | 6-266 |
| Secondary Quartz Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 10-423 |
| High albite, NaAlSi3O8 | 20-572 |
| Microcline, KAlSi3O8 | 19-932, |
| Tertiary Quartz Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 10-423 |
| Copper, Cu | 4-836 |
| Primary Cristobalite Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 11-500 |
| High albite, NaAlSi3O8 | 10-393, 20-572 |
| Secondary Cristobalite Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 10-423, 11-500 |
| High albite, NaAlSi3O8 | 10-393, 20-572 |
| Tertiary Cristobalite Line | |
| Interferent Name, Formula |
PDF No. |
| Aluminum Phosphate, AlPO4 | 11-500 |
PDF No. = JCPDS Powder Diffraction File Number
| Note: | The majority of the interferences listed above will
most likely not be present when sampling industrial operations which
produce quartz or cristobalite exposures. This list is presented as
|
One of the custom
- Uses a symmetric
five-point digital filter (a running average with weights = 0.6, 0.8, 1.0, 0.8, 0.6) to smooth the spectral count data. - Identifies peaks by maximum counts.
- Determines upper and lower 2q integration limits.
- Chooses the integration method (either valley to valley or perpendicular drop) by observation of background and signal counts.
- Integrates the peak by summing counts over the selected integration range.
- Calculates the amount of analyte in total µg and %.
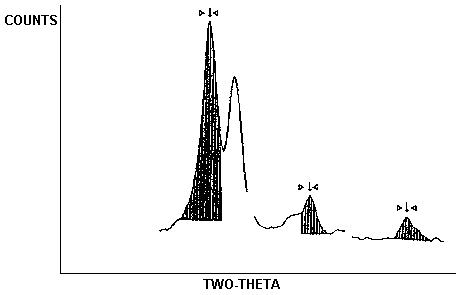
- Generates a hard copy report. Examples of reports used at
OSHA-SLTC are shown in Figures 1 and 2.
A custom
The analyst can choose different limit settings or change the
integration method and produce a new interpretation of the data as shown
in Figure 2.
This scan of a sample has been
The abbreviation "NORM CNTS" contained within the Figures stands for normalized counts (total counts/counting time).
Other
STANDARD
| AIR VOL. | 1.00 | UG |
| SAMPLE WT. | 100. |
AG CAL. 16505 COUNTS AT 44.45 DEG.
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
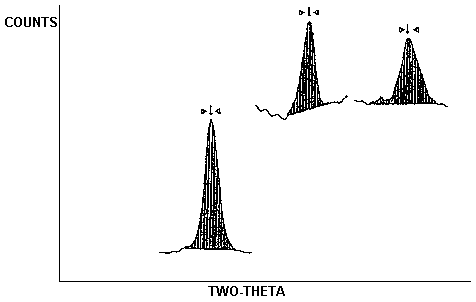
SAMPLE
| AIR VOL. | 1.00 | L |
| SAMPLE WT. | 100. | UG |
AG CAL. 12120 COUNTS AT 44.51 DEG.
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||