|
| Method no.: |
ID-105 |
| |
|
| Matrix: |
Air, Wipes (Smear Tabs), or
Bulks |
| |
|
OSHA Permissible Exposure
Limit: |
0.01 mg/m3 |
| |
|
| Collection Procedure: |
Inorganic arsenic1
particulate in the air is collected by drawing a known volume of the
air through a 0.8-µm mixed-cellulose ester
(MCE) filter and backup pad using a calibrated personal sampling
pump. A chemically-treated backup pad is used if
volatile inorganic arsenic species are suspected. If arsine is also
suspected, a sampling train is used (see Section 5). Wipe and bulk
materials are collected using grab sampling techniques. |
| |
|
Recommended Air Volume
Ranges
MCE Filter:
Sampling
Train (Section 5): |
I480 L to 960 L
120 to 240
L |
| |
|
Recommended Sampling Rates
MCE Filter:
Sampling Train (Section 5): |
2.0 L/min
0.5 L/min |
| |
|
| Analytical Procedure: |
Air filters, backup pads, wipes (smear
tabs), and bulks are digested with nitric acid and stabilized by
addition of nickel. After digestion, a small amount of hydrochloric
acid is added. Arsine collected on charcoal is extracted using a
dilute nitric acid/nickel solution. All samples are then diluted to
volume and analyzed by atomic absorption spectroscopy using a heated
graphite atomizer. |
| |
|
Detection Limits
Qualitative:
Quantitative: |
0.003 µg/mL arsenic
0.01 µg/mL
arsenic |
| |
|
Precision and Accuracy
Validation Level:
CVT
Bias
Overall Error |
0.006 to 0.04 mg/m3
0.10
+0.004
±20% |
| |
|
| Method Classification: |
Validated Analytical Method |
| |
|
| Chemist: |
Steven Edwards |
| |
|
| Date (Date Revised): |
1982 (May, 1991) |
| |
|
| 1 |
Inorganic arsenic means copper acetoarsenite
and all inorganic compounds containing arsenic (except arsine)
and measured as arsenic (8.1.). | |
| |
|
Commercial manufacturers and products mentioned in this method are
for descriptive use only and do not constitute endorsements by
USDOL-OSHA.
Similar products from other sources can be
substituted.
|
| |
|
Division of Physical Measurements and Inorganic
Analyses
OSHA Technical Center
Sandy, Utah
|
1.
Introduction
1.1. Scope
This method
describes the collection and analysis of inorganic arsenic for
airborne, wipe, and bulk material samples. Air samples can be
taken for particulate and volatile inorganic arsenic. Sample
preparation at the laboratory involves mineral acid digestion and
nickel stabilization. The analysis is performed with an atomic
absorption spectrometer (AAS) utilizing a heated graphite atomizer
(AAS-HGA). Additional analytes (Cd, Cu, Fe, Pb, and
Zn) can also be analyzed from the same sample media using flame
AAS techniques.
In addition, samples previously prepared
for ICP analysis by OSHA method no. ID-125G (8.2.)
can also be determined for arsenic using the analytical technique
described herein.
1.2. History
Previously, arsenic
was analyzed at the OSHA Analytical Laboratory using an arsine
generation procedure (8.3.). The method required special gas
generation equipment and was time-consuming. An early
AAS-HGA method without the addition of nickel as a
stabilizer was considered; however, this approach had decreased
sensitivity, poor reproducibility, and was subject to potential
interferences and loss of arsenic during analysis. The addition of
nickel to samples minimizes these problems by the apparent
formation of a stable nickel arsenide complex (8.4.). This complex
allows the use of a higher charring temperature during
AAS-HGA analysis and minimizes interferences caused
by incomplete volatilization of any organic substances contained
in the sample matrix (8.5.).
Compared to arsine
generation, the AAS-HGA procedure offers the
following advantages:
- a simple digestion procedure,
- increased ability to analyze other analytes from the same
sample,
- a decrease in sample loss and an increase in sample
throughput.
1.3. Analytical Principle
This method uses a HGA with a Zeeman/L'vov configuration
to analyze arsenic and reduce background contributions. Other
background compensation techniques can be used.
1.3.1. The Zeeman electromagnet technique assists in
minimizing background without the use of continuum sources such
as the deuterium arc. A magnetic field is provided during the
analytical atomization step and results in a "splitting" of the
atom's energy levels. The capability of measuring the atomic
absorption with and without the magnetic field applied during
the atomization step provides a "clean" signal. This "clean"
signal is the net difference between the signal produced with
the magnetic field turned off and then on.
1.3.2. The
L'vov platform is a pyrolytically-coated graphite support
inserted into a graphite tube which is also
pyrolytically-coated. This assembly offers a more
uniform temperature distribution inside the graphite tube,
increased sensitivity, and less opportunity for matrix effects
from molecular formation and absorption during atomization.
1.4. Uses
Arsenic has metallurgical
applications in industry where it is used for hardening lead and
enhancing the toughness and corrosion resistance of copper.
Arsenic compounds are used in medicine, glass manufacture, pigment
production, rodent poisons, insecticides, fungicides, weed
killers, semiconductor manufacture, and tanning processes.
1.5. Physical and Chemical Properties (8.6.)
Metallic arsenic is a steel gray, brittle metal, with a
density of 5.7. It also exists as yellow crystal, As4,
having a density of 2.0.
Some physical properties of
arsenic (CAS #7440-38-2) are:
| Atomic weight |
74.9216 |
| Specific Gravity |
5.727 |
| Melting Point |
sublimes without melting at 613 °C |
| Solubility |
insoluble in H2O; soluble in
HNO3 | 2. Range and Detection Limit
(8.7.)
2.1. For this method, the working range is
0.01 to 0.5 µg/mL arsenic. For a 480-L air
volume and 25-mL solution volume, this range permits
quantitation without sample dilution from approximately 0.0005 to
0.03 mg/m3 arsenic.
2.2. Calculated
quantitative detection limits (DL) are:
Sample Type
|
|
Air Vol
|
|
Flow rate
|
|
Solution Vol
|
|
DL
|
| Air |
480 L |
2 L/min |
25 mL |
0.0005 mg/m3 |
| Air |
120 L |
0.5 L/min |
10 mL |
0.0008 mg/m3 |
| Wipe or Bulk |
----- |
------- |
25 mL |
0.25 µg
|
2.3. The range and detection limits of the other
metal analytes (Cd, Cu, Fe, Pb, and Zn) should be unaffected by
this sample preparation. Detection limits and analytical
parameters for these and other elements can be found in references
8.2. or 8.8. 3. Precision and Accuracy
3.1. Previous and recent quality control samples
(8.9.) containing arsenic in the approximate range of 0.5 to 4
times the OSHA PEL (assuming 960-L air volumes), gave
the following data:
|
Sample Set #1
|
|
Sample Set #2
|
| Bias |
-0.024 |
+0.004 |
| CV |
0.097 |
0.10 |
| Overall analytical error |
±21.8% |
±20.0% |
| Analysis period |
2/1982-4/1982 |
1/1989-12/1990 |
| N |
78
|
100
|
| Analytical technique |
HGA/D2
|
HGA/LD2
(5%) HGA/ZL (95%) |
Where:
|
HGA/D2 |
= |
Heated Graphite Atomizer with deuterium
arc background correction. |
| HGA/LD2 |
= |
Heated Graphite Atomizer/L'vov Platform
with deuterium arc with background correction. |
| HGA/ZL |
= |
Heated Graphite Atomizer with Zeeman/L'vov
Platform |
Approximately 95% of the samples from Set #2 were
analyzed using the HGA Zeeman/L'vov platform approach mentioned in
this method. The remaining samples were analyzed with a HGA/L'vov
platform and deuterium arc background correction only. A
significant difference in results was not noted.
3.2.
Recovery data for arsenic analyzed in an "ICP digest" is presented
in reference 8.7. No significant loss of arsenic was noted when
using the "ICP digest" and a HGA equipped with a Zeeman/L'vov
system.
3.3. For precision and accuracy data for other
metals (Cd, Cu, Fe, Pb, and Zn) analyzed with arsenic, also see
reference 8.7. Recoveries for these metals analyzed by flame
atomic absorption were adequate. 4. Interferences
Sampling
Non-volatile organic arsenic-containing
compounds will provide a positive interference when sampling for
particulate arsenic. The industrial hygienist should make note of
any organo-arsenic use in the area sampled.
Analysis
The analysis of arsenic in an "ICP-type digestion"
matrix (8% HCl/ 4% H2SO4 as mentioned in
reference 8.2.) may require the use of significant background
correction due to the contribution from sulfuric acid. In these
cases, it is recommended to minimize background by using a
Zeeman-type graphite furnace assembly with a L'vov
platform inserted in pyrolytically-coated graphite
tubes; other techniques can be used to diminish background effects
provided they are evaluated using spiked samples and analytical
recovery is adequate.
Perchloric acid (HClO4)
should not be used for sample digestions and subsequent analysis
using this analytical technique. Inhibition of the arsenic signal
after digestion of polyvinyl chloride filters and rapid graphite
tube deterioration from HClO4 have been noted (8.10.).
5. Sampling
When other
compounds or elements are known or suspected to be present in the
sampled air, such information should be transmitted with the sample.
Sampling for arsenic in air is dependent on the operation. If the
operation being sampled has the potential for producing inorganic
arsenic vapor and arsine, a sampling train (Sampling Media II) is
used to capture the vapor and particulate. Some examples of
operations potentially producing arsenic vapor are welding and
torching (8.11.). Arsine can be formed from arsenic when sufficient
hydrogen is present with arsenic [i.e. lead-acid
battery manufacturing plants (8.11.)]. Sampling can be accomplished
using one of two different approaches:
Suspected Form
|
Sampling Media
|
|
Flow Rate (L/min)
|
| Particulate ( + Vapor) |
I |
2 |
| Particulate + Vapor
+ Arsine |
II |
0.5 |
If
possible, all samples should be taken for at least 240 min.
5.1. Equipment
5.1.1. Sampling Media I for
particulate arsenic:
Mixed cellulose ester (MCE)
filters (0.8 µm pore size), cellulose backup pads, and two- or
three-piece cassettes, 37-mm diameter,
(part no. MAWP 037 A0, Millipore Corp., Bedford, MA). If
volatile inorganic arsenic is suspected, the following is used:
For sampling particulate and volatile
inorganic arsenic compounds (i.e. heated arsenic
sources): The cellulose backup pad is chemically treated
and the pads and MCE filters are contained in
three-piece cassettes. This chemical treatment
ensures capture of volatile inorganic arsenic in the backup pad
(8.11.). The backup pads are treated using an impregnation
solution:
Pipettes, 0.5 mL
Sodium carbonate
(Na2CO3)
Glycerol
(C3H8O3)
Impregnation
solution [Na2CO3 solution with glycerol]
- prepare by dissolving 4.0 g Na2CO3 in
50 mL deionized water, add 2 mL glycerol, and dilute this
solution to 100 mL with deionized water. Remove
filters from the three-piece cassettes and use the opened
cassettes as supports for backup pad impregnation. Each backup
pad should be resting on the ridge of the middle insert of the
cassette and not in contact with the cassette base. Slowly
pipette 0.5 mL of the impregnation solution over the entire
backup pad, let dry overnight, and then place the MCE filters on
top of the backup pads and assemble the cassettes.
5.1.2. Sampling Media II (for sampling
when arsine is also suspected to be present): Sampling
media I with the chemically treated backup pad is used in series
with an arsine sampling tube. This tube is composed of glass and
contains 400 mg (front) and 200 mg (backup) sections of
activated coconut shell charcoal. This sampling train is
necessary if volatile inorganic arsenic species and arsine are
suspected to be present in the air.
5.1.3. Sampling
pumps capable of sampling at 2 L/min (Sampling Media I) or 0.5
L/min (Sampling Media II).
5.1.4. Assorted flexible
tubing.
5.1.5. Stopwatch and bubble tube or meter for
pump calibration.
5.1.6. Gel bands (Omega Specialty
Instrument Co., Chelmsford, MA) for sealing cassettes.
5.1.7. Scintillation vials, 20 mL, (part no. 74515 or
58515, Kimble, Div. of Owens-Illinois Inc., Toledo,
OH) with polypropylene or Teflon cap liners. If possible, submit
bulk or wipe samples in these vials.
5.1.8. Smear tabs,
(Whatman 50, part no. 225-24, SKC Inc., Eighty Four, PA) for
wipe sampling. 5.2. Sampling Procedure - Air Samples
5.2.1. Place an MCE filter and a cellulose backup
pad in each two- or three-piece cassette. The
backup pad should be chemically-treated if volatile
inorganic arsenic compounds are suspected. Seal each cassette
with a gel band.
5.2.2. Attach calibration sampling
media to the pump using flexible tubing. Depending on the
sampling media in use, follow the sampling scheme shown:
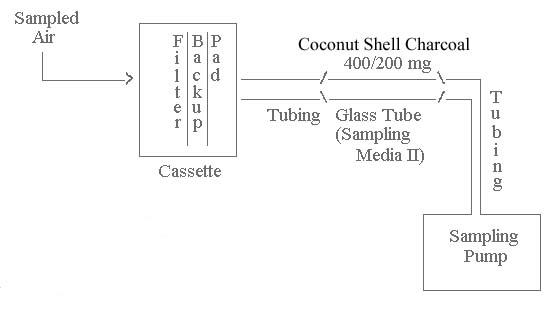 |
| Text Version:
Sampling Media I of the sampling train is a 37-mm mixed
cellulose ester (MCE) filter (0.8 μm pore size) and a
backup pad contained in a two- or a three-piece 37-mm
diameter plastic filter cassette. The backup pad is
chemically treated if volatile inorganic arsenic
substances are suspected to be present. This
treatment for the backup pad is described in Section
5.1.1. The MCE filter and chemically-treated backup
pad are available from SKC, Inc. as catalog no. 225-9001.
The filter cassette is connected to sampling Media II, a
coconut shell charcoal sampling tube (400/200 mg
sections), with a short length of plastic tubing.
The charcoal sampling tube is used if arsene is suspected
to be present. The sampling train is connected to
the sampling pump with flexible plastic
tubing. |
If arsine is suspected, use a
minimum amount of tubing to connect the
cassette to the arsine sampling tube.
5.2.3. Calibrate
each personal sampling pump with prepared sampling media
in-line to within ±10% of the recommended flow rate
of 2 L/min (Sampling Media I) or 0.5 L/min (Sampling Media II).
Remove the calibration media and attach new sampling media to
the calibrated pump.
5.2.4. Place the sampling
media/pump assembly in appropriate positions on the employee or
the workplace area.
5.2.5. If possible, collect
full-shift samples. The minimum recommended air volume is 480 L
(120-L for Sampling Media II). Take samples to
cover the workshift.
5.2.6. If the filter becomes
overloaded while sampling, prepare and use another filter
cassette. Take consecutive samples using shorter sampling
periods if overloading occurs.
5.2.7. Place plastic end
caps on each sampling media after sampling.
5.3. Sampling Procedure -
Wipe Samples
5.3.1. Wear clean, impervious, disposable gloves
when taking each wipe sample.
5.3.2. Moisten the wipe
filters with deionized water prior to use.
5.3.3. If
possible, wipe a surface area covering 100 cm2.
5.3.4. Fold the wipe sample with the exposed side in.
5.3.5. Transfer the wipe sample into a 20-mL
scintillation vial and seal with vinyl or electrical tape.
5.4. Sampling Procedure - Bulk Samples
In
order of laboratory preference, bulk samples may be one of the
following:
- a high-volume filter sample,
- a representative settled dust (rafter) sample,
- a sample of the bulk material in the workplace.
Transfer the bulk material into a
20-mL scintillation vial and seal with vinyl or electrical tape.
5.5. Shipment
5.5.1. Submit at least one blank sample with each
set of air, charcoal, or wipe samples. Blank samples should be
handled in the same manner as other samples, except that no
monitoring is performed with these samples.
5.5.2.
Attach an OSHA-21 seal around each cassette, scintillation vial,
and glass tube (if used) in such a way as to secure the end
caps. Document the industrial operation(s) the samples were
taken from. Send the samples along with any blank samples to the
laboratory with the OSHA-91A paperwork requesting
arsenic analysis. Also note whether volatile
arsenic or arsine was suspected and which Sampling Media was
used.
5.5.3. If desired, specify other elements
of interest. At the OSHA Technical Center the following elements
are analyzable on the same filter, wipe, or bulk with arsenic:
Technique
|
|
Element
|
|
| Atomic Absorption |
Cd, Cu,
Fe, Pb, Zn |
|
| ICP |
Be,
Cd, Cr, Co, Cu, Fe, Mn,
Mo, Ni, Pb, Sb, V,
Zn |
Choose any combination of three elements
listed for Atomic Absorption or choose arsenic/ICP analysis if
more than three elements are desired.
5.5.4. The type of
bulk sample should be stated on the OSHA-91A and
cross-referenced to the appropriate air sample(s).
5.5.5. Ship bulk samples separately from air samples.
They should be accompanied by Material Safety Data Sheets, if
available. Check current shipping restrictions and ship to the
laboratory by the appropriate method.
6. Analysis
6.1. Safety Precautions
6.1.1. Arsenic is considered a human carcinogen
(8.1., 8.6.). Use extreme care when handling arsenic or
arsenic-containing compounds.
6.1.2. All
work with concentrated acids is potentially hazardous. Care
should be exercised when handling any acidic solutions. Acid
solution contact with work surfaces should be avoided. If any
acid contacts the eyes, skin, or clothes, flush the area
immediately with copious amounts of water. Medical treatment may
be necessary.
6.1.3. Always wear safety glasses and
protective clothing when using chemicals. Prepare all mixtures,
samples, or dilutions in an exhaust hood. To avoid exposure to
acid vapors, do not remove any beakers
from the hoods until they have returned to room temperature and
have been diluted.
6.1.4. Use a pipette bulb, never
pipette by mouth.
6.1.5. When scoring glass sampling
tubes to remove the sorbent before analysis, score with care.
Apply only enough pressure to scratch a clean mark on the glass.
Use a paper towel or cloth to support the opposite side while
scoring. Moisten the mark with DI H2O and wrap the
tube in cloth before breaking. If the tube does not break
easily, re-score. Dispose of glass in a waste
receptacle specifically designed and designated for
broken-glass.
6.1.6. Consult the Standard
Operating Procedure (SOP) (8.12.) and any instrument manuals
before using any instrument.
6.1.7. Since metallic
elements and other toxic substances are vaporized during HGA
operation, it is imperative that an exhaust hood is installed
and used directly above the graphite furnace. Always ensure the
exhaust system is operating before proceeding with the analysis.
6.1.8. Do not look directly at the furnace during the
atomization step or at the emission of an electrodeless
discharge lamp.
6.2. Equipment
6.2.1. Atomic absorption
spectrophotometer consisting of a(an):
- Heated graphite furnace atomizer with argon purge system
and graphite tubes
| Note: |
If samples are analyzed in matrices
other than recommended in this method (4% HNO3,
200 µg/mL Ni), or matrix-matching samples and standards is
difficult, it is recommended to use an HGA capable of
significant resolution of background, such as a
Zeeman/L'vov Platform-type HGA
(Perkin-Elmer, Norwalk, CT) with
pyrolytically-coated graphite
tubes. |
- Pressure-regulating devices capable of maintaining
constant argon purge pressure.
- Optical system capable of isolating the desired wavelength
of radiation.
- Adjustable slit.
- Light measuring and amplifying device.
- Display, strip chart, or computer interface for indicating
the amount of absorbed radiation.
- Deuterium Arc Background Corrector (if Zeeman background
correction is unavailable).
- Electrodeless Discharge Lamp (EDL) for arsenic and an EDL
power supply (Note: A modulated system is necessary when using
a Zeeman HGA.).
- Automatic sampler.
6.2.2.
Glassware
- Phillips beakers, 125- and 250-mL
- Volumetric flasks, Class A: 10-, 25-, 50- and 100-mL
- Pipettes, Class A: Assorted sizes
- Scintillation vials, 20-mL (for desorbing charcoal)
6.2.3. Forceps.
6.2.4.
Exhaust hood and hotplate, or microwave digestion system (model
no. MDS-81, CEM Corp., Matthews, NC).
6.2.5. Filtering apparatus consisting of MCE filters,
0.45-µm pore size, 47-mm diameter (cat. no. HAWP
047 00, Millipore Corp., Bedford, MA) and filtering apparatus
(cat. no. XX15 047 00, Millipore).
6.2.6. Automatic
pipets, adjustable, 0.1 to 5.0 mL range (models P-1000 and
P-5000, Rainin Instruments Co., Woburn, MA).
6.2.7. Glass tube scorer, or needle, 21 to 25 gauge -
for glass wool, foam, and sorbent from glass tubes. A piece of
bent wire can also be used.
6.2.8. Exhaust vent.
6.2.9. Ultrasonic bath (for arsine samples).
6.2.10. Analytical balance (0.01 mg).
6.2.11.
Arsine sampling media (for standard preparation if arsine has
been collected): Obtain six sampling tubes each
containing 400 mg (front) and 200 mg (backup) sections of
activated coconut shell charcoal. 6.3. Reagents (All chemicals should be reagent grade
or better.)
6.3.1. Deionized water (DI H2O) with a
specific conductance of less than 10 µS.
6.3.2. Mineral
acids (used for digestions and dilution solution preparation)
CAUTION: Refer to Sections 6.1.2.-6.1.3. before
using acids.
- Hydrochloric acid (HCl), concentrated (36.5 to 38%).
- Nitric acid (HNO3), concentrated (69 to 71%).
6.3.3. Mineral acids (used for
cleaning glassware)
CAUTION: Refer to Sections
6.1.2.-6.1.3. before using acids.
- Nitric acid, 1:1 HNO3/DI H2O
mixture: Carefully add a measured volume of concentrated
HNO3 to an equal volume of DI H2O.
- Nitric acid 10% v/v: Carefully add 100 mL of concentrated
HNO3 to 500 mL of DI H2O and then dilute
to 1 L.
6.3.4. Nickelous nitrate
[Ni(NO3)2·
6H2O].
6.3.5. Stabilizer, 1,000 µg/mL Ni
solution - Dissolve 5.0 g nickelous nitrate in 100 mL of DI
H2O, add 5 mL concentrated HNO3, and
dilute to 1-L with DI H2O.
6.3.6. Mixed cellulose ester (MCE) filters, 0.8-µm pore
size, 37-mm diameter.
| (Note: |
These filters are used for
matrix-matching standards with samples. If possible, use
the same brand and lot of filters for air sampling and
matrix-matching.) |
6.3.7. Diluting solution: Place 20 blank
MCE filters in a cleaned 250-mL Phillips beaker and
carefully add 100 mL of concentrated HNO3 and
100 mL of the 1,000 µg/mL nickel solution. Digest this mixture
on a hot plate until about 20 to 40 mL of solution remain.
Transfer the solution to a cleaned 500-mL
volumetric flask, add 2 mL of concentrated HCl, and dilute to
volume with DI H2O.
6.3.8. Standard solution,
1,000 µg/mL arsenic: If possible, use commercially available
aqueous standards. Observe expiration dates; if none, properly
dispose the standard after 1 year.
6.3.9. If a
commercial standard (Section 6.3.8.) is not available, a 1,000
µg/mL solution can be prepared as follows:
- Sodium hydroxide (NaOH).
- Arsenic trioxide (As2O3).
- Sodium hydroxide, 10% solution: Dissolve 10 g of NaOH in
about 75 mL of DI H2O. Dilute to 100 mL.
In a cleaned 1-L volumetric
flask, dissolve 1.320 g As2O3 in 25 mL 10%
NaOH. Dilute to volume with DI H2O, and mix. Dispose
of properly after 1 year.
6.3.10. Argon, compressed gas
(for HGA tube purges). 6.4. Glassware Preparation
6.4.1. Place the Phillips beakers in an exhaust hood
and add approximately 10 mL of a 1:1 HNO3/DI
H2O mixture in each 125- or 250-mL
Phillips beaker. Using a hot plate, apply moderate heat to the
beakers until refluxing occurs. Carefully decant the acid
mixture into a waste container and allow the beakers to cool
before removing from the hood. Rinse the beakers thoroughly with
DI H2O.
6.4.2. Rinse all volumetric flasks
with 10% v/v HNO3 and then rinse thoroughly with DI
H2O.
6.4.3. Allow all glassware to air dry
before proceeding. 6.5. Standards
6.5.1. Dilute stock solutions:
Prepare
dilute arsenic stock solutions (0.1-, 1-, and 10-µg/mL) by
diluting aliquots of the 1,000-µg/mL standard
solution with DI H2O. Prepare the diluted stock
solutions on the same day the working standards are prepared.
6.5.2. Working standards:
A dilution scheme
using 0.1-, 1-, and 10-µg/mL stock solutions is
proposed below.
Working
Standard
(µg/mL) |
Stock
Solution
(µg/mL) |
Stock solution
Aliquot
(mL) |
Final Volume*
(mL) |
|
0.01
0.02
0.05
0.1
0.2
0.5 |
0.1
0.1
1
1
10
10 |
10
20
5
10
2
5 |
100
100
100
100
100
100 |
|
| * Diluent is the
diluting solution (Section 6.3.7.) |
Dilute all working standards to volume
using the diluting solution. This will assure the matrix (acid,
sample filter, and nickel content) of the samples (air and wipe)
and standards are closely matched. Dispose working standards
after 6 months.
6.5.3. Standards for arsine
determinations
Remove the 400-mg section of charcoal
sorbent from six arsine sampling tubes. Place each
400-mg section in a separate vial. Pipet a
3-mL aliquot from each working standard (prepared
in Section 6.5.2.) into each vial such that six standards
ranging in concentration from 0.01 to 0.5 µg/mL arsenic are
prepared with a charcoal matrix.
6.6. Sample Preparation
| Note: |
Always prepare blank samples with every
sample set. Prepare an additional blank media sample any
time an extra procedure is used (i.e. wiping out the
particulate contained inside a cassette with an MCE filter
or preparing a contaminated backup pad). If possible, this
blank media should be from the same manufactured lot as
the prepared filter, tube, or backup
pad. |
- Preparation of air and wipe sample
filters
- Open the filter cassette or scintillation vial,
carefully remove the sample filter with forceps, and place
in a labeled Phillips beaker. Use 125-mL
beakers for air samples and smear tabs. If the cassette or
vial contains loose dust, carefully rinse the dust into the
beaker with DI H2O. If necessary, wipe out the
dust with a clean MCE filter and place this filter in the
sample beaker.
- Prepare and analyze backup pads if:
- volatile inorganic arsenic species are suspected, or
- the backup pad appears to be discolored. Discoloration
may be due to leakage of air around the filter during
sampling.
If analysis of the backup pad is
necessary, place each backup pad in a separate beaker.
- Preparation of bulk samples
- Review any available material safety data sheets to
determine safe bulk handling. The data may also offer a clue
regarding the aliquot amount needed for adequate detection.
- Measure by volume or weight an appropriate aliquot of
any liquid bulk sample. Weigh the appropriate amount of any
solid bulk sample.
| Note: |
Aliquot amounts of bulks are dependent
on the analytical sensitivity, detection limit, and
solubility of the material used. If uncertain, a 20- to
50-mg aliquot of a solid material can be
taken as a starting point. Make sure the aliquot taken is
representative of the entire bulk sample. If necessary,
use a mortar and pestle to grind any nonhomogenous
particulate bulk samples in an exhaust
hood. |
After measuring, transfer the aliquot to a 250-mL Phillips
beaker.
- Preparation of arsine (charcoal)
samples
- Score the tube with a glass tube cutter (also see
Section 6.1.5.) and then break open the front section of the
tube above the glass wool. An alternative approach to
scoring and breaking is to carefully remove the glass wool
with a bent wire or needle.
- Carefully transfer each section of the sorbent to
separate 20-mL scintillation vials without losing any
particles.
6.7. Sample
Digestion or Extraction
- MCE air filters and smear tabs
Place the beakers in an exhaust hood and carefully add
3 to 5 mL concentrated HNO3 and the appropriate
amount of Stabilizer (Section 6.3.5.) as shown below. Place
the beakers on a hot plate and heat the samples until the
appropriate amount of solution remains as shown below.
Air Vol (L)
|
Stabilizer (mL)
|
Digestion Vol
(mL)
|
| <200 |
2.0 |
0.5 |
| ³ 200 |
5.0 |
1.0 |
| Smear tabs |
5.0 |
1.0 |
| Note: |
If the sample solution is not clear, add
a second portion of approximately 1 to 2 mL of
concentrated HNO3. Apply heat until the
appropriate digestion volume listed above remains.
|
Remove the beakers from the hotplate. Allow
beakers to cool, then add 100 µL (~2 drops) of HCl to each and
swirl the contents.
- Polyvinyl chloride filters, or backup
pads
| Note: |
Polyvinyl chloride (PVC) filters are not
routinely used for arsenic sample collection and analysis.
In some cases the industrial hygienist will sample for
total or respirable dust using PVC filters and also submit
these samples for analysis. The PVC filter will not be
completely digested using the acid digestion listed in
this method; rather, the particulate is
acid-extracted from the filter.
Perchloric acid should not be used to digest
arsenic samples collected on PVC filters or backup pads;
low recoveries for arsenic were noted when PVC filters
were digested using an
H2SO4/HCl/HClO4 acid
matrix (8.10.). In addition, graphite tube degradation is
greatly accelerated from perchloric
acid. |
Place the beakers in an exhaust hood and
add the following amount of concentrated HNO3 to
the beakers:
| Backup pads |
|
10 to 15 mL |
| PVC filters |
|
3 to 5
mL |
Follow the digestion procedure mentioned above (Section
6.7., MCE air filters and smear tabs)
and determine the amount of Stabilizer needed, and digestion
volumes. After heating on a hot plate and subsequent cooling,
each PVC filter should be thoroughly rinsed with DI
H2O during quantitative transfer of the sample
solution.
- Bulk samples
If necessary,
use a microwave digestion system to facilitate digestion [For
further information regarding microwave digestion, see the
Microwave Standard Operating Procedure (8.13.)].
Add
10 to 30 mL HNO3, 5 mL of Stabilizer, and place the
beaker on a hot plate. Digest the bulk sample until the
material dissolves and approximately 1 mL of solution remains.
Remove the beakers from the hot plate. Allow beakers to cool,
then add 40 µL (~2 drops) of HCl to each and swirl the
contents.
- Arsine (charcoal) samples
To each scintillation vial add 3 mL of Stabilizer
solution (Section 6.3.5.). Cap and sonicate each vial contents
for 10 min. 6.7.1. Filtration - any solutions samples containing
particulate
Digested samples: If particulate
matter is present after digesting, allow the sample to cool, add
approximately 10 mL DI H2O, then filter the
solution through a 0.45-µm MCE filter. Save the
filtrate for analysis. Repeat the digestion procedure above for
the filter containing the particulate.
Arsine samples:
If particulate is present after extraction (i.e. charcoal
fines), filter the 3-mL solution through a
0.45-µm MCE filter, and analyze the filtrate.
6.7.2. Dilution - all samples
Digested samples: Allow all beakers to cool to
room temperature in an exhaust hood before proceeding. Carefully
add about 5 mL of DI H2O to each beaker, rinsing down
the insides of each beaker. Quantitatively transfer each sample
solution to individual volumetric flasks. Dilute to volume with
DI H2O and mix well. Recommended final sample
solution volumes are:
| Air (³200-L), wipe, and bulk samples |
|
25 mL |
| Air volumes <200-l |
|
10 mL |
Larger dilution volumes can be used for bulk
samples; however, the final solution volume should contain 4%
HNO3 and 200 µg/mL Ni.
Arsine samples:
For charcoal samples, further dilution is not necessary.
6.7.3. Samples previously prepared for
ICP analysis
For samples already prepared and
analyzed using OSHA method no. ID-125G, no
additional sample preparation is necessary.
6.8. Instrument Setup and
Analysis
6.8.1. Set up the spectrometer and HGA according to
the SOP (8.12.) or the manufacturer's instructions. Suggested
parameters for two specific instruments are shown in Appendix A.
- Install an EDL for arsenic and allow to stabilize.
- Optimize conditions such as lamp position, furnace
alignment, etc. as mentioned in the SOP (8.12.).
- Be sure cooling water is circulating around the furnace
before heating it and if deuterium (D2) arc
background correction is used, assure the purge air is
circulating around the D2 components before
lighting the D2 lamp.
- Only for those samples previously
prepared using OSHA Method ID-125G:
Set
up the instrument such that a nickel spike is added to each
sample or standard immediately prior to HGA initiation. A
10-µL aliquot of the sample can be injected, then
overlay 5 µL of Stabilizer (Section 6.3.5.) on the sample
before starting the HGA cycle. Standards prepared in Section
6.5. can be used during analysis of these samples.
6.8.2. Inject an aliquot of a
standard into the HGA and measure the absorbance of the standard
using peak height or area. The standard concentration should be
within the linear range. If possible, compare this absorbance to
a value from a previous analysis. Measure other prepared working
standards first to assure proper instrument operation.
6.8.3. Analyze samples and blanks. Analyze a standard
after every four or five samples. Standards should bracket the
sample concentrations. Standard readings should be within 10 to
15% of the readings obtained at the beginning of the analysis.
6.8.4. If any samples exceed the linear range, dilute
with diluting solution (Section 6.3.7.) to bring them into the
working range.
6.8.5. Cadmium, copper, iron, lead, and
zinc can be analyzed in conjunction with the arsenic analysis
using an atomic absorption spectrophotometer (air/acetylene
flame) and direct aspiration. Analytical conditions for flame
analysis of these elements are shown in Appendix B. Additional
information can be found in OSHA Method No. ID-121
or instrument manufacturers' manuals. 6.9. Analytical Recommendations
6.9.1. The amount of nickel added to each sample can
vary slightly from the standards without producing a significant
matrix effect. An excess of nickel always needs to be present.
(Note: A common range is to have from 100 to 2,000 µg/mL Ni
present in the samples and standards.)
6.9.2. When
standards are prepared, analyze the old and new standards and
compare results to verify the new standard is correct. If two or
more 1,000 µg/mL arsenic solutions are available for standard
preparations, rotate the preparation from one stock solution to
the next to verify the quality.
6.9.3. Keep a permanent
record of all standard preparation and comparison data. Assign
and follow expiration dates for all standards.
6.9.4.
Always analyze blank samples along with the other samples. Treat
blanks in the same fashion as samples, including any filtration
steps.
6.9.5. If possible, analyze quality control
samples from an independent source. The quality control samples
should be freshly prepared if they are derived from liquid
spikes on MCE filters. 7. Calculations
If
sample or standard injection volumes are not constant, the
differences need to be considered before establishing a curve and
calculating results.
7.1. Plot the peak height or area versus the standard
concentrations in µg/mL. Using a least squares method, determine
the equation for the best curve fit.
7.2. Use the equation
to calculate the concentration of arsenic in µg/mL for each
sample.
7.3. Calculate the concentration of each air
sample as:
| C = |
[(A × SA × D) -
(B × SB)]
air volume |
| Where: |
|
C |
= |
arsenic (mg/m3) |
| A |
= |
concn of arsenic in the sample solution
(µg/mL) |
| B |
= |
concn of arsenic in the blank solution
(µg/mL) |
| SA |
= |
sample solution volume (mL) |
| SB |
= |
blank solution volume (mL) |
| D |
= |
sample dilution factor (if any) |
| Air Vol |
= |
air volume sampled
(L) |
7.4. For
wipe or bulk samples, calculate the total amount (in µg) of
analyte in each sample using the equation above without air
volumes. Convert bulk sample analytes to % composition using:
| Arsenic %(w/w)
= |
(C)(100%)
(sample weight)(1000 µg/mg) |
(Bulk
Samples) |
| Where: |
| C |
= |
arsenic amount (µg) |
| Sample wt |
= |
aliquot (in mg) of bulk taken in Section
6.6. |
7.5.
Analytes other than arsenic are calculated in the same fashion as
described above. For the charcoal sampling media results from
Sampling Media II, multiply the arsenic found by 0.326 to obtain
ppm arsine values. For air samples, multiply any results for zinc
or iron by the appropriate gravimetric factor (ZnO/Zn =
1.2447, Fe2O3/Fe =
1.4298).
7.6. With the exception of arsine sample results,
combine results from sampling trains or filtrate/particulate
samples to give a single arsenic result per sample. As examples:
Total As exposure
|
|
Results
|
| Sampling Media I or II |
= |
filter + backup pad* |
| Samples containing undigested
particulate |
= |
filtrate +
redigest |
| * |
If the chemically-treated pad was used or
if the air sample leaked onto the
pad. |
7.7.
Reporting Results to the Industrial Hygienist
7.7.1. Report air sample results as mg/m3
arsenic.
7.7.2. Report wipe sample concentrations as
total micrograms or milligrams arsenic.
7.7.3. Report
bulk sample results as approximate percent by weight arsenic
(Note: Sample results for bulk liquids may be reported as
approximate percent by volume if volumetric aliquots were taken
during sample preparation.) Due to differences in sample
matrices between bulks and standards, bulk results are
approximate.
Analytes other than arsenic are reported in
the same fashion as described above. Arsine results (in ppm) are
reported separately. Air sample results for zinc and iron are
reported to the industrial hygienist as oxides.
8. References
8.1. "Inorganic arsenic," Code of
Federal Regulations 29 CFR 1910.1018. 1989.
142-155.
8.2. Occupational
Safety and Health Administration Technical Center: Metal and Metalloid Particulate in Workplace
Atmospheres (ICP) by J. Septon
(USDOL/OSHA-SLTC Method No. ID-125G).
Salt Lake City, UT. Revised 1991.
8.3. Occupational Safety and Health Administration
Analytical Laboratory: OSHA Manual of
Analytical Methods edited by R.G. Adler (Method No.
I-2). Salt Lake City, UT. 1978.
8.4. Ediger, R.D.: Atomic Absorption Analysis with
the Graphite Furnace using Matrix Modification. Atomic Absorption Newsletter 14(5):
127-130 (1975).
8.5. Edwards, S.E.: "The Determination of Arsenic and
Lead on a Single Personal Air Sample." Paper presented at American
Industrial Hygiene Association National Conference, Houston, TX,
1980.
8.6. Hawley, G.G.: The Condensed Chemical Dictionary. 11th ed.
New York: Van Nostrand Reinhold Co., 1987.
8.7. Occupational Safety and Health Administration Technical
Center: Arsenic Backup Data Report
(ID-105). Salt Lake City, UT. 1991.
8.8. Occupational Safety and Health
Administration Technical Center: Metal
and Metalloid Particulate in Workplace Atmospheres (Atomic
Absorption) (USDOL/OSHA-SLTC Method No.
ID-121). Salt Lake City, UT. Revised 1990.
8.9. Occupational Safety and Health
Administration Technical Center: OSHA
Laboratory Quality Control Division Data by B. Babcock.
Salt Lake City, UT, 1990 (unpublished).
8.10. Occupational Safety and Health Administration
Analytical Laboratory: As on FWSB
filters by ICP digest by C. Merrell. Salt Lake City,
UT. 1989 (unpublished).
8.11. Costello,
R.J., P.M. Eller, and R.D. Hull: Measurement of Multiple
Inorganic Arsenic Species. Am. Ind. Hyg. Assoc.
J. 44(1): 21-28 (1983).
8.12. Occupational Safety and Health Administration Technical
Center: AAS-HGA Standard
Operating Procedure. Salt Lake City, UT. In progress
(unpublished).
8.13. Occupational Safety
and Health Administration Analytical Laboratory: Standard Operating Procedure for Microwave
Digestions. by D. Cook. Salt Lake City, UT. 1989
(unpublished).
Appendix
A
Typical Instrumental Parameters*
|
| Instrument |
Zeeman*
|
PE 5000*
|
|
Wavelenth+
Slit
Signal
Mode
D2 Background Correction
Integration Time
Sample Injection Vol
Automatic sampler |
197.3 nm
0.7 nm
Peak Area
6 s
10 µL
AS60** |
197.3 nm
0.7 nm low
Peak Area
AA-BG/ABS
Yes
6 s
10 µL
AS40** |
| Zeeman* or PE
5000* wiht L'vov Platform |
|
Furnace |
Time |
Internal Argon |
|
Temperature
(°C) |
Ramp
(s) |
Hold
(s) |
Flow
(mL/min) |
|
1) Pre-dry
2)
Dry
3) Char
4) Cool
Down
5) Atomize
6) Burn
out
|
90
140
1,300
30
2,300
2,600 |
5
30
10
1
0
1 |
10
10
20
8
5
6 |
300
300
300
300
0
300 |
|
| * Instruments are: |
| Zeeman |
= |
Model 5100 Zeeman Atomic Absorption
Spectrophotometer equipped with a model 600 HGA controller
(Perkin-Elmer, Norwalk, CT) |
|
| PE 5000 |
= |
Model 5000 Atomic Absorption
Spectrophotometer equipped with a model 500 HGA controller
(Perkin-Elmer) |
|
| ** Model numbers of
automatic samplers (Perkin-Elmer) |
|
| + |
Secondary wavelength is used to
increase the upper linear range. Primary wavelength of 193.7
nm can be used to increase sensitivity; however, a decrease
in the upper range may be
noted. |
Appendix B
Cd, Cu, Fe, Pb, Zn
Analysis |
The following parameters were used for the
validation (8.7.) (atomic absorption-air/acetylene
flame) of Cd, Cu, Fe, Pb, and Zn:
|
| Metal |
Wavelength
(nm) |
Slit Setting
(nm) |
Light
Source |
Comments |
|
Cd
Cu
Fe
Pb
Zn |
228.8
324.7
248.3
283.3
213.9 |
0.7
0.7
0.2
0.7
0.7 |
HCL
HCL
HCL
HCL
HCL |
*
|
HCL = Hollow Cathode Lamp
*
When Fe is determined in the presence of Ni and
HNO3, a reduction in sensitivity is observed. This
effect can be controlled by using a very lean (hot) flame.
All analytes were analyzed using an oxidizing
air/acetylene (lean-blue) flame.
| |

