1. General Discussion
1.1 Background
1.1.1 History of
Procedure.
Recently, the OSHA Analytical Laboratory received a
set of field samples requesting analysis for Acid Blue 9. The air
samples had been collected on glass fiber filters, at 1 Lpm for a
total of about 90 liters air. This report describes the analytical
procedure developed and the preliminary validations of the sampling
method.
Acid Blue 9 is a widely used food dye. There has been
many schemes proposed for the qualitative analysis of food dyes, most
of which depended on paper and thin-layer chromatography. Less
attention has been given to the quantitative analysis of dyes. Some of
the methods attempted were: (a) comparison of spot intensities
on TLC plates with those of a range of standards, (b)
spectrophotometric quantitation, (c) titration with titanous chloride
solution, and (d) electrophoresis on polyacrylamide gel. More
recently, HPLC has been applied for dye analysis, using anion-exchange
columns or, more satisfactorily, by ion-pairing. Paired-ion HPLC
affords a means of separating a mixture of food dyes in a single run
(Ref. 5.2.). Preliminary search did not reveal an air sampling method
for Acid Blue 9. Judging from its physical properties, glass fiber
filters may be a suitable collection medium.
1.1.2 Toxic
Effects.
(This section is for information only and should not
be taken as the basis of OSHA policy.)
Acid Blue 9 is
carcinogenic in rats after its subcutaneous injection: it produced
fibrosarcomas following repeated injections. It also produced an
increased incidence of kidney tumors in mice after its oral
administration (Ref. 5.1.).
1.1.3 Potential Workplace
Exposure.
Acid Blue 9 is an FDA certified food dye and is used
in such products as gelatin desserts, ice cream and sherbets,
carbonated beverages, dry drink powders, candy and confectionary
products when they do not contain oils and fats, bakery products and
cereal, puddings, aqueous drug solutions, tablets, capsules, bath
salts, and hair rinses (Ref. 5.3.). Acid Blue 9 has been produced in
the U.S. for over sixty years. In 1975, three U.S. companies produced
622,000 Kg of the general dye grade, and another four companies
produced 56,000 Kg of he food, drug, and cosmetic grade (Ref. 5.1.).
Preliminary literature searches did not reveal any estimate on the
extent of worker exposure.
1.1.4 Physical
Properties
Color Index Names: Acid Blue 9, Food Blue 2
Color
Index Number: 42090
Cas Reg. Number: 2650-18-2
(3844-45-9 )
Chem. Abstr. Names:
N-Ethyl-N-(4[(4-(ethyl[(3-sulfophenyl)methyl]amino)
phenyl)-(2-sulfophenyl)methylene]-2,
5-cyclohexadien-1-ylidene)3-sulfobenzenemethanaminium hydroxide
inner salt, disodium salt; C.I. Acid Blue 9, disodium salt; D and C
Blue No.1; D and C Blue No.4; ethyl(4-(p[ethyl (m-sulphobenzyl)amino]-α
-(o-sulphophenyl)benzylidene)-2,5-cyclohexadiene-l-ylidene)- (m-sulphobenzyl) ammonium hydroxide inner salt,
disodium salt; FD and C Blue 1; FD and C Blue. No.1; FDC Blue No.1;
Acid Sky Blue A; Acilan Turquoise Blue AE; A. F. Blue No.1; Aizen
Brilliant Blue FCF; Aizen Food Blue No.1; Alphazurine; Alphazurine
FG; Alphazurine FGND; Amacid Blue FG; Amacid Blue FG Conc; 1206
Blue, 11388 Blue; Blue Dye Number 1 food additive; Brilliant Blue;
Brilliant Blue FCF; Brilliant Blue Lake; Bucacid Azure Blue;
Calcocid Blue EG; Calcocid Blue 2G; Canacert Billiant Blue FCF,
Cogilor Blue 512.12; Cosmetic Blue Lake; Dispersed Blue 12195;
Disulphine Lake Blue EG; Dolkwal Brilliant Blue; Edicol Blue Cl 2;
Edicol Supra Blue E6; Erioglaucine ; Erioglaucine A; Erioglaucine E;
Erioglaucine G; Eriosky Blue; Fenazo Blue XI; Fenazo Blue XR; Food
Blue 1; Hexacol Brilliant Blue A; Hidacid Azure Blue; Intracid Pure
Blue L; Kjtoc Blue AR; Kiton Pure Blue L; Maple Brilliant Blue FCF;
Merantine Blue EG; Neptune Blue BRA; Concentration; Patent Blue AE;
Patent Blue 2Y; Peacock Blue X-1756; Usacert Blue No.1; Xylene Blue
VSG. Appearance:
Reddish-violet powder or granules with a metallic
luster.
Spectroscopy Data: λ max 630 nm.
Chemical Formula and Molecular
Weight:
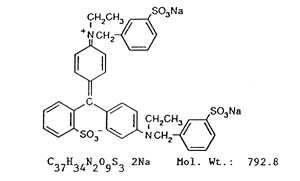
| Solubility: Soluble in water and ethanol;
insoluble in vegetable oils.
1.2 Limit Defining Parameters
1.2.1 Detection Limit of the
Analytical Procedure
The detection limit of the analytical
procedure is 0.83 ng Acid Blue 9 per injection. This is the amount of
analyte which will give a peak whose height is approximately five
times the amplitude of the baseline noise. See Figure
1.
1.2.2 Detection Limit of the Overall
Procedure
The detection limit of the overall procedure is
estimated to be 0.2 µg per sample or 0.002 mg/cu m based on the
recommended air volume, assuming 100% recovery from the sampling
device. The recovery test at this level has not been
performed.
1.2.3 Sensitivity
The sensitivity of
the analytical procedure over a concentration range of 0.395 to 11.9
µg/mL is 19,280 area units per µg/mL of Acid Blue 9. The sensitivity
is determined by the slope of the calibration curve. See Figure
2. 1.3
Advantages
The analytical procedure is rapid, sensitive, and
reproducible.
1.4 Disadvantages
The method has not
been fully validated. 2.
Sampling Procedure
2.1 Apparatus
2.1.1 An air sampling pump with
a flow rate which can be calibrated to within ±5% of the recommended 1
Lpm flow rate while the sampler is in line.
2.1.2 Glass
fiber filter, 37-mm diameter, Gelman Type A, or
equivalent.
2.1.3 Filter holder for 37-mm filters,
Millipore M000037AO, or equivalent. 2.2 Sampling Technique
2.2.1 Assemble the filter in the
two-piece cassette holder and close firmly. The filter is supported by
a backup pad. Secure the cassette holder together
with
tape.
2.2.2 Attach the outlet of the filter cassette to
the personal sampling pump inlet with flexible
tubing.
2.2.3 Air being sampled should not pass through
any hose or tubing before entering the filter
cassette.
2.2.4 A sample size of 100 liters is
recommended. Sample at a flow rate of 1.0 liter/minute. The flow rate
should be known with an accuracy of ±5%.
2.2.5 With each
batch of samples, submit a blank filter from the same lot of filters
used for sample collection. This filter must be subjected to exactly
the same handling as the samples except that no air is drawn through
it. Label this filter as the blank.
2.2.6 The cassette
should be shipped in a suitable container designed to prevent damage
in transit. The samples should be shipped to the laboratory as soon as
possible.
2.2.7 A sample of the bulk material should be
submitted to the laboratory in a glass container with a Polyseal cap.
Never transport, mail, or ship the balk sample in the same container
as the sample or blank filter. 2.3 Retention Efficiency
Two glass fiber
filters were spiked with 1.1.5 µg of Acid Blue 9. Humid air (87%
relative humidity) 140 liters was drawn through the filters at 1 Lpm.
The average recovery of the two filters was 101%.
| Sample |
Spiked Amount |
Treatment |
Peak Height |
Recovery |
|
YC5
YC6
YC7
YC8 |
41.5 µg on GFF
41.5 µg on
GFF
41.5 µg; control
41.5 µg; control |
140 L humid air
140 L humid
air
none
none |
138.0 mm
131.5 mm
131.0
mm
136.0 mm |
103.4%
98.5%
----
---- |
|
|
Average
recovery 100.9% |
2.4 Extraction Efficiency
The average
extraction efficiency from the glass fiber filters spiked with 41.5 µg
of Acid Blue 9 was 95.9%.
| Sample |
Spiked Amount |
Peak Height |
Recovery |
|
YC3
YC4
YC7
YC8 |
41.5 µg on GFF
41.5 µg on
GFF
41.5 µg; control
41.5 µg; control |
126.5 mm
129.5 mm
131.0
mm
136.0 mm |
94.8%
97.0%
----
---- |
|
|
Average
recovery 95.9% |
2.5 Storage
Two glass fiber filters were
spiked with 41.5 µg of Acid Blue 9 and stored at room temperature in the
dark for two days. The average recovery was 100.2%.
| Sample |
Spiked Amount |
Storage Days |
Peak Height |
Recovery |
|
YC3
YC4
YC7
YC8
YC1
YC2
YC7
YC8 |
41.5 µg
41.5 µg
41.5
µg
41.5 µg
41.5 µg
41.5 µg
41.5 µg
41.5 µg |
0
0
control
control
2
2
control
control |
126.5 mm
129.5 mm
131.0
mm
136.0 mm
168.0 mm
157.0 mm
162.0 mm
158.0
mm |
94.8%
97.0%
----
----
105.0%
98.1%
----
---- |
2.6 Recommended Air Volume and Sampling Rate
2.6.1 The recommended air volume
is 100 liters.
2.6.2 The recommended sampling rate is 1
Lpm. 2.7
Interferences
There are no known interferences associated with
the sampling procedure.
2.8. Safety Precautions
2.8.1 Attach the sampling
equipment to the worker in such a manner that it will not interfere
with work performance or safety.
2.8.2 Follow all safety
practices that apply to the work area being sampled
3. Analytical
Method
3.1 Apparatus
3.1.1 High performance liquid
chromatograph equipped with pump, sample injector, variable wavelength
detector, chart recorder, and other necessary
hardware.
3.1.2 HPLC reverse phase C18 analytical column.
Dupont Zorbax ODS column was used for this study.
3.1.3
An electronic integrator or other suitable method to measure detector
response.
3.1.4 Microliter syringe or automatic sampling
device for making sample injections.
3.1.5 Volumetric
flasks of convenient sizes for preparing standards.
3.1.6
Shaking device for extraction of samples. 3.2 Reagents
3.2.1 Acid Blue 9
(Erioglaucine)
3.2.2 Tetrabutylammonium phosphate,
reagent grade
3.2.3 Methanol, HPLC
grade
3.2.4 Water, HPLC grade
3.2.5
Phosphoric Acid 3.3 Sample
Preparation
3.3.1 Remove the filter form the
cassette clean tweezers and place it in a 20-mL scintillation
vial.
3.3.2 Add 5 mL of methanol/water (1:1) to the vial
and cap it.
3.3.3 Shake the vials vigorously on a shaker
for 30 minutes. 3.4
Standard Preparation
3.4.1 Standard of Acid Blue 9 is
prepared by dissolving 8 to 12 mg (accurately weighed) of Acid Blue 9
in water in a 10-mL volumetric flask and making it
to
volume.
3.4.2 Dilute to the working range of 0.1 to
12 µg/mL with water.
3.4.3 Store standards in dark
bottles under refrigeration. 3.5 Analysis
3.5.1 HPLC
Conditions
| Column: |
Zorbax ODS (25 cm x 4.6 mm) |
| Mobile phase: |
55% methanol, 45% water, 0.005 M
tetrabutylammonium phosphate |
| Flow Rate: |
1.0 mL/minute |
| Variable Wavelength Detector: |
650 nm |
| Injection Volume: |
20 µL |
| Retention Time: |
7.8
minutes |
3.5.2
Chromatogram
See Figure 1.
3.5.3. Peak magnitude is
measured by electronic integrator or other means.
3.5.4
An external standard procedure is used to prepare a calibration curve
from the analysis of at least three different concentrations from two
separate
weighings.
3.5.5 Bracket the sample with
analytical standards. 3.6
Interferences (Analytical)
3.6.1 Any collected compound
that has the same LC retention time as analyte and absorbs at 650 nm
is an interference.
3.6.2 HPLC parameters may be varied
to circumvent most interferences.
3.6.3 Retention time
alone is not proof of a chemical identity. Confirmation by other means
should be sought when possible. 3.7 Calculations
3.7.1 The integrator value in
area units for each standard is plotted against its concentration in
µg/mL and a calibration curve using the best fit straight line through
the points is obtained.
3.7.2 Sample concentration is
calculated from the calibration curve.
3.7.3 The air
concentration of Acid Blue 9 for a sample is calculated by the
following equation:
| mg/m3
= |
(µg/mL in
sample)(extraction volume, mL)
(Air volume, L) |
3.8 Safety Precautions
3.8.1 Confine the use of
solvents to a fume hood.
3.8.2 Wear safety glasses in all
laboratory areas. 4.
Recommendations for Further Study
4.1 Preparation of pure
standard
The commercially available Acid Blue 9 is not pure. The
U.S. specification for the food grade is 85% minimum. Purification of
the standard should be attempted either by preparative TLC or
preparative HPLC.
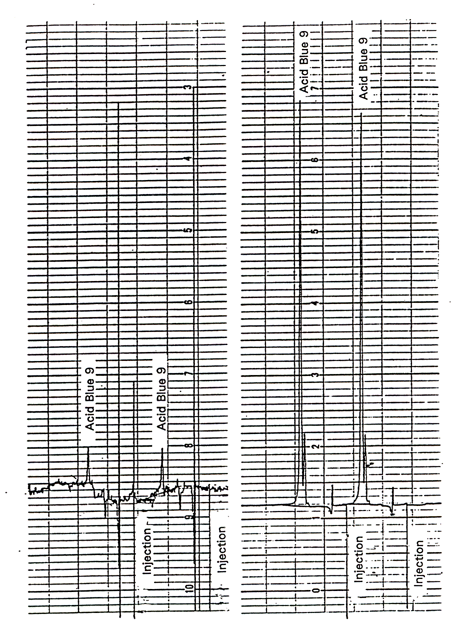
Figure
1. Chromatogram of Acid Blue 9 at Target Concentration and at
Detection Limit. |
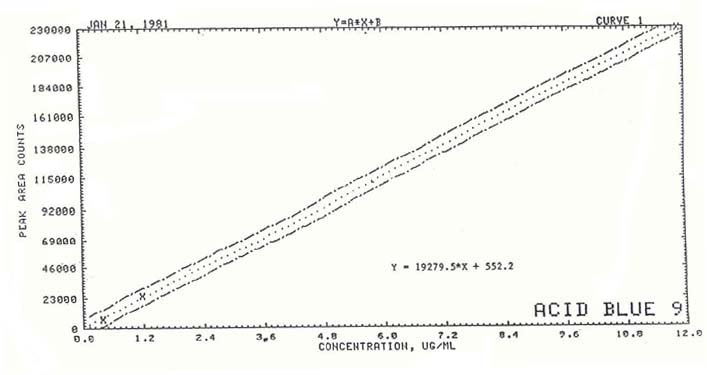
Figure 2. Calibration of Acid Blue
9. |
5.
References
5.1 WHO, International Agency for
Research on Cancer, IARC Monograph on the Evaluation
of the Carcinogenic Risk of Chemicals to Man. Some Aromatic Amines and
Related Nitro Compounds -- Hair Dyes, Colouring Agents and Miscellaneous
Industrial Chemicals. Vol. 16, pp. 171-86.
5.2 J.
Chudy, N.T. Crosby, and I. Patel, J. Chromatogr., 154, (1978), p
306-312.
5.3 A Standen, ed., Kirk-Othmer
Encyclopedia of Chemical Technology, Second Ed., Vol. 5, pp.
865-66. Interscience Publishers, New York, N.Y.,
1963.
|

