1. General Discussion
1.1 Background
1.1.1 History
The fully
validated NIOSH Method S-150 for morpholine suggests that morpholine
can be collected on silica gel tubes1.
Silica gel has a high affinity for water and the water may displace
any morpholine collected from the silica gel. In PV2016
cyclohexylamine showed good retention and storage efficiency when
collected on tubes containing 10% phosphoric acid coated XAD-7 tubes,
which had 10 L of humid air (83%@ 23°C) drawn through them2.
This evaluation uses tubes containing 10% phosphoric acid coated XAD-7
tubes. Morpholine collected on these tubes also indicate good
extraction, retention and storage efficiency.
1.1.2 Toxic
effects (This section is for information only and should not be taken
as the basis of OSHA policy.)
Morpholine causes visual
disturbance, nose irritation, coughing respiratory irritation, liver
and kidney damage. When guinea pigs and rats inhaled 12,000 ppm
morpholine for 8 hours obvious ocular and upper respiratory tract
irritation were recorded. Cloudy swelling of the renal tubular
epithelium and of the interstitial hepatic parenchyma and cellular
debris in the bronchi were noted at necropsy3.
The single oral LD50 for morpholine is 1.05 g/kg body
weight and the single dermal LD50 for 24 hour skin contact
is 0.5 mL/kg4.
Workers exposed for several hours at low concentrations complained of
foggy vision with rings around lights, resulting from a transient
corneal ederma, which cleared within 2 to 4 hours after cessation of
exposure5.
1.1.3
Workplace exposure
Morpholine is a cheap solvent for resins,
waxes, casein and dyes. Morpholine fatty acid salts are used as
surface agents and emulsifiers. Other morpholine compounds are used as
corrosion inhibitors, antioxidants, plasticizers, viscosity improvers,
insecticides, fungicides, herbicides, local anesthetics and
antiseptics6.
1.1.4
Physical properties and other descriptive information7
| CAS number: |
110-91-8 |
IMIS number8: |
1797 |
| Molecular weight: |
87.12 |
Density: |
0.994 |
| Vapor density: |
3 |
Odor: |
weak ammonia like |
| Vapor pressure: |
10 kPa (mmHg) |
Flash point: |
38oC |
| Molecular formula: |
C4 H9NO |
Boiling point: |
128.9oC |
| Melting point: |
4.9oC |
Corrosive material |
|
| Hydroscopic |
|
Flammable liquid |
|
| Miscible in: |
water; methanol;
ethanol; ethyl acetate; acetone |
| Synonyms: |
tetrahydro-2H-1,4-oxazine; diethylene oximide;
tetrahydro-1,4-oxazine diethylenimide oxide;
tetrahydro-4H-1-4,oxazine; 1-oxa-4-azacyclohexane.
|
Structure:
|
 |
|
|
| |
|
|
This method was evaluated according to the OSHA SLTC
"Evaluation Guidelines for Air Sampling Methods Utilizing Chromatographic
Analysis"9.
The Guidelines define analytical parameters, specify required laboratory
tests, statistical calculations and acceptance criteria. The analyte air
concentrations throughout this method are based on the recommended
sampling and analytical parameters. Air concentrations listed in ppm are
referenced to 25°C and 101.3 kPa (760 mmHg).
1.2 Detection limit of the overall
procedure (DLOP) and reliable quantitation limit (RQL)
The DLOP
is measured as mass per sample and expressed as equivalent air
concentrations, based on the recommended sampling parameters. Ten
samplers were spiked with equal descending increments of analyte that
the highest sampler loading was 4.55 µg/sample. This is the amount
spiked on a sampler that would produce a peak approximately 10 times the
response for a sample blank. These spiked samplers and the sample blank
were analyzed with the recommended analytical parameters, and the data
obtained used to calculate the required parameters (standard error of
estimate and slope) for the calculation of the DLOP. Values of 446 and
68.95 were obtained for the slope and standard error of estimate,
respectively. DLOP was calculated to be 0.464 µg/sample (13 ppb, 46
µg/m³).
Table
1.2
Detection Limit of the Overall Procedure
|
mass per
sample
(µg) |
area
counts
(µV-s) |
|
0
0.93
1.24
1.76
2.30
2.67
3.05
3.53
4.07
4.55 |
0
302
411
614
846
1016
1196
1440
1735
2012 |
| |
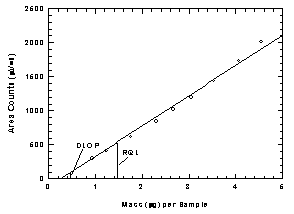
Figure
1.2.1 Plot of data to determine the DLOP/RQL. (Y=446X -
117) |
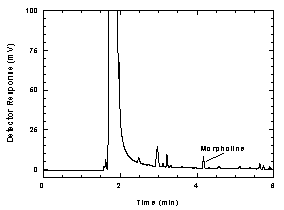
Figure
1.2.2 Chromatogram of the RQL. |
The RQL is considered the lower limit for precise
quantitative measurements. It is determined from the regression line
parameters obtained for the calculation of the DLOP, providing 75% to
125% of the analyte is recovered. The RQL is 1.55 µg per sample ( 45
ppb, 160 µg/m³). Recovery at this concentration is 90.0%.
2. Sampling Procedure
2.1 Apparatus
Samples are
collected using a personal sampling pump calibrated, with the sampling
device attached, to within ±5% of the recommended flow
rate.
Samples are collected on 10% phosphoric acid coated XAD-7
tubes, lot 2409 containing 80 mg adsorbing section with a 40 mg backup
section separated by a 2-mm portion of urethane foam, with a silanized
glass wool plug before the adsorbing section and a 3-mm plug of urethane
foam at the back of the backup section. The ends are flame sealed and
the glass tube containing the adsorbent is 7-cm long, with a 6-mm o.d.
For this evaluation, commercially prepared samplers were purchased from
SKC, Inc. (Cat. No. 226-98).
2.2 Reagents
None
required.
2.3 Technique
Immediately before sampling, break
off the ends of the flame-sealed tube as to provide an opening
approximately half the internal diameter of the tube. Wear eye
protection when breaking ends. Use tube holders to minimize the hazard
of broken glass. All tubes should be from the same lot.
The
smaller section of the adsorbent tube is used as a back-up and is
positioned nearest the sampling pump. Attach the tube holder to the
sampling pump so that the adsorbent tube is in an approximately vertical
position with the inlet facing down during sampling. Position the
sampling pump, tube holder and tubing so they do not impede work
performance or safety.
Draw the air to be sampled directly into
the inlet of the tube holder. The air being sampled is not to be passed
through any hose or tubing before entering the sampling
tube.
After sampling for the appropriate time, remove the
adsorbent tube and seal it with plastic end caps. Seal each sample
end-to-end with an OSHA-21 form as soon as possible.
Submit at
least one blank sample with each set of samples. Handle the blank sample
in the same manner as the other samples except draw no air through
it.
Record sample air volume (liters), sampling time (minutes)
and sampling rate (mL/min) for each sample, along with any potential
interferences on the OSHA-91A form.
Submit the samples to the
laboratory for analyses as soon as possible after sampling. If delay is
unavoidable, store the samples at refrigerator temperature. Ship any
bulk samples separate from air samples.
All safety practices that
apply to the work area being sampled should be followed. The sampling
equipment should be attached to the worker in such a manner that it will
not interfere with the work performance or safety.
2.4 Extraction
efficiency
The extraction efficiencies of morpholine were
determined by liquid-spiking media with the morpholine at 0.5 to 2 times
the target concentration. These samples were stored overnight at ambient
temperature and then extracted and analyzed. The mean extraction
efficiency over the studied range was 102% for
morpholine.
Table 2.4
% Extraction
Efficiency of Morpholine
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
0.1
0.5
1.0
2.0
Wet
1.0 |
69.6
398
696
392
696 |
102
101
101
104
98.8 |
104
100
104
97.4
100 |
104
100
106
97.8
98.4 |
105
103
102
103
100 |
104
101
103
101
99.3 |
|
2.5 Retention
efficiency
Six sampling tubes were spiked with 696 µg (69.6
mg/m³) morpholine, allowed to equilibrate for 6 h, and then had 10 L
humid air (absolute humidity of 15.9 mg/L of water, about 80% relative
humidity at 22.2°C) pulled through them. The samples were extracted and
analyzed. The mean retention efficiency is 94.0%. There was no
morpholine found on the backup portion of the sampler.
Table 2.5
% Retention
Efficiency of Morpholine
|
|
sample number
|
|
| section |
1 |
2 |
3 |
4 |
5 |
6 |
mean |
|
front
rear
total |
95.0
0
95.0 |
93.4
0
93.4 |
95.0
0
95.0 |
95.0
0
95.0 |
93.3
0
93.3 |
92.0
0
92.0 |
94.0
0
94.0 |
|
2.6 Sample storage
Fifteen
sampling tubes were each spiked with 695.8 µg (19.6 ppm) of
morpholine. They had 10 L of air with an absolute humidity of 15.7
milligrams of water per liter of air (about 80% relative humidity
at 22.2°C) drawn through them. Six were sealed and stored at room
temperature. Six were sealed and stored at 5 degrees centigrade.
Immediately 3 were analyzed. After eight days, three at room
temperature and three at refrigeration were analyzed and the
remaining six after 14 days of storage. The amounts recovered,
which are corrected for extraction efficiency, indicate good
storage stability for the time period studied. |
Table
2.6
Storage Test for Morpholine
|
|
sample no.
|
|
| time (days) |
1 |
2 |
3 |
mean |
|
Immediate
8
ambient
refrigerated
14 ambient
refrigerated |
101
104
107
95
87 |
97
99
97
89
91 |
97
102
102
87
lost |
98
102
102
91
89 |
| |
2.7 Recommended air volume and sampling rate
Based
on the data collected in this evaluation, 10-L air samples should be
collected at a sampling rate of 0.1L/min. 3. Analytical Procedure
Adhere to the rules set down
in your Chemical Hygiene Plan10.
Avoid skin contact and inhalation of all chemicals and review all
MSDSs.
3.1 Apparatus
Gas chromatograph
equipped with an FID. A Hewlett-Packard Model 6890 was used in this
evaluation.
3.2 Reagents
Methanol, (CAS 67-56-1).The
methanol used in this evaluation was HPLC grade (lot no. B0502782)
purchased from Acros (New Jersey).
Morpholine, (CAS no.110-91-8).
The morpholine used in this evaluation was 99.5+% (Lot No. 10131EO)
purchased from Aldrich (Milwaukee, Wisconsin).
Deionized water,
Nanopure Diamond filtered by a Barnstead deionizer in the SLTC
laboratory.
Sodium hydroxide, (CAS no. 1310-73-2) (Lot no.
X20944) purchased from J.T. Baker.
Extraction solution: 20%
deionized water in methanol.
3.3 Standard
preparation:
Prepare concentrated stock solutions of morpholine
in methanol. The PEL of morpholine is 70 mg/m³: 70 µg/L x 10 L = 700 µg
and the density of morpholine is 994 µg/µL. Dilute this 994 µg/µL
solution 0.1 to 99.4 µg/µL , 0.01 to 9.94 µg/L and 0.001 to 0.994 µg/µL.
Prepare working analytical standards by injecting microliter amounts of
concentrated stock solutions into 2-mL vials containing 1 mL of
extracting solution. Prepare standards by injecting 14 µL, 7µL and 4µL
of the 99.4 µg/µL solution ; 7 µL of the 9.94 µg/µL solution and 7µL of
the 0.994 µg/uL solution. The concentrations are: 1391.6, 695.8, 397.6,
69.6 and 6.96µg/mL. To analyze compliance samples dilutions of 2
concentrated stock solutions at the 2X PEL could be made. Dilutions of
these stock solutions to approximately 7µg/mL level could be
made.
3.4 Sample preparation
Remove the plastic end caps
from the sample tube and carefully transfer each section of the
adsorbent to separate 2-mL vials. Include the glass wool plug in the
front section of the sample media.
Add 1.0 mL of extracting
solution to each vial and immediately seal the vials with
polytrafluoethylene-lined caps.
Extract the samples on a
rototurner for 0.5 hr.
Transfer 0.5 mL of sample to a 2 mL vial
and add 0.5 mL of 20% 1.0N NaOH in methanol. Shake. The solid that forms
doesn’t interfere with the analysis. The samples are centrifuged before
analysis.
3.5 Analysis
| column: |
MDN-1; 30-m; 0.32 mm i.d.; 1.0
µm df |
| GC conditions: |
|
| temperature
program: |
50°C at 10°C per minute to
110°C |
| zone temperatures: |
200°C (injector)
250°C
(detector) |
| run time: |
6 minutes |
| column gas flow: |
2.0 mL/min |
| injection size: |
1.0 µL, 10:1 split |
| retention times: |
1.67 min methanol
4.45 min
morpholine |
| FID conditions |
|
| hydrogen flow: |
40.0 mL/min |
| air flow: |
450 mL/min |
| nitrogen makeup flow: |
15 mL/min | |
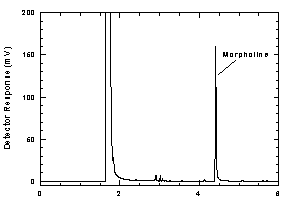
Figure
3.5 Chromatogram at the target concentration |
3.6 Analytical
Interferences
Any compound that produces a FID response and
has a similar retention time as morpholine or internal standard is
a potential interference. If any potential interferences were
indicated by the industrial hygienist, they should be considered
before samples are extracted. Generally, chromatographic
conditions can be altered to separate an interference from
morpholine. |
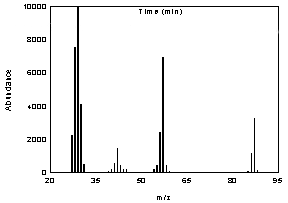
Figure 3.6
Mass spectrum of morpholine. | 3.7 Calculations
The amount of morpholine per
sampler is obtained from the appropriate calibration curve in terms of
micrograms per sample, uncorrected for extraction efficiency. The back
section is analyzed primarily to determine the extent of sampler
saturation. If any morpholine is found on the back section, it is added
to the amount on the front section. This total amount is then corrected
by subtracting the total amount (if any) found on the blank. The air
concentration is calculated using the following formulas.
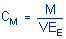 |
where |
CM is concentration by weight
(mg/m³)
M is micrograms per
sample
V is liters of air
sampled
EE is extraction
efficiency, in decimal form
|
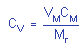 |
where |
CV is concentration by volume
(ppm)
VM is molar volume
at 25°C and 760 mmHg (NTP)
CM is concentration by
weight
Mr is molecular
weight (87.12) |
3.8 Safety precautions
Avoid skin contact and
inhalation of all chemicals. Wear safety glasses, gloves and a
laboratory coat at all times while in laboratory areas.
4. Recommendations for further
study:
Collection studies need to be performed.
1. Morpholine, NIOSH Method S-150, NIOSH Manual of
Analytical Methods, 2nd Edition, Volume 3, Cincinnati, OH
(1977).
2. Eide, Mary E. Cyclohexylamine ( OSHA method PV 2016)
Organic Service Branch 1, OSHA Salt Lake Technical Center, Salt Lake City,
Utah 84165 (1994).
3. Shea, T.E.: The Acute and Subacute Toxicity
of Morpholine. J. Ind. Hyg Toxoxicol. 21: 236-245 (1939)
4. Smyth,
Jr., H.F.; Carpenter, C.P. ; Weil, C.S.; Pozzani, U.C.: Range-Finding
Toxicity Data. List V. Arch. Ind. Hyg. Occup. Med. 10:61-68
(1954)
5. Proctor, N.H.; Hughes, J.P.; Fischman, M.L.: Morpholine.
In: Chemical Hazards of the workplace, 2nd ed., pp.353-354, J.B.
Lippincott Co. Philadelphia, Pennsylvania.(1988).
6. Merck Index:
12th Ed. 1996 page 1074-5: Review of Morpholine and its derivatives.
(1996)
7. Budavari, S., Ed. Merck Index 12th ed. Merck Research
Laboratories; Whitehouse Station, N.J., 1996, page 6359.
8. OSHA
Chemical Sampling Information (accessed 5/14/03)
9. Burright, D. ;
Chan, Y ; Eide, M. ; Elskamp, C. ; Hendricks, W. ; Rose, M.C. Evaluation
Guidelines for AirSampling Methods Utilizing Chromatographic Analysis,
OSHA, Salt Lake Technical Center, U.S. Department of Labor: Salt Lake
City, Ut 1999
10. Occupational Exposure to Hazardous Chemicals in
Laboratories. Code of Federal Regulations, Part1910,1450, Title 29,
1998.
|

