| Method No.: |
PV2120 |
| |
| Control No.: |
T-PV2120-01-0305-ACT |
| |
| Matrix: |
Air |
| |
| Procedure: |
A sample is collected by drawing air
through an orifice into an evacuated fused silica-lined stainless
steel canister. The canisters are analyzed in the laboratory, where
they are first pressurized with nitrogen. Aliquots of the air sample
are withdrawn, cryofocused, and analyzed by gas chromatography/mass
spectrometry to determine the concentrations of compounds
collected. |
| |
| Recommended sampling volume
and sampling time: |
The canister volume is
approximately 400 mL. Short-term sampling orifices allow the
canister to be filled in less than one minute. Long-term area
samples or personal air samples may be collected over periods up to
8 hours or longer. |
| |
| Detection Limits: |
Detection and reliable
quantitation limits will vary with analyte response factor.
Detection limits of low ppb levels are possible for most common
analytes. |
| |
| Status of method: |
Partially Validated. This
method has been subjected to the established evaluation procedures
of the SLTC Methods Development Team. |
| |
| Date: |
May, 2003 |
| |
| Chemist: |
Patrick Hearty
|
Applied IH
Chemistry Team
Program Support Division
OSHA Salt Lake
Technical Center
Salt Lake City, Utah
84115-1802
| 1. General Discussion
1.1 Background and
History
Evacuated stainless steel canisters with electro-polished
inner surfaces, called SUMMA canisters, are widely used in EPA
applications when sampling for volatile organic compounds (VOC's) in the
environment (1).
Canisters have been evaluated for use with a range of volatile organics,
including aliphatic and aromatic hydrocarbons, and chlorinated compounds
(2).
This technique has also been applied to a variety of practical
applications, such as indoor air quality problems (3),
and other situations involving low levels of volatile contaminants,
including the docking of the Russian Priroda module with Space Station
Mir (4).
The evacuated canisters offer a number of advantages, including
elimination of the need for a sampling pump, avoidance of questions
concerning sorbent tube collection and recovery, and the ability to make
replicate injections and dilutions during analysis.
The
development of a process which coats stainless steel with fused silica
has led to important advances in chemical sampling and analysis. This
material offers the structural strength and impermeability of steel
combined with the inertness of fused silica. Entech, Inc., Simi Valley,
CA, has combined the fused silica coating with the polished canister
technology in a smaller MiniCan, 400-mL capacity, for use as a personal
sampler in industrial hygiene. This new technique is leading to improved
analytical methods for a variety of reactive and labile compounds of
interest at very low levels (5,
6).
Short-term area samples are collected by attaching a sampling
orifice to the inlet of the MiniCan. Sampling begins immediately, and is
completed when the pressure inside the canister is equal to the
atmospheric pressure on the outside, or when the sampling orifice is
detached from the canister. For personal air samples, a MiniCan is
mounted in a holster attached to a belt fastened around the waist of the
worker. A sampling orifice with regulator is attached to the inlet of
the canister, and a length of inert tubing leading from the breathing
zone of the worker is connected to the inlet of the orifice. In the
laboratory, the canister is pressurized with nitrogen, and the contents
are analyzed by gas chromatography/mass spectrometry.
The data
presented in this method were produced during an evaluation study
conducted at OSHA's Salt Lake Technical Center (7).
1.2 Limit Defining Parameters.
1.2.1 Detection
Limits
Standards of n-hexane (5.3
ppb), tetrachloroethylene (5.2 ppb), toluene (5.3 ppb), and p-xylene (5.3 ppb) were analyzed for determination
of detection limits. Lower limits of detection are estimated to be:
0.2 ppb for n-hexane, 0.4 ppb for
tetrachloroethylene, 1 ppb for toluene, and 4 ppb for p-xylene. (Section 4.1)
1.2.2 Minimum
Injection Volume
Using canisters spiked with standards of 50
ppb of the four analytes listed above, injection volumes from 5 mL to
200 mL were analyzed. Results indicate that a 10 mL injection is the
smallest volume which provides acceptable precision. (Section
4.2)
1.2.3 Storage Stability
Canisters spiked with each
of the four test compounds at 25 ppb were stored at room temperature
for up to 14 days. The average of four replicates was as follows:
n-hexane 125%; toluene 97.4%;
tetrachloroethylene 89.7%; p-xylene 100%.
(Section 4.3)
To test recovery at higher levels, a single test
was run by spiking one canister with trichloroethane at 100 ppm. The
measured value after 5 days was 102%.
The draft NIOSH method
for canister sampling of VOC's indicates 30-day sample stability for
most compounds (8).
Some compounds have been reported stable for up to 4 months (2).
1.2.4
Precision
Five canister replicates were spiked with the four
test analytes each at a level of 50 ppb. The coefficients of variation
for the four analytes were as follows: n-hexane 13.4%; toluene 6.7%; tetrachloroethylene
7.3%; p-xylene 6.8%. Data were taken from
Table 4.2. Statistical analyses may be found in Tables 4.4.1 through
4.4.4. 1.3 Advantages
1.3.1 Problems with collection
efficiency and analyte recovery, which may be encountered with
sorbents or filters, are avoided. Evacuated canister sampling is a
whole-air sampling technique.
1.3.2 When collecting short-term
samples, no sampling error is associated with this
method.
1.3.3 Sampling pumps are not needed.
2. Sampling Procedure
2.1 Apparatus
2.1.1 Entech Minicans, 400-mL volume
(Entech P/N 29-MC400), were used in this study. Sampling canisters may
be obtained from a contract laboratory, and returned to the contract
lab after samples are collected. Canisters obtained from the contract
lab will be certified clean (9).

Figure 2.1.1
Four
400 mL Minicans. |
2.1.2 Samples are collected by filling
evacuated canisters through a sampling orifice. Orifices are available
which provide practically instant grab sampling (Entech P/N 39-QFS).
Pressure regulated orifices (Entech P/N CS1200E) offer sampling times
as short as 2 minutes, or as long as 8 hours or more. These sapphire
orifices are said to provide superior flow stability, compared to
needle valve or frit-regulated controllers.

Figure
2.1.2.1
Short-term sampling orifice. |

Figure
2.1.2.2
Minican with pressure regulated
orifice. |
2.1.3 For personal sampling, a holster
and belt (Entech P/N 39-35000) can be used to attach the canister to
the waist of an employee. An inert inlet line (Entech P/N 39-36010) is
used to draw air from the employee's breathing zone.

Figure
2.1.3
Minican with pressure regulated orifice and personal
sampling belt and inlet line. |
2.1.4 End caps are removed from the
canisters prior to attachment of the sampling regulators, and replaced
when sampling is complete.
Figure
2.1.4
Minican inlet with end cap removed and in
place. | 2.2 Reagents
None needed. 2.3 Sampling
Technique
2.3.1 Choose the time-release
regulator, either short- or long-term, appropriate for the desired
application.
2.3.2 Holding the sampling regulator in one hand,
slide back the knurled collar with thumb and index finger.
2.3.3
Hold the canister in the other hand, with protective end cap removed,
and with tip of canister facing sampling regulator.
2.3.4 Insert
the canister tip into the regulator, and release the knurled collar. No
gap should be observable between the regulator and the fitting at the
end of the canister.
2.3.5 Sampling begins immediately. (Note the
time of day.)
2.3.6 Bear in mind that this is a whole air
sampling technique. Lack of selectivity is inherent in this method. If
for example, the person performing the sampling, or the person being
sampled should be wearing perfume or cologne, volatile components of
these will also be sampled.
2.3.7 When sampling is complete,
reverse above steps to disengage the canister from the regulator. Slide
back the knurled collar with thumb and index finger, and separate the
canister from the regulator. Release the knurled collar.
2.3.8
Replace the protective end cap onto the canister, and seal each canister
with an OSHA Form 21.
2.3.9 Record sampling time. (Note the time
of day when sampling is completed.)
2.3.10 No sample blank is
necessary if the canisters were assured to be clean at the outset of
sampling. A sample collected in a control area may be included if
desired. 2.4 Safety Precautions
(sampling)
2.4.1 Follow all safety procedures which
apply in the work area being sampled.
2.4.2 If personal sampling
is being conducted, attach sampling equipment to the employees in such a
manner that it will not interfere with work performance or
safety. 3. Analytical Procedure
It is possible to conduct sampling using this method even if your
laboratory is not equipped with apparatus for cleaning and analysis of
canister samples. Contract laboratories will provide loan of cleaned and
evacuated canisters followed by GC/MS analysis of your samples (9).
3.1 Apparatus
3.1.1 Entech Canister System
consisting of a Model 7032L 21-Position Loop Autosampler and Model
7100 Preconcentrator, connected to a GC/mass spectrometer system. A
Hewlett-Packard 5973 GC/mass spectrometer was used in this
evaluation.
3.1.2 Entech Model 4600 Dynamic Dilution
System.
3.1.3 Entech Model 3100 Canister Cleaning
System.
3.1.4 Summa Canisters 6-liter volume, Silonite
coated.
3.1.5 A GC column capable of providing adequate
separation of the analytes of interest must be chosen. A 30-m DB-1-MS
column, 0.32-mm i.d. with df 0.25 microns (J&W Scientific, catalog
#1230132) was used in this study. 3.2 Reagents
3.2.1 Standard gas mixture according
to the compounds to be analyzed.
3.2.2 Liquid
nitrogen.
3.2.3 Helium (ulta high purity).
3.3 Canister Cleaning
3.3.1 The Entech Model 3100 is used to
clean canisters prior to sampling. Canisters are evacuated to 13 kPa
(2 psi), then filled with clean, humidified nitrogen to 172 kPa (25
psi), while heated to 80°C.
3.3.2 This process is repeated
until no residual contaminants remain. Canisters are pressurized to
207 kPa (30 psi) with nitrogen, and an aliquot is withdrawn for
analysis (Section 3.6) to ascertain cleanliness.
3.3.3
Canisters which are to be used to sample relatively high (ppm)
concentrations of analytes are usually adequately cleaned after 3
cleaning cycles. If a canister which has previously been used for
sampling of ppm-level contaminants is to be used to sample low (ppb)
concentrations of analytes, more rigorous cleaning will be required.
Up to 100 cleaning cycles may be necessary. An effective and more
efficient approach, however, is to put the contaminated canisters
through 3 cleaning cycles, allow the canisters to sit for a couple of
days, then repeat cleaning through three cycles, and check for
cleanliness. Repeat this clean, store, and clean sequence as many
times as necessary.
3.3.4 Canisters should be evacuated to a
pressure of 6.7 Pa or less prior to sampling. It is recommended that
cleaning and evacuation be conducted as near to the time of use as
practical.
3.3.5 Canisters may be checked for leaks by
pressurizing with clean nitrogen to 207 kPa, rechecking pressure after
24 hours. A pressure drop greater than 14 kPa indicates a leak.
3.4 Standard Preparation
3.4.1 Using nitrogen as the diluent
gas, standards of the desired analytes plus internal standards are
prepared in 6-liter Summa canisters using the Entech Model 4600
Dynamic Dilution System.
3.4.2 If electropolished Summa
canisters are used, be sure to use humidified nitrogen as diluent gas,
especially if polar compounds are being analyzed. When using fused
silica-coated canisters, the requirement for humidity is not critical,
since contaminant molecules have lower affinity for the silica-coated
surface than for bare stainless steel surfaces.
3.5 Sample Preparation
Prior to
analysis, the pressure in each sample canister is increased to twice its
original value, using zero-grade nitrogen as the diluent gas. After
equilibration, this elevated pressure allows measured aliquots of the
sample gas to be easily withdrawn for analysis.
3.6
Analysis
3.6.1 For samples expected to contain
relatively high levels of contaminants (e.g., ppm levels), the Entech
Model 7032L Loop Autosampler is used to withdraw approximately 1-mL
aliquots of the sample air, diluted with nitrogen. This aliquot is
cryofocused prior to introduction on to the GC/MS column.
3.6.2
For samples expected to contain levels of contaminants less than 1
ppm, the Entech Model 7032L Loop Autosampler, is used to withdraw 10-
to 100-mL aliquots of the sample air, diluted with nitrogen. These
aliquots are drawn into a sampling loop, then concentrated and
cryofocused prior to introduction on to the GC/MS column.
3.6.3
Replicate analyses or subsequent aliquots of a different size may be
drawn from a sample canister.
3.6.4 Mass spec conditions
| GC Column: |
DB-1-MS, 30m x 0.320mm i.d. |
| Initial temperature: |
35°C, hold for 5 minutes |
| Program rate: |
10°C/minute |
| Final temperature: |
280°C |
| |
|
| zone temperatures: |
GC injector: 250°C |
|
Transfer line: 280°C |
|
Source: 230°C |
|
Analyzer: 150°C |
| |
|
| Electron energy: |
70 eV |
| Scan range: |
24-250
AMU | 4. Back-up Data
4.1 Detection Limits
Figure 4.1
shows chromatograms of 20-mL and 10-mL injections of a standard of
approximately 5 ppb each of the four test compounds. Based on a 100-mL
injected sample volume and a two-fold dilution, the estimated limits of
detection are: n-hexane 0.2 ppb;
tetrachloroethylene 0.4 ppb; toluene 1 ppb; and p-xylene 4 ppb. Detection limits were calculated
based on peak heights which are three times the baseline noise.
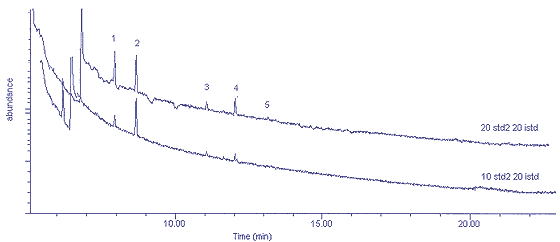
Figure
4.1 Chromatograms of 20-mL and 10-mL volumes of standard 2 (5 ppb). 1 =
n-hexane (5.3 ppb), 2 = internal standard
(1,1,1-trichloroethane), 3 = toluene (5.3 ppb), 4 = tetrachloroethylene
(5.2 ppb), 5 = p-xylene (5.3 ppb)
4.2
Minimum Injection Volume
Table 4.2 shows the results of
injections of a standard of approximately 50 ppb of each of the four
test compounds, with injection volumes varying from 5 to 200 mL. Due to
lack of acceptable reproducibility of the results from injections of 5
mL, it was concluded that 10 mL is the minimum injection volume which
produces reliable results.
Table 4.2 Instrument response of 50-ppb
standard (area counts)
|
| mL |
hexane |
toluene |
tetrachloro-ethene |
xylene |
ISTD |
|
| 5 |
135213 |
77835 |
244387 |
48399 |
1174510 |
| 5 |
299161 |
191667 |
426274 |
121338 |
1111884 |
| 10 |
4459700 |
4534540 |
6104070 |
4245197 |
1318405 |
| 10 |
3690372 |
3667848 |
5004722 |
3339190 |
1112885 |
| 20 |
12708131 |
14293658 |
18675442 |
13303503 |
789727 |
| 20 |
12661318 |
13970257 |
18132314 |
12841465 |
1073221 |
| 50 |
31717802 |
45084647 |
64610183 |
41904604 |
2195409 |
| 50 |
30678491 |
43439246 |
63863835 |
40392582 |
1716193 |
| 50 |
30201947 |
43346349 |
63159344 |
40179816 |
1569277 |
| 50 |
29278081 |
40377803 |
58893541 |
37513228 |
1636387 |
| 50 |
39971450 |
38043062 |
54059640 |
35324003 |
834744 |
| 100 |
61064562 |
126363870 |
244037259 |
122562739 |
2049239 |
| 100 |
56598966 |
111798212 |
213612977 |
109534835 |
2245114 |
| 150 |
161232524 |
221738723 |
451565957 |
267346044 |
1743758 |
| 150 |
160587701 |
213603130 |
438511070 |
249931430 |
1948079 |
| 200 |
109569656 |
300828355 |
618466534 |
346332656 |
2497457 |
| 200 |
211332078 |
310746877 |
617009392 |
322998984 |
2033987 |
|
4.3 Storage
Stability
For storage stability studies, canisters were spiked
with standards at approximately 25 ppb of each compound and stored at
ambient temperatures. Two canisters were spiked with hexane and toluene,
and two with tetrachloroethylene and xylene. Aliquots were analyzed on
day 0, and subsequently on days 3, 9, and 14. Table 4.3.1 shows the
results, in percentages of theoretical amounts, for hexane and toluene.
Similar data for tetrachloroethylene and xylene are shown in Table
4.3.2. These results are represented graphically for hexane in Figure
4.3.1.1, for toluene in Figure 4.3.1.2, for tetrachloroethylene in
Figure 4.3.2.1, and for xylene in Figure 4.3.2.2.
Table 4.3.1. Storage data for n-hexane and toluene at 25 ppb
|
| time (days) |
| |
n-hexane recovery (%) |
| |
toluene recovery (%) |
|
| 0 |
| |
98.4 |
102.1 |
101.6 |
98.0 |
| |
99.7 |
104.3 |
100.4 |
95.5 |
| 3 |
| |
98.5 |
99.5 |
- |
- |
| |
99.8 |
101.3 |
- |
- |
| 9 |
| |
109.6 |
112.6 |
98.7 |
97.8 |
| |
101.1 |
102.4 |
100.9 |
102.7 |
| 14 |
| |
119.8 |
115.3 |
134.3 |
131.2 |
| |
95.3 |
91.3 |
102.7 |
100.3 |
|
Table 4.3.2. Storage data for tetrachloroethene
and p-xylene at 25 ppb
|
| time (days) |
| |
tetrachloroethene recovery (%) |
| |
p-xylene recovery (%) |
|
| 0 |
| |
98.9 |
104.9 |
100.8 |
95.4 |
| |
98.9 |
105.1 |
100.4 |
95.6 |
| 3 |
| |
101.8 |
105.3 |
- |
- |
| |
100.4 |
103.2 |
- |
- |
| 9 |
| |
81.9 |
80.0 |
87.3 |
94.0 |
| |
96.3 |
99.4 |
102.7 |
102.4 |
| 14 |
| |
93.4 |
87.5 |
89.9 |
88.1 |
| |
96.5 |
93.1 |
106.1 |
104.2 |
|
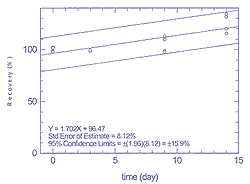
Figure
4.3.1.1
Storage test for 25 ppb of n-hexane |
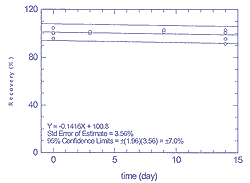
Figure
4.3.1.2
Storage test for 25 ppb of toluene |
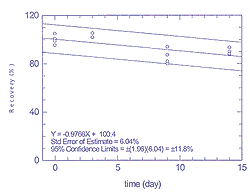
Figure
4.3.2.1
Storage test for 25 ppb of tetrachloroethane |
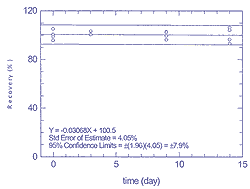
Figure
4.3.2.2
Storage test for 25 ppb of p-xylene |
A single canister was spiked with 100 ppm trichloroethane.
This was analyzed immediately, and after storage up to 13 days. Results
are tabulated in Table 4.3.3, and shown graphically in Figure
4.3.3.
Table 4.3.3 Storage test for
1,1,1-tricholoroethane at 100 ppm
|
| | |
time (days) |
| |
recovery (%) |
| |
|
| | |
0 |
| |
102.0 |
98.0 |
| |
| | |
3 |
| |
100.4 |
93.3 |
| |
| | |
5 |
| |
107.2 |
96.1 |
| |
| | |
7 |
| |
96.8 |
90.0 |
| |
| | |
12 |
| |
91.4 |
80.3 |
| |
| | |
13 |
| |
91.3 |
80.3 |
| |
| | |
17 |
| |
98.3 |
120.7 |
| |
|
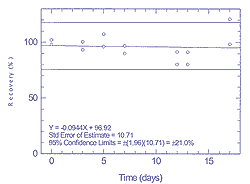
Figure
4.3.3
Storage test for 100 ppm of
1,1,1-trichloroethane. |
4.4 Precision
The data in Section 4.4 were
extracted from Table 4.2, and show the results of five replicate
analyses of a canister spiked with 50 ppb of each of the 4 test
compounds. Table 4.4.1 shows results for n-hexane along with statistical analysis. Table
4.4.2 shows similar results for toluene, Table 4.4.3 for
tetrachloroethylene, and Table 4.4.4 for o-xylene.
Table 4.4.1
Precision data for n-hexane, 50-mL injection, 50 ppb
|
| | |
Area Counts X 10-7 |
| |
|
| |
|
| | |
3.1718 |
| |
|
| |
| | |
3.0627 |
| |
mean = 3.2370 |
| |
| | |
3.0202 |
| |
SD = 0.4339 |
| |
| | |
2.9278 |
| |
CV = 13.40 % |
| |
| | |
3.9971 |
| |
|
| |
|
Table 4.4.2
Precision data for
toluene, 50-mL injection, 50 ppb
|
| | |
Area Counts X 10-7 |
| |
|
| |
|
| | |
4.5085 |
| |
|
| |
| | |
4.3439 |
| |
mean = 4.2058 |
| |
| | |
4.3346 |
| |
SD = 0.2814 |
| |
| | |
4.0378 |
| |
CV = 6.69 % |
| |
| | |
3.8043 |
| |
|
| |
|
Table 4.4.3
Precision data for
tetrachloroethylene, 50-mL injection, 50 ppb
|
| | |
Area Counts X 10-7 |
| |
|
| |
|
| | |
6.4610 |
| |
|
| |
| | |
6.3864 |
| |
mean = 6.0917 |
| |
| | |
6.3159 |
| |
SD = 0.4429 |
| |
| | |
5.8894 |
| |
CV = 7.27 % |
| |
| | |
5.4060 |
| |
|
| |
|
Table 4.4.4
Precision data for xylene,
50-mL injection, 50 ppb
|
| | |
Area Counts X 10-7 |
| |
|
| |
|
| | |
4.1905 |
| |
|
| |
| | |
4.0393 |
| |
mean = 3.9603 |
| |
| | |
4.0180 |
| |
SD = 0.2690 |
| |
| | |
3.7513 |
| |
CV = 6.79 % |
| |
| | |
3.5324 |
| |
|
| |
|
4.5 Canister
cleaning.
4.5.1 Figure 4.5.1 shows a
chromatogram after 3 cleaning cycles of a canister which had contained
33 ppm trichloroethane. A 1-mL aliquot was sampled by loop injection
without pre-concentration. The canister is adequately clean for
sampling and analysis at the ppm level.
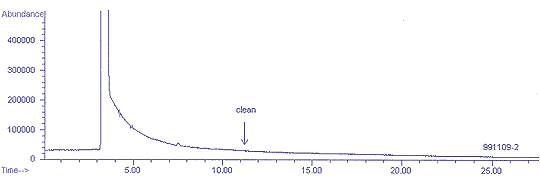
Figure 4.5.1 Total ion
chromatogram of a MiniCan that had contained 33 ppm of
1,1,1-trichloroethane and then was cleaned 3 cycles.
Analysis by loop injection. Arrow shows the retention time of
1,1,1-trichloroethane.
4.5.2 Figure 4.5.2 shows a
chromatogram after 100 cleaning cycles of a canister which had
contained 100 ppm of trichloroethane. A 100-mL aliquot was sampled for
pre-concentration prior to injection. The canister is adequately clean
for sampling and analysis at the ppb level. It is recommended that
highly contaminated canisters be cleaned for 3 cycles, allowed to sit
for several days, then cleaned for 3 more cycles and tested for
cleanliness. Repeat this sequence until acceptable cleanliness is
achieved.
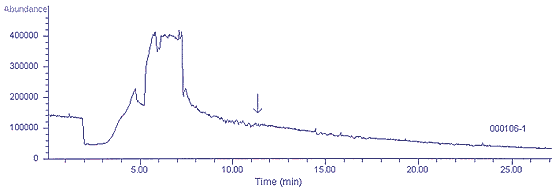
Figure 4.5.2 Total ion
chromatogram of a MiniCan that had previously contained 100 ppm of
1,1,1-Trichloroethane and had been cleaned 100 cycles.
1,1,1-trichloroethane elutes at 11.4 min.
- Methods TO14A and TO15, Compendium of Methods for the Determination
of Toxic Compounds in Air, Second Edition. Center for Environmental
Research Information - Office of Research and Development, U.S.
Environmental Protection Agency, 1999.
- Wai-mei Sin, D., Wong, Y-C., Sham, W-C., and Wang, D., Development
of an Analytical Technique and Stability Evaluation of 143 C3-C12
Volatile Organic Compounds in SUMMA Canisters by Gas Chromatography-Mass
Spectrometry, The Analyst, 126, pp 310-21, 2001.
- Farant, J-P., and Simon, P. An Approach to the Resolution of
Sporadic Gaseous Indoor Air Pollution Events, Proceedings of the 2nd
European Conference on Energy Performance and Indoor Climate in
Buildings and the 3rd International Conference on Indoor Air Quality,
Ventilation and Energy Conservation in Buildings, pp. 952-56, 1998.
- Cole, H., Ward, S., Manuel, S., Rather, D., Simon, P., Farant, J-P.,
Krasnec, J., Gouzenberg, A., Moukhamedieva, L., and Mikos, K. The
Application of Grab and Time Integrated Sampling to the Characterization
of Trace Contaminants Contributed by the Docking and Integration of the
Priroda Module to Space Station Mir, Proceedings of the 28th
International Conference on Environmental Systems, 1998.
- Formaldehyde and VOC's in Indoor Air Quality Determinations by
GC/MS, Entech Instruments Applications Note 101, Entech Instruments,
Inc., Simi Valley, CA.
- Ochiai, N., Takino, M., Daishima, S., and Cardin, D. Analysis of
Volatile Sulfur Compounds in Breath by Gas Chromatography-Mass
Spectrometry using a Three-Stage Cryogenic Trapping Preconcentration
System, Journal of Chromatography B, 762, pp. 67-75, 2001.
- Chan, Y., and Hearty, P. Evaluation of the Entech Canister System,
2000. Unpublished.
- Draft method, "Volatile Organic Compounds in Air, GC/MS." Steven P.
Sanders, 3M Corporation, 2001.
- Certified laboratories which will analyze Entech Canisters on a
fee-for-analysis basis include Galson Laboratories, East Syracuse, NY,
and Aerotech Laboratories, Inc., Phoenix, AZ.
|

