1. General Discussion
1.1 Background
1.1.1 History
A request from an
OSHA industrial hygienist for a sampling procedure for l -thyroxine was received at SLTC. l -Thyroxine is a drug used to treat dysfunctions
of the thyroid gland. l -Thyroxine is
prescribed in dosages ranging from 25 to 300 µg, depending on the
person’s weight and the ability of their thyroid gland tofunction. A
target concentration of 30 µg/m³ was based on the maximum recommended
dosage of 300 µg and 10 m³, which is the amount of air the average
person breathes in eight hours. Other hormones have been collected on
glass fiber filters and analyzed by liquid chromatography (LC) using
an ultraviolet detector (see PV2001 Estradiol, etc.)1
A C18 column with a mobile phase 80:20:0.2 methyl alcohol:DI
water:phosphoric acid at 1mL/min gave a good separation of the l -thyroxine peak from the methyl alcohol. The
samples were extracted with methyl alcohol, and had extraction
efficiencies averaging 100.2% for the concentration range of 0.72 to
14.4 µg/filter. The retention efficiency study showed no l -thyroxine on the back up filter for two filters
in series that had front filters spiked with 14.4 µg, and had 240-L
(at 1 L/min average recovery of 100.4%) or 480-L (at 2 L/min average
recovery of 99.5%) humid air drawn through them. The storage study
showed no loss for samples stored for up to 14 days under both
refrigerated and ambient conditions.
1.1.2 Toxic effects (This
section is for information only and should not be taken as the basis
of OSHA policy.)2
l -Thyroxine is used to treat thyroid gland
deficiencies. Overdose symptoms include weight loss, palpitations,
nervousness, diarrhea or abdominal cramps, sweating, tachycardia,
cardiac arrhythmias, angina pectoris, tremors, headache, insomnia,
intolerance to heat, and fever. Prolonged or high overexposure may
result in death due to cardiac arrhythmia or failure.
1.1.3
Workplace exposure3
l -Thyroxine and l
-thyroxine sodium are prescribed for over 9 million patients in the
United States. Most tablets consumed in the United States are produced
in the United States. Some tablets are exported to other countries. No
current data could be found for the number of workers exposed to l -thyroxine in the manufacturing process, as the
number of companies manufacturing it is ever-changing due to recent
FDA regulations. In August 1997, FDA announced that all l -thyroxine sodium products were considered new
drugs and must undergo the approval process for new drugs even though
they were currently being sold. At the time this method was written
there were only three forms of l -thyroxine
sodium that had completed that process, though old stocks of
unapproved formulations we still being used.
1.1.4 Physical
properties and other descriptive information4,5
| CAS number: |
51-48-9 |
IMIS:6
|
L200 |
| molecular weight: |
776.93 |
melting point: |
223ºC (decomposes) |
appearance:
|
off-white to beige solid |
molecular formula: |
C15H11I4NO4
|
solubility:
|
water, alcohol, acetone |
|
|
synonyms:
|
Levothyroxine; l -T4; T4 (hormone); tetraiodothyronine;
3,3',5,5'-tetraiodo-l -thyronine;
l -thyroxin; Thyroxinal; Thyroxine;
Thyreoideum
|
structural formula:
|
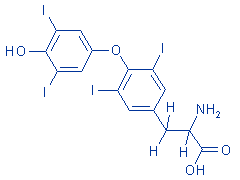 |
|
| I-Thyroxine sodium
pentahydrate7
is the alternate form sold in many pharmaceutical
formulations
| CAS: |
6106-07-6 |
MW: |
888.94 |
| appearance: |
off-white solid |
|
|
| solubility: |
water, alcohol, acetone |
|
|
| synonyms: |
Dathroid;
Eltroxin; Euthyrox; Laevoxin; Levaxin; Levoroxine;
Levothyroxine sodium; monosodium thyroxine; sodium l -thyroxine; synthroid; Synthroid R;
3,3',5,5'-tetraiodo-Lthyronine, sodium salt; Thyroxevan;
Unithroid |
|
|
structural
formula:
|
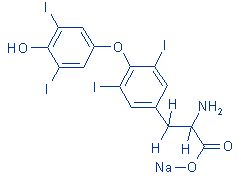
|
|
This method was evaluated according to the OSHA SLTC
"Evaluation Guidelines for Air Sampling Methods Utilizing Chromatographic
Analysis"8.
The Guidelines define analytical parameters, specify required laboratory
tests, statistical calculations and acceptance criteria. The analyte air
concentrations throughout this method are based on the recommended
sampling and analytical parameters.
1.2 Detection limit of the overall
procedure (DLOP) and reliable quantitation limit (RQL)
The DLOP
is measured as mass per sample and expressed as equivalent air
concentrations, based on the recommended sampling parameters. Ten
samplers were spiked with equal descending increments of analyte, such
that the highest sampler loading was 1.06 µg of l -thyroxine. This is the amount spiked on a sampler
that would produce a peak at least 10 times the response for a sample
blank. These spiked samplers were analyzed with the recommended
analytical parameters, and the data obtained used to calculate the
required parameters (standard error of estimate (SEE) and slope) for the
calculation of the DLOP. The slope was 5.18E4 and the SEE was 1169. The
RQL is considered the lower limit for precise quantitative measurements.
It is determined from the regression line parameters obtained for the
calculation of the DLOP, providing 75% to 125% of the analyte is
recovered. The DLOP and RQL were 0.0677 µg and 0.266 µg respectively.
The recovery at the RQL was 99.5%.
Table 1.2
Detection Limit of the Overall
Procedure for l -thyroxine
|
| mass per
sample (µg) |
area counts
(µV·s) |
|
0.00
0.106
0.212
0.318
0.424
0.530
0.636
0.742
0.848
0.954
1.060
|
0
3806
8629
13406
19021
25092
30573
35053
43231
48901
52931
|
| |
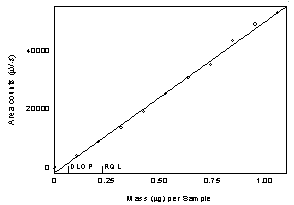
Figure
1.2. Plot of data to determine the DLOP/RQL for l - thyroxine at 230 nm. (y=5.18E4x-1927;
SEE=1169) |
Below is the
chromatogram of the RQL level.
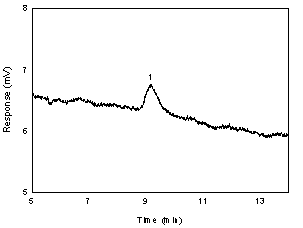
Figure
1.2.2. Chromatogram of the l
- thyroxine peak in
a standard near the RQL at 230 nm. (1=l-thyroxine)
| 2. Sampling
Procedure
All safety practices that apply to the work area being
sampled should be followed. The sampling equipment should be attached to
the worker in such a manner that it will not interfere with work
performance or safety.
2.1 Apparatus
2.1.1 Samples are collected using a
personal sampling pump calibrated, with the sampling device attached,
to within ±5% of the recommended flow rate.
2.1.2 Samples are
collected with three-piece polystyrene cassettes containing a
binderless A/E glass fiber filter. For this evaluation glass fiber
filters were purchased from SKC, Inc. (catalog no. 225-7).
2.2 Reagents
None required.
2.3
Technique
2.3.1 Immediately before sampling,
remove the end plugs from the cassette.
2.3.2 Attach the
cassette to the sampling pump so that it is in an approximately
vertical position with the inlet facing up during sampling. Position
the sampling pump, cassette and tubing so it does not impede work
performance or safety.
2.3.3 Air being sampled should not pass
through any hose or tubing before entering the cassette.
2.3.4
After sampling for the appropriate time, remove the cassette, and
replace the top and end plug. Wrap each sample end-to-end with a Form
OSHA-21 seal.
2.3.5 Submit at least one blank sample with each
set of samples. Handle the blank sample in the same manner as the
other samples except draw no air through it.
2.3.6 Record
sample volumes (in liters of air) for each sample, along with any
potential interferences.
2.3.7 Ship any bulk samples separate
from the air samples.
2.3.8 Submit the samples to the
laboratory for analysis as soon as possible after sampling. If delay
is unavoidable, store the samples in a refrigerator.
2.4 Extraction efficiency
The
extraction efficiency of l -thyroxine was
determined by liquid-spiking glass fiber filters with the analyte at 0.1
to 2 times the target concentration. These samples were stored overnight
at ambient temperature and then extracted and analyzed. The mean
extraction efficiency over the studied range was 100.2%. The wet
extraction efficiency was determined at 1 times the target concentration
by liquid spiking the analyte onto glass fiber filters which had 240-L
humid air (absolute humidity of 15.9 mg/L of water, about 80% relative
humidity at 22.2ºC) drawn through them at 1 L/min immediately before
spiking. The mean recovery for the wet samples was
99.8%.
Table 2.4
Extraction
Efficiency (%) of l -Thyroxine
|
| level |
sample number |
|
| × target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
5 |
6 |
mean |
|
0.1
0.25
0.5
1.0
1.5
2.0
1.0
(wet) |
0.72 1.8
3.6
7.2
10.8 14.4
7.2 |
101.5 99.9 101.4 100.9
101.1 100.4
99.1 |
99.4 100.3 100.3 99.2
100.1 100.2
101.0 |
100.9 101.2 100.4 101.5
100.4 99.3
99.9 |
98.7 100.1 99.5 101.0
99.9 100.6
100.1 |
99.1 99.8 99.9 101.4
100.7 99.9
98.7 |
99.6 98.9 99.3 100.8
100.2 100.6
100.0 |
99.9 100.0 100.1 100.8
100.4 100.2
99.8 |
|
2.5 Retention
efficiency
Six glass fiber filters were spiked with 14.4 µg (60
µg/m³)of l -thyroxine and allowed to
equilibrate for 4 h. Each spiked filter was placed in a cassette and
placed in series with a cassette containing an unspiked glass fiber
filter. Each sampling train had 240-L humid air (absolute humidity of
15.9 mg/L of water, about 80% relative humidity at 22.2ºC) pulled
through them at 1 L/min. The samples were extracted and analyzed. The
mean recovery was 100.4%. There was no analyte found on the back
filters.
Table
2.5.1
Retention Efficiency (%) of l
-Thyroxine Sampled at 1 L/min
|
|
sample
number |
|
| section |
1 |
2 |
3 |
4 |
5 |
6 |
mean |
|
spiked filter
back up
filter
total |
100.8
0.0
100.8 |
101.3
0.0
101.3 |
99.8
0.0
99.8 |
100.2
0.0
100.2 |
99.5
0.0
99.5 |
100.6
0.0
100.6 |
100.4 0.0 100.4 |
|
A test to see if a
higher sampling rate could be used was conducted by spiking six glass
fiber filters with 14.4 µg (60 µg/m³) of l
-thyroxine and allowing them to equilibrate for 4 h. Each spiked filter
was placed in a cassette and placed in series with a cassette containing
an unspiked glass fiber filter. Each sampling train had 480-L humid air
(absolute humidity of 15.9 mg/L of water, about 80% relative humidity at
22.2ºC) pulled through them at 2 L/min. The samples were extracted and
analyzed. The mean recovery was 99.4%. There was no analyte found on the
back filters.
Table
2.5.2
Retention Efficiency (%) of l
-Thyroxine Sampled at 2 L/min
|
|
sample
number |
|
| section |
1 |
2 |
3 |
4 |
5 |
6 |
mean |
|
spiked filter
back up
filter
total |
100.5
0.0
100.5 |
97.6
0.0
97.6 |
100.6
0.0
100.6 |
99.4
0.0
99.4 |
100.2
0.0
100.2 |
98.9
0.0
98.9 |
99.5
0.0
99.5 |
|
2.6 Sample
storage
Fifteen glass fiber filters were each spiked with 7.2 µg
(30 µg/m³) of l -thyroxine. They were allowed
to equilibrate for 4 h, then 240-L of air, with an absolute humidity of
15.7 milligrams of water per liter of air (about 80% relative humidity
at 23ºC), was drawn through them. Three samples were analyzed
immediately. The rest of the samples were sealed, and six were stored at
room temperature (23ºC), while the other six were stored at refrigerated
temperature (4ºC). Three samples stored at room temperature and three
samples stored at refrigerated temperature were analyzed after 7 days
and the remaining samples after 14 days. The amounts recovered indicate
storage was stable at both temperatures for the time period studied.
Table 2.6
Storage Test
for l -thyroxine
|
time
(days) |
ambient
storage
recovery (%) |
|
refrigerated
storage
recovery (%) |
|
0
7
14 |
101.3
100.8
101.4 |
100.4
100.5
101.1 |
101.5
101.8
100.1 |
|
101.3
101.6
100.4 |
100.4
101.1
101.3 |
101.5
100.9
101.5 |
|
2.7 Recommended air
volume and sampling rate
Based on the data collected in this
evaluation, 240-L air samples should be collected at a sampling rate of
1 L/min for 240 minutes, and for greater sensitivity a higher sampling
rate of 2 L/min may be used for 240 minutes for a 480-L air
sample.
2.8 Interferences (sampling)
2.8.1 There are no known compounds
which will severely interfere with the collection of l -thyroxine. Tablets of l -thyroxine usually contain a filler. If high
loadings of l -thyroxine or
l -thyroxine and filler are
expected, sample for less time to avoid clogging the filter with the
filler.
2.8.2 Suspected interferences should be reported to the
laboratory with submitted samples.
3. Analytical Procedure
Adhere to the rules
set down in your Chemical Hygiene Plan. Avoid skin contact and inhalation
of all chemicals and review all appropriate MSDSs.
3.1 Apparatus
3.1.1 A liquid chromatograph equipped
with a UV detector. For this evaluation, a Waters 600 Controller and
pump were used, with a Waters 2487 Dual Wavelength Absorbance
Detector, and a Waters 717 Plus Autosampler was used in this
evaluation.
3.1.2 An LC column capable of separating l -thyroxine from the extraction solvent and any
potential interferences. A 4.6 × 250 mm column packed with 5-µm
Bakerbond C18 (JT Baker, Phillipsburg, NJ) was used in this
evaluation.
3.1.3 An electronic integrator or some other
suitable means of measuring peak areas. A Waters
Millennium32 Data System was used in this
evaluation.
3.1.4 Glass vials with
poly(tetrafluoroethylene)-lined caps. For this evaluation 4-mL vials
were used.
3.1.5 A dispenser capable of delivering 2.0 mL of
extracting solvent to prepare standards and samples. If a dispenser is
not available, a 2.0-mL volumetric pipet may be used.
3.1.6
Volumetric flasks - 10-mL and other convenient sizes for preparing
standards. 3.2 Reagents
3.2.1 l
-Thyroxine sodium. Spectrum 99-103% (lot QK0730) was used in this
evaluation.
3.2.2 Methyl alcohol, HPLC grade. Fisher 99.9% (lot
023066) was used for this evaluation.
3.2.3 Deionized water (DI
water). A Barnstead NANOpure Diamond water deionizer was used in this
evaluation.
3.2.4 Mobile phase was 80:20:0.2 methyl alcohol: DI
water:phosphoric acid. 3.3
Standard preparation
3.3.1 Freshly prepare two stock
standards. A stock standard may be prepared by weighing out 28.8 mg of
l -thyroxine in a 10-mL flask, then fill to
the mark with methyl alcohol.
3.3.2 Diluted standards are
prepared by serial dilution with methyl alcohol. Bracket sample
concentrations with working standard concentrations. If sample
concentrations are higher than the concentration range of prepared
standards, either analyze higher standards, or dilute the sample. The
higher standards should be at least as high in concentration as the
highest sample. The range of standards used in this study was from 0.1
to 28.8 µg/mL. 3.4 Sample
preparation
3.4.1 Open the cassette and carefully
transfer the glass fiber filter to a labeled 4-mL vial. Wipe the
inside of the cassette with a glass fiber filter and place into a
separate labeled 4-mL vial.
3.4.2 Add 2.0 mL of methyl alcohol
to each vial using the same dispenser as used for preparation of
standards or use a volumetric pipet.
3.4.3 Immediately seal the
vials with poly(tetrafluoroethylene)-lined caps.
3.4.4 Shake
the vials on a shaker, or rotate on a rotator, for 30 minutes.
3.5 Analysis
3.5.1 HPLC conditions
column:
|
Bakerbond C18 column 4.6
× 250 mm |
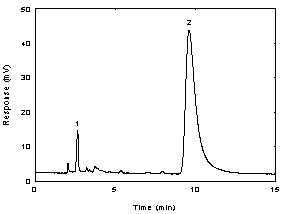
Figure
3.5.1. A chromatogram of 14.4 ug/mL l
- thyroxine in methyl alcohol at 230 nm. Key:(1) methyl
alcohol; (2) l -
thyroxine.
|
| injection size: |
10 µL |
mobile
phase:
|
1 mL/min 80:20:0.2
methyl alcohol: water: phosphoric acid |
| detector: |
UV at 230 and 254
nm |
| run time: |
15 min
|
retention
times:
|
2.6 min methyl
alcohol
9.8 min l -thyroxine
|
|
|
|
|
3.5.2
Peak areas are measured by an integrator or other suitable
means.
3.5.3 An external standard (ESTD) calibration
method is used. A calibration curve can be constructed by
plotting response of standard injections versus micrograms of
analyte per sample. Bracket the samples with freshly prepared
analytical standards over a range of concentrations. |
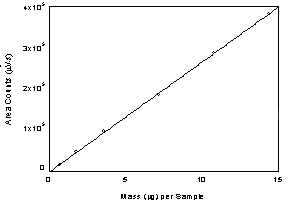
Figure
3.5.2. Calibration curve of l - thyroxine at 230 nm.
(Y=2.74E4x - 1.2.3E4) | 3.6 Interferences (analytical)
3.6.1 Any compound that produces an LC
response and has a similar retention time as the analyte is a
potential interference. If any potential interferences were reported,
they should be considered before samples are extracted. Generally,
chromatographic conditions can be altered to separate an interference
from the analyte.
| 3.6.2 When necessary,
the identity or purity of an analyte peak may be confirmed by a
photodiode array scan of the peak, by wavelength ratioing, or by
LC/mass spec. |
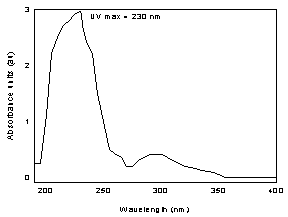
Figure 3.6.2. The UV spectrum of l - thyroxine in methyl
alcohol | 3.7
Calculations
The amount of analyte per sampler,
including the amount found in the cassette wipe, is obtained from the
appropriate calibration curve in terms of micrograms per sample,
uncorrected for extraction efficiency. This total amount is then
corrected by subtracting the total amount (if any) found on the blank.
The air concentration is calculated using the following formulas.
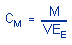 |
where |
CM is
concentration by weight (mg/m³)
M
is micrograms per sample
V is
liters of air sampled
EE is extraction efficiency,
in decimal form | 4. Recommendations for further study
Collection, reproducibility, and other
detection limit studies need to be performed to make this a validated
method.
1OSHA Sampling and
Analytical Methods. http://www.osha-slc.gov/ (accessed
6/24/2002).
2Arky, R., Physicians’ Desk Reference, Medical Economics Company:
Montvale, NJ, 1997, p 1015.
3WebMD.
http://www.webmd.com (accessed 6/25/02).
4Lewis, R., Sax’s Dangerous Properties
of Industrial Materials, Van Nostrand Reinhold: New York, 2002, p
176.
5Cheminfo
http://ccohs.ca/products/databases/cheminfo.html (accessed
5/21/2002).
6OSHA Chemical Sampling
Information
http://www.osha.gov (accessed 5/21/2002).
7MSDS Sigma-Aldrich http://www.sigmaaldrich.com (accessed
5/21/2002).
8Burright, D.; Chan, Y.; Eide,
M.; Elskamp, C.; Hendricks, W.; Rose, M. C.
Evaluation Guidelines for Air Sampling Methods
Utilizing Chromatographic Analysis;
OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake City,
UT, 1999.
|

