1. General Discussion
1.1 Background
1.1.1 History of procedure
This evaluation was
undertaken because the OSHA Salt Lake Technical Center laboratory
received samples requesting the analysis of methidathion. This report
describes the analytical method developed for the sampling and the
analysis of methidathion.
1.1.2 Toxic effects (This section is
for information only and should not be taken as the basis of OSHA
policy.)
Methidathion is a hydrazine derivative (thiadiazole).
Hydrazine derivatives retain some of hydrazine's toxicity. This is the
basis for perhaps the fastest growing use of hydrazine derivatives as
pesticides in agriculture. Hundreds, perhaps thousands, of hydrazine
derivatives have been suggested or patented for pesticidal
applications. (Ref.5.1) Methidathion is also an organophosphate
(cholinesterase-inhibiting) insecticide and its toxicity is related to
the organophosphate properties.
Symptoms of acute
exposure
Symptoms of organophosphate poisoning include
headache, giddiness, blurred vision, weakness, nausea, diarrhea,
cramps, discomfort in the chest, nervousness, sweating, miosis
(pinpoint), tearing, salivation, uncontrollable muscle twitches,
convulsions, coma, and loss of reflexes and sphincter control. If
swallowed and aspirated into lungs, chemical pneumonia can occur.
(Ref. 5.2)
Chronic toxicity studies
In long term feeding
studies with mice, rats and dogs, high doses of Methidathion Technical
caused hepatotoxic effects. In mice, lifetime feeding at high doses
produced liver tumors. In a reproductive study with rats,
cholinesterase inhibition caused impaired mating performance,
decreased pup body weights and decreased pup viability at the highest
dose level administered. Methidathion was not teratogenic in rats or
rabbits, and various mutagenicity studies show that methidathion is
not genotoxic. (Ref. 5.2)
1.1.3 Potential workplace
exposure
Methidathion is an insecticide and acaricide. It is
used to control alfalfa weevils and certain other insects in alfalfa,
scales in citrus, spider mites, bollworms, budworms, lygus bug, pink
bollworms, and whiteflies in cotton. It is also used on sunflower,
artichokes, apples, almonds, cherries, apricots, pears, nectarines,
plums, prunes, walnuts, peaches and pecans. (Ref. 5.3) There was no
information available on the number of workers potentially exposed to
methidathion.
1.1.4 Physical properties (Refs. 5.3 and 5.4
unless otherwise noted)
| Chemical name: |
O,O-dimethyl phosphorodithioate, S-ester
with 4-(mercaptomethyl)-2-methoxy- 21,3,4-thiadiazolin-5-one; Phosphorodithioic
acid O,O-dimethyl ester S-ester with
4-(mercaptomethyl)-2-methoxy- 21,3,4-thiadiazolin-5-one; Phosphorodithioic
acid O,O-dimethyl ester S-ester with
4-(mercaptomethyl)-2-methoxy- 21,3,4-thiadiazolin-5-one; dithiophosphoric acid
O,O'-dimethyl-S-[(2-methoxy-1,3,4-thiadiazol-5(4H)-on-4-yl)-methyl]
ester; dithiophosphoric acid
O,O'-dimethyl-S-[(5-methoxy-1,3,4-thiadiazol-2(3H)-one-3-yl)methyl]
ester;
O,O'-dimethyl-S-[(2-methoxy-1,3,4-thiadiazole-5(4H)-one-4-yl)methyl]
dithiophosphate 21,3,4-thiadiazolin-5-one; dithiophosphoric acid
O,O'-dimethyl-S-[(2-methoxy-1,3,4-thiadiazol-5(4H)-on-4-yl)-methyl]
ester; dithiophosphoric acid
O,O'-dimethyl-S-[(5-methoxy-1,3,4-thiadiazol-2(3H)-one-3-yl)methyl]
ester;
O,O'-dimethyl-S-[(2-methoxy-1,3,4-thiadiazole-5(4H)-one-4-yl)methyl]
dithiophosphate |
| Common name: |
Methidathion |
| Synonyms: |
Ultracide; Supracide; somonil; Fisons NC
2964; DMTP; ENT 27193 |
| CAS number: |
950-37-8 |
| Molecular formula: |
C6H11N2O4PS3 |
Structural formula: |
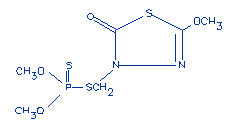 |
| Molecular weight: |
302.31 |
| Melting point: |
39-40°C |
| Vapor pressure: |
3.33 x 10-7 kPa (2.5 x
10-6 mmHg) at 25°C (technical) |
| Solubility: |
Readily soluble in benzene, acetone,
methanol, xylene and other organic solvents; solubility in water
less than 1% |
| Description: |
Colorless crystals |
| Stability: |
Relatively stable to hydrolysis in
neutral or slightly acidic media, less stable in more acidic (pH 1)
or alkaline media (pH 13, 50% loss in 30 min @ 25°C.) (Ref.
5.5) |
|

