| Method number: | PV2010 |
| Matrix: | Air |
| Target Concentration: | 800 ppm (1900 mg/m3) ACGIH threshold limit value (TLV). |
| Procedure: | Samples are collected by drawing known volumes of air through two Carbosieve S-III tubes in series. Samples are desorbed with desorbing solution (carbon disulfide/internal standard) and analyzed by gas chromatography (GC) using a flame ionization detector (FID). |
| Recommended air volume and sampling rate: |
3 L at 0.05 L/min |
| Detection limit of the overall procedure (based on the recommended air volume and the analytical detection limit): |
0.79 ppm (1.88 mg/m3) |
| Status of method: | Partially evaluated method. This method has been partially evaluated and is presented for information and trial use only. |
| August 1993 (Final) | Chemist: Ing-Fong Chan |
Organic Service Branch II
OSHA Technical Center
Salt
Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
This evaluation was undertaken to develop a sampling and analytical procedure for butane at the ACGIH TLV 800 ppm level (Ref. 5.1.).
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
Butane is a simple asphyxiant and irritant. It is an anesthetic at high concentrations. (Ref. 5.2. and Ref. 5.3.)
1.1.3. Potential workplace exposure
The following paragraph is excerpted from the book Kirk-Othmer Encyclopedia of Chemical Technology, (Ref. 5.2.)
Butanes are used primarily as gasoline blending components and less so as liquefied fuel and in the manufacture of chemicals.n-Butane and small amounts of isobutane are used for direct blending in motor-fuel gasoline to control the volatility of the finished product. The butane content of motor gasolines is ca6-8 vol%. United States consumption ofn-butane for this purpose was ca 84% of supply in 1977. --- Nonmotor-fuel uses of butanes represent ca 10% of the total consumption. Liquid petroleum gas (LPG) is a mixture of butane and propane, typically in a ratio of 60:40 butane-propane. LPG is consumed as fuel in engines and in home, commercial, and industrial applications. --- Butane also is used as a solvent in liquid-liquid extration of heavy oils in deasphalting processes (Ref. 5.2.). No data is available on the extent of work place exposure.
1.1.4. Physical properties (Ref. 5.2. and 5.3.)
| CAS number: | 106-97-8 |
| IMIS number: | 0420 |
| Molecular weight: | 58.12 |
| Molecular formula: | C4H10 |
| Boiling point: | -0.5°C at 101.3 kPa (760 mmHg) |
| Flash point: | -138°C at 101.3 kPa (760 mmHg) |
| Autoignition point: | 420°C at 101.3 kPa (760 mmHg) |
| Explosive limit: | 1.8-8.4% by volume |
| Vapor density: | 2.046 (air = 1) |
| Appearance: | colorless, and flammable gas |
| Structure: | CH3CH2CH2CH3 |
1.2. Limit defining parameters
The detection limit of the analytical procedure, including a 15:1 split ratio, is 0.333 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by using a personal sampling pump
that can be calibrated to within ± 5% of the recommended flow rate
with the sampling device in line.
2.1.2. Samples are collected with two Carbosieve
2.2. Reagents
No sampling reagents are required.
2.3. Sampling technique
- 2.3.1. The ends of the Carbosieve S-III tubes are opened
immediately before sampling.
2.3.2. Connect two Carbosieve S-III tubes in series and connect the second tube to the sampling pump with flexible tubing.
2.3.3. Attach the sampler vertically in the employee's breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the sampling device and seal the tube with plastic end caps.
2.3.5. Wrap each sample end-to-end with an OSHA Form-21 seal.
2.3.6. Record the air volume for each sample and list any possible interferences.
2.3.7. Submit at least one blank with each set of samples. Handle the blank in the same manner as the other samples, except that no air is drawn through it.
2.3.8. If bulks are submitted for analysis, they must be shipped in a container separate from the samples.
2.4. Desorption efficiency
An amount of adsorbent equal to the sampling section (130 mg) of a
Carbosieve
Desorption Efficiency
|
| |||
Sample # |
Amount Spiked, mg |
Amount Found, mg |
% Recovered |
| D1 D2 D3 |
0.564 0.564 0.564 |
0.599 0.606 0.617 |
106.2 107.4 109.4 |
| Average of 0.1× TLV = 107.7% | |||
| D4 D5 D6 |
2.926 2.926 2.926 |
2.940 3.046 3.088 |
100.5 104.1 105.5 |
| Average of 0.5× TLV = 103.4% | |||
| D7 D8 D9 |
5.643 5.643 5.643 |
5.768 5.772 5.716 |
102.2 102.3 101.3 |
| Average of 1.0× TLV = 101.9% | |||
| D10 D11 D12 |
10.450 10.450 10.450 |
10.698 10.356 10.954 |
102.4 99.1 104.8 |
| Average of 2.0× TLV = 102.1% | |||
|
| |||
2.5. Retention efficiency
Three Carbosieve S-III tubes were each vapor spiked with 2.7 mL (1×
TLV) of butane. Five tubes were each vapor spiked with 5.0 mL (2× TLV)
of butane. The tubes were allowed to equilibrate overnight in a drawer
at room temperature. Each tube was connected in series with another
Carbosieve
Retention Efficiency
|
| ||||||
| % Recovered | ||||||
| Front tube | Second tube | |||||
| Sample # | 'A' | 'B' | 'A' | 'B' | Total | |
| RE1 | 98.9 | 0.0 | 0.0 | 0.0 | 98.9 | |
| RE2 | 88.7 | 12.2 | 0.0 | 0.0 | 100.9 | |
| RE3 | 90.8 | 8.3 | 0.0 | 0.0 | 99.1 | |
| ||||||
| R1 | 95.4 | 8.6 | 0.0 | 0.0 | 104.0 | |
| R2 | 72.5 | 19.3 | 14.9 | 0.0 | 106.7 | |
| R3 | 74.1 | 17.7 | 8.2 | 0.0 | 100.0 | |
| R4 | 67.0 | 22.8 | 16.5 | 0.0 | 106.3 | |
| R5 | 64.8 | 25.3 | 10.2 | 0.0 | 100.3 | |
| ||||||
|
| ||||||
2.6. Sample storage
Eight Carbosieve S-III tubes were each vapor spiked with 2.7 mL (1× TLV) of butane. The tubes were allowed to equilibrate overnight in a drawer at room temperature. Each tube was connected in series with another Carbosieve S-III tube. Three liters of humid air (~80% relative humidity) were drawn through each tube at 0.05 L/min. The eight tubes were divided into two groups of four each. The first group was stored in a drawer at ambient temperature, and the second group was stored in a freezer (-5°C). After seven days they were extracted and analyzed as in Section 3. No analyte was observed in backup sections of the second tubes. The results are given in Tables 2.6.1. and 2.6.2.
Ambient Storage
|
| |||
| Days Stored |
Amount Spiked, mg |
Amount Found, mg |
% Recovered |
| 7 7 7 7 |
5.643 5.643 5.643 5.643 |
5.333 5.512 5.097 4.754 |
94.5 97.7 90.3 84.2 |
| Average = 91.7% | |||
|
| |||
Freezer Storage
|
| |||
| Days Stored |
Amount Spiked, mg |
Amount Found, mg |
% Recovered |
| 7 7 7 7 |
5.643 5.643 5.643 5.643 |
5.506 5.564 5.544 5.427 |
97.6 98.6 98.2 96.2 |
| Average = 97.7% | |||
|
| |||
2.7. Recommended air volume and sampling rate
- 2.7.1. The recommended air volume is 3 L.
2.7.2. The recommended flow rate is 0.05 L/min.
2.8. Interferences (sampling)
It is not known if any compounds will interfere with the collection of butane. Any suspected interferences should be reported to the laboratory with submitted samples.
2.9. Safety precautions (sampling)
- 2.9.1. Attach the sampling equipment in such a manner that it
will not interfere with work performance or employee safety.
2.9.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with an FID. A Hewlett-Packard 5890A GC
equipped with both an FID and a
3.1.2. A GC column capable of separating butane from any interferences. A 60 m × 0.32 mm i.d. (1.0 µm film) DB-1 capillary column was used in this evaluation.
3.1.3. An electronic integrator or some other suitable means to measure detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4. Volumetric flasks, pipets, and syringes for preparing standards, making dilutions and performing injections.
3.1.5. Vials, 2-mL with PTFE-lined caps.
3.2. Reagents
- 3.2.1. Hydrogen, air and nitrogen, GC grade.
3.2.2. Butane. The butane used in this evaluation was 99.9% pure and purchased from Matheson CO., Inc.
3.2.3. Carbon disulfide. Reagent grade or better carbon disulfide should be used. The carbon disulfide used in this evaluation was purchased from EM Science.
3.2.4. p-Cymene. The p-Cymene used as an internal standard (ISTD) was purchased from Aldrich Chemical Company Inc.
3.2.5. Desorbing solution. The desorbing solution is prepared by adding 250 µL of p-Cymene to 1 L of carbon disulfide.
3.3. Standard preparation
- 3.3.1. Standards are prepared by diluting a known quantity of
butane with the desorbing solution. A standard of 1000 µL/ml butane
in the desorbing solution at 664 mmHg and 23°C would be 2.09 mg/mL.
This was calculated as follows:
| (1000 µL)(P)(298°K)(µ
mole)(58.12 µg)(1 mg)
(mL)(760 mm)(T)(24.45 µL)(µ mole)(1000 µg) |
= 2.09 mg/mL |
| P T |
= = |
Pressure at time of standard preparation = 664
mmHg. Temperature at time of standard preparation = 296°K. |
3.3.2. At least two separate standards should be made. A third standard at higher concentration should be prepared to check the linearity of the detector response for butane.
3.4. Sample preparation
- 3.4.1. The front and back sections of each tube are placed in
separate 2 mL vials.
3.4.2. Each section is desorbed with 1.0 mL of the desorbing solution.
3.4.3. The vials are sealed immediately with PTFE-lined septa and allowed to desorb for 30 minutes with occasional shaking.
3.5. Analysis
- 3.5.1. Instrument conditions
| Column: | DB-1, 60 m × 0.32 mm i.d., 1.0 µm film |
| Head pressure: | 8.5 psi |
| Injector temperature: | 150°C |
| Detector temperature: | 250°C |
| Column temperature: | 50°C (initial temp.) |
| Temperature program: | hold initial temp. 5 min, increase temp. at 10°C/min to 170°C, hold final temp. 1.5 min |
| Dectector gas flow: | |
| hydrogen flow rate: | 30 mL/min |
| air flow rate: | 240 mL/min |
| nitrogen flow rate: | 30 mL/min |
| Injection volume: | 1 µL |
| Split ratio: | 15:1 |
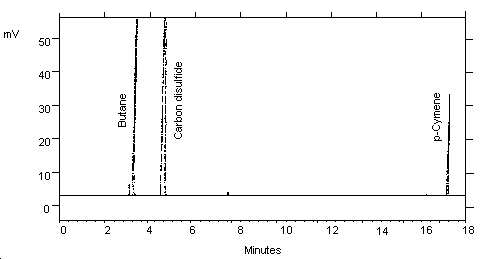
| Retention time: | 3.4 min (Butane) 17.3 min (p-Cymene) |
3.5.2. Chromatogram (See Figure 1.)
3.5.3. Measure detector response using a suitable method such as electronic integration.
3.6. Interferences (analytical)
- 3.6.1. Any collected compound which produces an FID response and
has a similar retention time as butane or the internal standard is a
potential interference.
3.6.2. GC conditions may generally be varied to circumvent interferences.
3.6.3. Retention time on a single column is not proof of chemical identity. Analysis by an alternate GC column, and confirmation by mass spectrometry are additional means of identification.
3.7. Calculations
- 3.7.1. An ISTD calibration method is used. The linear nature of
FID allows the use of single level calibration, but bracketing of
samples with analytical standards is a good practice.
3.7.2. Determine the µg/mL of butane in both sections of each sample and blank from the calibration curve. If butane is found on the backup section, it is added to the amount found on the front section. Blank corrections should be performed before adding the results together.
3.7.3. Determine the air concentration by using the following formulae.
| mg/m3 = | (µg/mL, blank corrected) ×
(desorption volume, mL)
(air volume, L) × (desorption efficiency, decimal) |
| ppm = | (mg/m)(24.45)
(58.12) |
| where | 24.45 58.12 |
= = |
molar volume (liters) at 101.3 kPa (760 mmHg)
and 25°C molecular weight of butane |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid air exposure to butane.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses in laboratory.
4. Recommendation for Further Study
This method should be fully validated.
5. References
- 5.1. "Threshold Limit Values for Chemical
Substances and Physical Agents and Biological Exposure Indices",
ACGIH., 1991-1992.
5.2. Grayson, M., Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., John Wiley & Sons Inc., New York, 1983; Vol. 12, pp. 910-919.
5.3. Windholz, M., Budavari, S., Blumetti, RF., and Otterbein, E., The Merck Index, 10th ed., Merck & CO., Inc., Rahway, N.J., 1983; p 210.