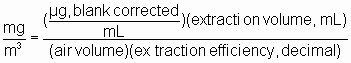|
| Method Number: | PV2067 |
| Control Number: | T-PV2067-01-8911-CH |
| Matrix: | Air |
| Target Concentration: | 1 mg/m3 (arbitrary level). There is no OSHA permissible exposure limit (PEL) or ACGIH threshold limit value (TLV) for glyphosate. |
| Procedure: | Samples are collected by drawing known volumes of air through glass fiber filters. Samples are desorbed with 0.025 M borate buffer, derivatized and analyzed by high performance liquid chromatography (HPLC) using an ultraviolet detector (UV). |
|
Recommended air volume and sampling rate: |
100 L at 1.0 L/min |
|
Detection limit of the overall procedure (based on the recommended air volume): |
1 µg/m3 |
| Status of method: | Stopgap method. This method has been only partially evaluated and is presented for information and trial use. |
| Date: November, 1989 |
Chemist: Duane Lee |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
1.1 Background
1.1.1 History of procedure
The OSHA Analytical Laboratory received samples on glass fiber filters and OVS-2 tubes requesting the analysis of Roundup which is the isopropylamine salt of glyphosate. A NIOSH procedure was tried but it did not yield a satisfactory separation. (Ref. 5.1) From a literature search there were procedures for the analysis of glyphosate in soil and water samples. (Refs. 5.2 to 5.4) These procedures were modified for the analysis of air samples. This report describes the preliminary validation of a sampling and analytical method using glass fiber filters. The OVS-2 tubes were examined but felt to be unnecessary since glyphosate is a solid with a melting point over 200ºC.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The acute oral LD50 for rats is 4300 mg/kg for glyphosate. (Ref. 5.6)
1.1.3 Potential workplace exposure
Glyphosate is used as a non-selective, postemergence herbicide. (Ref. 5.6) No information could be found on the number of workers exposed to glyphosate.
1.1.4. Physical properties (Refs. 5.5 to 5.7)
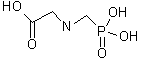
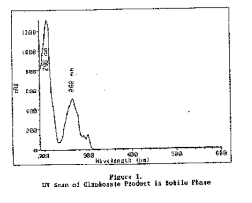
Molecular weight: 169.07 Molecular formula: C3H8NO5P CAS #: 1071-83-6 Melting point: 230ºC (decomposition) Solubility: soluble in water at 25ºC 12 g/L, insoluble in most organic solvents Chemical name: glycine, N-(phosphonomethyl)- Other names: Mon 0573; N-(phosphonomethyl)glycine; phosphonomethyliminoacetic acid Description: white solid Structure: UV scan: 1.2 Limit defining parameters
The detection limit of the analytical procedure is 0.84 ng per injection. This is the amount of analyte which will give a peak whose height is approximately five times the baseline noise.
2. Sampling Procedure
2.1 Apparatus
2.1.1 A personal sampling pump that can be calibrated to within ± 5% of the recommended flow rate with the sampling device in line.
2.1.2 Gelman type A/E 37-mm glass fiber filters. The filters were assembled in two-piece 37-mm polystyrene cassettes with backup pads. The cassettes are sealed with shrink bands and the ends are plugged with plastic plugs.
2.2 Reagents
No sampling reagents are required.
2.3 Sampling technique
2.3.1 Immediately before sampling, remove the plastic plugs from the filter cassettes.
2.3.2 Attach the cassette to the sampling pump with flexible tubing.
2.3.3 Attach the cassette vertically in the employee's breathing zone in such a manner that it does not impede work performance.
2.3.4 After sampling for the appropriate time, remove the cassette and seal with plastic plugs.
2.3.5 Wrap each sample end-to-end with an OSHA seal (Form 21).
2.3.6 Submit at least one blank for each set of samples. Handle the blank in the same manner as the samples, except no air is drawn through it.
2.3.7 Record the air volume (in liters of air) for each sample, and list any possible interferences.
2.3.8 Submit bulk samples for analysis in a separate container.
2.4 Extraction efficiency
Six treated glass fiber filters were each liquid spiked with 20 µL of a 5.22 mg/mL glyphosate standard. These were allowed to dry and placed in a drawer overnight. The next day each filter was extracted with 3.0 mL of 0.025 M borate buffer, shaken for 30 min and then analyzed as per section 3.5. The results are listed in the table below.
Table 2.5
Retention Efficiencyamount
spiked, µgamount
found, µg%
recovered104.4
104.4
104.4
104.4
104.4
104.4100.44
103.46
105.75
109.59
140.94
103.1
average96.2
99.1
101.3
105.0
100.5
98.8
100.2
2.5 Retention efficiency
Six glass fiber filters were liquid spiked with 20 µL of a 5.22 mg/mL standard and humid air (80% relative humidity) was drawn through each filter at 1 L/min for 100 minutes. The filters were extracted with 3 mL of 0.025 M borate buffer, shaken for 30 min and then analyzed as per section 3.5. The results are listed in the table below.
Table 2.4
Extraction Efficiencyamount
spiked, µgamount
found, µg%
recovered104.4
104.4
104.4
104.4
104.4
104.4103.91
87.77
107.5
99.68
107.39
103.34
average99.5
84.1
103.0
95.5
102.9
99.0
97.32.6 Sample storage
Twelve glass fiber filters were liquid spiked with 20 µL of a 5.22 mg/mL standard and humid air (80% relative humidity) was drawn through each filter at 1 L/min for 100 minutes. Six of the samples were stored at ambient temperature in a drawer, and six were stored in a freezer. After four days of storage, three samples from each group were extracted with 3 mL of 0.025 M borate buffer, shaken for 30 min and then analyzed as per section 3.5. The remaining samples were desorbed and analyzed after six days of storage. The results are given in the tables below.
Table 2.6.1
Ambient Storagedays
storedamount
spiked, µgamount
found, µg%
recovered4
4
4
6
6
6104.4
104.4
104.4
104.4
104.4
104.497.49
100.91
100.38
94.18
94.71
95.67
average of 4
average of 693.4
96.7
96.1
90.2
90.7
91.6
99.2
91.6
Table 2.6.2
Freezer Storagedays
storedamount
spiked, µgamount
found, µg%
recovered4
4
4
6
6
6104.4
104.4
104.4
104.4
104.4
104.4103.16
102.89
104.65
96.87
92.40
97.76
average of 4
average of 698.8
98.6
100.2
92.8
88.5
93.6
99.2
91.62.7 Recommended air volume and sampling rate
2.7.1 The recommended air volume is 100 L.
2.7.2 The recommended flow rate is 1.0 L/min.
2.8 Interferences (sampling)
It is not known if any compounds will interfere with the collection of glyphosate.
2.9 Safety precautions (sampling)
2.9.1 Attach the sampling equipment in such a manner that it will not interfere with work performance or employee safety.
2.9.2 Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
3.1 Apparatus
3.1.1 A balance capable of weighing to the nearest tenth of a milligram. A Mettler HL52 balance was used in this evaluation.
3.1.2 Mechanical shaker.
3.1.3 A high performance liquid chromatograph (HPLC) equipped with an ultraviolet (UV) detector. A Hewlett-Packard (HP) 1090M with a diode array detector was used in this evaluation.
3.1.4 An HPLC column capable of separating glyphosate from any interferences. A 25 cm × 4.6 mm i.d. Zorbax NH2 column was used in this evaluation.
3.1.5 An electronic integrator, or some other suitable method for measuring detector response. The Hewlett-Packard 3357 Laboratory Data System and the Hewlett-Packard 1090M system were used in this evaluation.
3.1.6 Volumetric flasks and pipets.
3.1.7 Vials, 4-mL with Teflon-lined caps.
3.1.8 Vials, 2-mL suitable for use on HPLC autosamplers.
3.2 Reagents
3.2.1 Acetonitrile, HPLC grade from Burdick and Jackson.
3.2.2 Glyphosate, Environmental Protection Agency (EPA #3801, 97.3% purity).
3.2.3 Borate, sodium borate (Na2B4O7·10H2O) from Mallinckrodt. The borate buffer was 0.025 M sodium borate with a pH = 9.
3.2.4 HPLC grade water, Milli-Q filtered water, Millipore Inc.
3.2.5 Acetone, high purity solvent from Burdick and Jackson.
3.2.6 9-Fluorenylmethyl chloroformate (FMOCCL), reagent grade obtained from Aldrich. This was made 0.002 M in acetone and used as the derivatizing reagent.
3.2.7 Potassium hydroxide, reagent grade from Baker. This was 7 N in water and used to adjust the pH of the mobile phase.
3.2.8 Potassium phosphate monobasic (KH2PO4), reagent grade from Mallinckrodt.
3.3 Standard preparation
Prepare stock glyphosate standards by weighing 10 to 15 mg of glyphosate. Transfer the glyphosate to separate 10-mL volumetric flasks, and add borate buffer to the mark. Make working range standards of 0.03 to 80 µg/mL by pipet dilutions of the stock standards with borate buffer. This range corresponds to 0.09 to 240 µg per sample when an extraction volume of 3 mL is used. Store stock and dilute standards in a freezer.
3.4 Sample preparation
3.4.1 Transfer the glass fiber filter of each cassette to a 4-mL vial.
3.4.2 Add 3.0 mL of borate buffer to each vial and seal with a Teflon-lined cap.
3.4.3 Shake the vials for 30 minutes on a mechanical shaker.
3.5 Derivatization of samples and standards
3.5.1 Transfer 1 mL of each sample and standard to 4-mL vials.
3.5.2 Add 1.0 mL of 0.002 M FMOCCL to each vial.
3.5.3 Cap the vials and then shake them for 10 to 15 seconds to ensure mixing and allow them to sit at room temperature for 30 min.
3.5.4 Transfer, if necessary, a portion of each sample and standard to separate 2-mL vials for the HP autosampler.
3.6 Analysis
3.6.1 Instrument conditions
Column: 25 cm × 4.6 mm i.d. Zorbax NH2 Mobile phase: 50% acetonitrile 50% water 0.05 M KH2PO4 pH adjusted to 6.0 with 7 N KOH Flow rate: 1.0 mL/min Column temperature: 40ºC Injection volume: 25.0 µL Retention time: 9.6 min Detectors: UV 206 nm Fluorescence excitation = 206 nm emission =
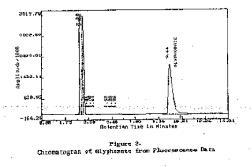
320 nm filter 3.6.2 Chromatogram
3.7 Interferences (analytical)
3.7.1 Any collected compound having a similar retention time and responds to a UV and a fluorescence detector is an interference.
3.7.2 Any compound that reacts with FMOCCL is an interference.
3.7.3 HPLC conditions may be varied to circumvent an interference.
3.7.4 Retention time alone is not proof of chemical identity. Analysis by an alternate HPLC column, ratioing between fluorescence and UV detectors and confirmation by mass spectrometry are additional means of identification.
3.8 Calculations
3.8.1 Construct a calibration curve by plotting detector response versus concentration (µg/mL) of glyphosate.
3.8.2 Determine the µg/mL of glyphosate in each sample and blank from the calibration curve.

3.8.3 Blank correct the samples by subtracting the µg/mL in the blank from each sample.
3.8.4 Use the following formula to determine the air concentration.
3.9 Safety precautions (analytical)
3.9.1 Avoid skin contact and exposure to glyphosate in air.
3.9.2 Avoid skin contact with all solvents.
3.9.3 Wear safety glasses at all times.
4. Recommendation for Further Study
The method should be fully validated.
5. References
5.1 Mosely, C. L. and Anderson, K.; Hazards Evaluations and Technical Assistance Branch, NIOSH, U.S. Department of Health and Human Services; Cincinnati, OH; Report No. HETA-83-341-1557.
5.2 Miles, C. J.; Moye, H. A. J. Agric. Food Chem. 1988, 36(3), 486-491.
5.3 Gauch, R.; Leuenberger, U.; Mueller, U. Z. Lebensm.- Unters. -Forsch. 1989, 188(1), 36-38.
5.4 Miles, C. J.; Wallace, L. R.; Moye, H. A. J. Assoc. Off. Anal. 1986, 69(3), 458-461.
5.5 Registry of Toxic Effects of Chemical Substances 1985-86 Edition; DHHS(NIOSH) Publication No. 87-114, U.S. Department of Health and Human Services: Cincinnati, OH, 1987; p 2551.
5.6 Farm Chemicals Handbook; Berg, Gordon L. Ed.; Meister: Willoughby, OH, 1989; p C147.
5.7 Merck Index, 10th ed.; Windholz, Martha Ed.; Merck: Rahway, NJ, 1983; p 648.