![]()
m-XYLENE-a,a'-DIAMINE (m-XYLYLENEDIAMINE; mXDA)
p-XYLENE-a,a'-DIAMINE (p-XYLYLENEDIAMINE; pXDA)
| Method number: | 105 | |
| Matrix: | Air | |
Target concentration: OSHA PEL: ACGIH TLV: |
mXDA 0.1 mg/m3 (15-min ceiling) None 0.1 mg/m3 (Ceiling) |
pXDA 0.1 mg/m3 (15-min ceiling) None None |
| Procedure: | Samples are collected closed-face by drawing known volumes of air through sampling devices consisting of three-piece cassettes, each containing two sulfuric acid-treated glass fiber filters separated by the ring section. Samples are analyzed by HPLC using an ultraviolet detector. | |
| Recommended air volume and sampling rate: |
15 L at 1.0 L/min | |
Reliable quantitation limit: the target concentration: |
mXDA 0.91 µg/m3 5.1% |
pXDA 1.12 µg/m3 5.1% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | |
| Date: December 1994 | Chemist: Carl J. Elskamp | |
Organic Methods Evaluation Branch
OSHA Salt Lake
Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
1.1 Background
1.1.1 History
There were no methods found in the literature for the determination of airborne levels of xylylenediamines. Sampling procedures evaluated at the OSHA Salt Lake Technical Center (SLTC) for a number of other aromatic amines involve collection with sulfuric acid-treated glass fiber filters. (Refs. 5.1-5.7) For those amines where the target concentrations were in the ppb range, quantitation was performed by analyzing the heptafluorobutyric acid anhydride derivatives by gas chromatography using an electron capture detector in order to achieve high sensitivities. Before the derivatization step is performed, the free amines are extracted from an aqueous system into toluene. Extraction of the xylylenediamines into toluene proved to be impossible because they are too water soluble. Analysis was thus performed by HPLC using paired-ion chromatography. Adequate sensitivity was obtained using an ultraviolet detector. Ion-pairing is necessary because the polar analytes are not otherwise sufficiently retained. A column designed for analysis of basic compounds proved useful in producing symmetrical peaks.
A target concentration of 0.1 mg/m3 with a 15-minute ceiling was chosen for both analytes because of the ACGIH TLV for m-xylene-a,a'-diamine (mXDA). (Ref. 5.8) The analytical procedure has adequate sensitivity for 15-minute samples (15-L, 1 L/min), but if desired, long-term sampling can be also done because the sampler has ample capacity. Samples are stable for at least 15 days, even when stored at room temperature.
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Both mXDA and pXDA are harmful if swallowed, inhaled, or absorbed
through the skin. They are extremely destructive to tissue of the
mucous membranes and upper respiratory tract, eyes and skin.
Symptoms of exposure may include burning sensation, coughing,
wheezing, laryngitis, shortness of breath, headache, nausea and
vomiting.
The following paragraphs are taken directly from the ACGIH Documentation of the Threshold Limit Values concerning the TLV for mXDA. (Ref. 5.8)
Two studies have indicated mXDA to have a rather low oral acute toxicity to the rat (1500 and 930 mg/kg, respectively), however to be strongly irritating to the skin. A dermal LD50 of 2000 mg/kg was found for rabbits. The undiluted compound was corrosive to the skin of guinea pigs, and a 50% emulsion in an acetone-dioxane mixture was severely irritating, but little effect was produced by a concentration of 10%. A 10% aqueous solution, however, caused severe erythema and irritation, yet repeated application of a 5% concentration was needed to produce swelling and redness.
In one study evidence of mild sensitization was found following repeated application to guinea pig skin, but this finding was not duplicated in the second investigation.
Exposure of rats for one hour to an aerosol of mXDA, at measured concentrations ranging from 1.74 to 6.04 mg/liter, resulted in eye irritation, lacrimation and labored breathing. No deaths occurred during exposure, but several animals died within 48 hours, and a few more later, up to 14 days, the end of the observation period. Of the animals which survived, female rats showed reduced weight gain, while that of males was near normal.
At necropsy macroscopic abnormalities were found chiefly in the lungs, however changes in liver and kidneys were also noted. The LC50 for a one-hour exposure and 14-day observation period was 3.75 mg/L, or about 700 ppm.
In comparison with the better known phenylene diamine (q.v.), the dermal effects of mXDA seem similar, but the oral toxicity appears less. By analogy, a ceiling limit of 0.1 ppm [sic], with a skin notation, is retained for the present, however, the Committee is currently reviewing this compound. At this concentration, the compound should be largely in the vapor state.
1.1.3 Workplace exposure
mXDA is used to make polyamide fibers and resins and as a curing agent for epoxy resins. It is also a source of m-xylene diisocyanate. (Refs. 5.8, 5.11) It is assumed that pXDA can be used similarly in industry. In one case an experimental nylon derived from pXDA was tested to improve the flat spotting performance of bias and bias-belted tires. (Ref. 5.12)
1.1.4 Physical properties (Ref. 5.9-5.10)
| mXDA | pXDA | |
| CAS number: | 1477-55-0 | 539-48-0 |
| molecular weight: | 136.20 | 136.20 |
| melting point: | 62-64°C | |
| boiling point: | 265°C at 99.3 kPa | 230°C at 1.3 kPa |
| vapor pressure: | 2.0 kPa at 145°C | |
| specific gravity: | 1.032 | |
| description: | colorless to yellow liquid | yellow crystals |
| synonyms: | m-xylylenediamine;
1,3-bis(aminomethyl)benzene; m-xylene-a,a'-diamine; m-phenylenebis(methylamine) |
p-xylylenediamine;
1,4-bis(aminomethyl)benzene; p-xylene-a,a'-diamine; p-phenylenebis(methylamine) |
| molecular formula: | C8H12N2 | C8H12N2 |
| structural formula: |  |
 |
The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters.
1.2 Limit defining parameters
1.2.1 Detection limit of the analytical procedure
The detection limits of the analytical procedure are 24.4 and 30.7 pg for mXDA and pXDA respectively. These are the amounts of each analyte that will give responses that are significantly different from the background response of a reagent blank. (Sections 4.1 and 4.2)
1.2.2 Detection limit of the overall procedure
The detection limits of the overall procedure are 4.1 ng per sample (0.27 µg/m3) and 5.0 ng per sample (0.33 µg/m3) for mXDA and pXDA respectively. These are the amounts of each analyte spiked on the sampler that will give responses that are significantly different from the background response of a sampler blank. (Sections 4.1 and 4.3)
1.2.3 Reliable quantitation limit
The reliable quantitation limits are 13.6 ng per sample (0.91 µg/m3) and 16.8 ng per sample (1.12 µg/m3) for mXDA and pXDA respectively. These are the amounts of each analyte spiked on a sampler that will give signals that are considered the lower limits for precise quantitative measurements. (Section 4.4)
1.2.4 Precision (analytical procedure)
The precisions of the analytical procedure, measured as the pooled relative standard deviations over a concentration range equivalent to 0.5 to 2 times the target concentration, are 0.37% and 0.93% for mXDA and pXDA respectively. (Section 4.5)
1.2.5 Precision (overall procedure)
The precision of the overall procedure at the 95% confidence level for the ambient temperature 15-day storage test (at the target concentration) is ±10.0% for both mXDA and pXDA. (Section 4.6). These include an additional 5% for sampling error.
1.2.6 Recovery
The recovery of analyte from samples used in a 15-day storage test remained above 95% and 97% for mXDA and pXDA respectively when the samples were stored at ambient temperatures. (Section 4.7)
1.2.7 Reproducibility
Six samples spiked by liquid injection, with a draft copy of this procedure, were submitted to an SLTC service branch for analysis. The samples were analyzed after nine days of storage at 0°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.5. (Section 4.8)
2. Sampling Procedure
2.1 Apparatus
 2.1.1 Samples are collected using a personal sampling pump
calibrated, with a sampling device attached, to within ±5% at the
recommended flow rate.
2.1.1 Samples are collected using a personal sampling pump
calibrated, with a sampling device attached, to within ±5% at the
recommended flow rate.
2.1.2 Samples are collected closed-face using a sampling device
consisting of two sulfuric-acid treated 37-mm Gelman type A/E glass
fiber filters contained in a three-piece polystyrene cassette. The
filters are prepared by soaking each filter with 0.5 mL of 0.26 N
sulfuric acid. (0.26N sulfuric acid can be prepared by diluting 1.5
mL of 36 N sulfuric acid to 200 mL with deionized water.) The
filters are dried in an oven at 100°C and then assembled into
three-piece
2.2 Reagents
None required
2.3 Technique
2.3.1 Remove the plastic end plugs from the sampling device immediately before sampling.
2.3.2 Attach the sampling device to the sampling pump with flexible tubing and place the device in the employee's breathing zone. Position the sampler so it does not impede work performance or safety.
2.3.3 Do not pass the sampled air through any hose or tubing before it enters the sampling device.
2.3.4 Immediately after sampling, seal the sampling device with plastic end plugs and seal and identify with an OSHA Form 21.
2.3.5 Submit at least one blank with each sample set. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.6 Record sample volumes (in liters of air) for each sample. Also list any compounds considered potential interferences that could be present in the sampling area.
2.3.7 If any bulk samples are submitted for analysis, ship them in separate containers from the air samples.
2.4 Sampler capacity
Collection efficiency studies were conducted by drawing humid air through a sampling device that was attached to a glass U-tube immersed in an oil bath heated to 40°C. Milligram amounts of mXDA and pXDA were added to the U-tube. The inlet of the U-tube was attached to a humid air generator so air at approximately 80% relative humidity could be drawn through it. Tests were done by drawing air for 15 minutes at 1.0 L/min and also for 200 minutes at 1.0 L/min. After sampling, the filters were analyzed. None of the amines were found on any of the back filters for any of the tests. There was an average of 7.4 µg of mXDA and 5.6 µg of pXDA found on the front filters for the 15-L samples, and 37.9 µg of mXDA and 26.1 µg of pXDA for the 200-L samples.
2.5 Extraction efficiency
2.5.1 The average extraction efficiency over the range of 0.5 to 2 times the target concentration is 98.8% and 98.6% for mXDA and pXDA respectively. (Section 4.9.1)
2.5.2 The extraction efficiency at 0.05, 0.1, and 0.2 times the target concentration was found to be 96.6%, 98.2%, and 96.7% respectively for mXDA and 97.6%, 98.6%, and 97.0% respectively for pXDA. (Section 4.9.1)
2.5.3 Extracted samples remain stable for at least 24 h. (Section 4.9.2)
2.6 Recommended air volume and sampling rate
2.6.1 For short-term and ceiling samples, sample 15 L of air at 1 L/min (15-min samples).
2.6.2 For long-term samples, sample 100 L of air at 1 L/min.
2.7 Interferences (sampling)
2.7.1 It is not known if any compounds will severely interfere with the collection of the analytes on sulfuric acid treated filters.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
2.8.1 Attach the sampling equipment to the employee so that it will not interfere with work performance or safety.
2.8.2 Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
3.1 Apparatus
3.1.1 An HPLC system equipped with an ultraviolet detector. A Hewlett-Packard 1050 Series HPLC consisting of a pumping system, programmable variable wavelength detector and an autosampler was used in this evaluation.
3.1.2 An HPLC column capable of separating the analyte of interest from any interferences. A 15-cm × 4.6-mm i.d. Supelcosil LC-ABZ column (Supelco, Inc., Bellefonte, PA, Catalog no. 5-9140) was used in this evaluation. It is critical that if this particular column will not be used for more than 6 h, it should be rinsed with water to remove any buffer salts and ultimately flushed with acetonitrile.
3.1.3 An electronic integrator or some other suitable means of measuring peak heights or areas. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4 Glass vials with Teflon®-lined caps capable of holding 4 mL.
3.1.5 A dispenser capable of delivering 2.0 mL of extraction solvent to prepare standards and samples. If a dispenser is not available, a 2.0-mL volumetric pipet may be used.
3.1.6 A test tube rocker to gently mix the samples during the extraction step. A Vari-Mix mixer (Thermolyne, Dubuque, IA) was used in this evaluation.
3.1.7 A laboratory centrifuge.
3.2 Reagents
3.2.1 m-Xylylenediamine (mXDA) and p-xylylenediamine (pXDA), reagent grade. Aldrich Chemical (Milwaukee, WI) Lot KY00202DP mXDA and Lot PF10421AF pXDA were used in this evaluation. Both of these compounds are corrosive and must be stored under a blanket of nitrogen.
3.2.2 Acetonitrile, methanol, and water, HPLC grade. The acetonitrile and methanol used in this evaluation were "Optima" brand from Fisher Chemical (Fair Lawn, NJ) and the water was from a Millipore Milli-Q water purification system.
3.2.3 Sodium phosphate, monobasic monohydrate (NaH2PO4·H2O), reagent grade. Fisher Lot 704979 was used in this evaluation.
3.2.4 1-Heptanesulfonic acid, sodium salt, HPLC grade. Aldrich Lot HF06915BF was used in this evaluation.
3.2.5 Phosphoric acid, reagent grade.
3.2.6 Extraction solvent/mobile phase. The extraction solvent is the same as the mobile phase used in the HPLC analysis. It consists of 50 mM of 1-heptanesulfonic acid and 50 mM of NaH2PO4·H2O in 75/25, water/acetonitrile adjusted to pH 3.0 with phosphoric acid. To prepare 1 L of the extraction solvent/mobile phase, dissolve (expedite using sonication) 10.1 g of 1-heptanesulfonic acid, sodium salt and 6.9 g of NaH2PO4·H2O into 750 mL of HPLC grade water and adjust the pH of the solution to 3.0 with phosphoric acid. Add, with thorough mixing, 250 mL of acetonitrile to the pH-adjusted aqueous solution.
3.3 Standard preparation
3.3.1 Prepare concentrated standards by accurately weighing approximately 20 mg of each amine into a 25-mL volumetric flask. Dissolve the amines with methanol. Dilute to the mark with additional methanol and thoroughly mix the solution. Stock standards are stable for at least 6 months when stored in brown bottles.
3.3.2 Prepare analytical standards by injecting microliter amounts of stock standards into 4-mL vials containing 2.0 mL of extraction solvent delivered from the same dispenser or pipet used to extract samples.
3.3.3 Bracket sample concentrations with analytical standard concentrations. If samples fall outside of the concentration range of prepared standards, prepare and analyze additional standards at the appropriate concentrations to ascertain the linearity of response.
3.4 Sample preparation
3.4.1 Transfer front and back filters to individual 4-mL vials.
3.4.2 Add 2.0 mL of extraction solvent to each vial using the same dispenser or pipet as used for preparation of standards.
3.4.3 Cap the vials and gently rock them for 15 min.
3.4.4 Centrifuge the sample vials for 10 min at 2000 rpm. Analyze the samples by making direct injections of the centrifuged extracts.
3.5 Analysis
3.5.1 HPLC conditions
| mobile phase: | 50 mM of 1-heptanesulfonic acid and 50 mM of phosphate buffer in 75/25, water/acetonitrile at pH 3.0. See 3.2.6 for preparation instructions. |
| flow rate: | 0.8 mL/min |
| UV detector wavelength: | 208 nm |
| output range: | 0.1 absorbance units full-scale (AUFS) |
| output signal: | recorder output at 1 volt |
| response: | 1 second |
| injection volume: | 25 µL |
| column: | 15-cm × 4.6-mm Supelcosil 5-µm LC-ABZ (Supelco, Inc., Bellefonte, PA, Catalog No. 5-9140) This column must be stored in 100% acetonitrile. (See 3.1.2.) |
| retention times: | pXDA, 4.1 min mXDA, 4.6 min |

Figure 3.5.1. Chromatogram at the target
concentrations.
Key: (1) pXDA, (2) mXDA.
3.5.2 Peak heights or areas are measured by an integrator or other suitable means.
3.5.3 An external standard (ESTD) calibration method is used. Calibration curves are prepared by plotting micrograms of analyte per sample versus peak heights or area counts of the standards. Sample concentrations must be bracketed by standards.

Figure 3.5.3.1. Calibration curve from the data in
Table 4.5.1.
The equation of the line is Y=36419X.
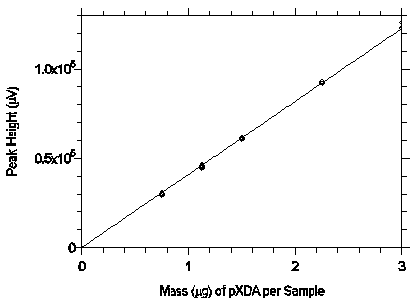
Figure 3.5.3.2 Calibration curve from the data in
Table 4.5.2.
The equation of the line is Y=40879X.
3.6 Interferences (analytical)
3.6.1 Any compound that produces a response on a UV detector at 208 nm and has the same general retention time of any of the analytes of interest is a potential interference. Possible interferences should be reported to the laboratory with submitted samples by the industrial hygienist. These interferences should be considered before samples are extracted.
3.6.2 HPLC parameters may be changed to possibly circumvent interferences.
3.6.3 When necessary, the identity or purity of an analyte peak may be confirmed with additional analytical data, such as wavelength ratioing. As an aid in choosing appropriate wavelengths to ratio, the UV spectra for both analytes is given in Section 4.10.
3.7 Calculations
The analyte concentration for samples is obtained from the appropriate calibration curve in terms of micrograms of analyte per sample. The back filter of each sampler is analyzed primarily to determine if there was any breakthrough from the front filter during sampling. If a significant amount of analyte is found on the back filter (e.g., greater than 25% of the amount found on the front filter), this fact should be reported with sample results. If any analyte is found on the back filter, it is added to the amount found on the front filter. This total amount is then corrected by subtracting the total amount (if any) found on the blank. The air concentration is calculated using the following formula.
mg/m³ = (µg of analyte per sample)/[(L of air sampled)(extraction efficiency)]
3.8 Safety precautions (analytical)
3.8.1 Adhere to the rules set down in your Chemical Hygiene Plan.
3.8.2 Avoid skin contact and inhalation of all chemicals.
3.8.3 Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
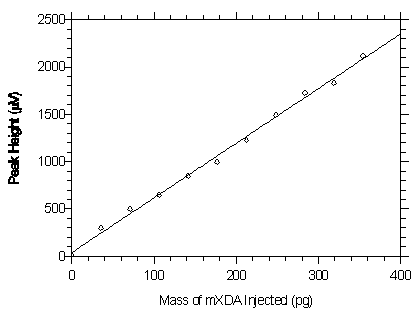
Figure 4.2.1. Plot of the data from Table 4.2.1 to determine the DLAP of 24.4 pg for mXDA. The equation of the line is Y = 5.78X + 39.0.
Figure 4.2.2. Plot of the data from Table 4.2.2 to determine the DLAP of 30.7 pg for pXDA. The equation of the line is Y = 6.84X + 75.2.4.3 Detection limit of the overall procedure (DLOP)
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based on the recommended sampling parameters. Ten samplers were spiked with equal descending increments of mXDA and pXDA such that the highest sampler loading was 28.32 and 28.61 ng/sample respectively. These are the amounts, when spiked on a sampler, that would produce peaks approximately 10 times the baseline noise for a sample blank. These spiked samplers, plus a sample blank, were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOPs. Values of 77.7 and 73.4 for A and 106 and 123 for SEE were obtained for mXDA and pXDA respectively. The DLOPs were calculated to be 4.1 ng/sample (0.27 µg/m3) and 5.0 ng/sample (0.33 µg/m3) for mXDA and pXDA respectively.
Table 4.3.1 DLOP for mXDA
mass (ng)
per samplepeak height
(µV)
0.00 0 2.832 483 5.664 490 8.496 668 11.33 939 14.16 1209 16.99 1314 19.82 1607 22.66 1747 25.49 1965 28.32 2450

Figure 4.3.1. Plot of data from table 4.3.1 to determine the DLOP of 4.1 ng/sample (0.27 µg/m3) for mXDA. The equation of the line is Y = 77.7X + 70.1.Table 4.3.2 DLOP for pXDA
mass (ng)
per samplepeak height
(µV)
0.00 745 2.861 705 5.722 880 8.583 1111 11.44 1288 14.30 1478 17.17 1725 20.03 2020 22.89 2156 25.75 2325 28.61 2852

Figure 4.3.2. Plot of data from Table 4.3.2 to determine the DLOP of 5.0 ng/sample (0.33 µg/m3) for mXDA. The equation of the line is Y = 73.4X + 522.4.4 Reliable quantitation limit (RQL)
The RQL is considered the lower limit for precise quantitative measurements. It is determined from the regression line data obtained for the calculation of the DLOPs (Section 4.3). The RQL is defined as the amount of analyte that gives a response (YRQL) such that
YRQL - YBR = 10(SDBR)
therefore
RQL = 10(SEE)
A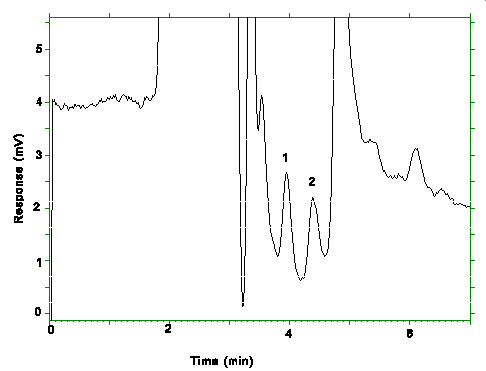 Figure 4.4. Chromatogram of the RQLs. Key: (1)pXDA, (2)mXDA.
Figure 4.4. Chromatogram of the RQLs. Key: (1)pXDA, (2)mXDA.
The RQLs were calculated to be 13.6 ng/sample (0.91 µg/m3) and 16.8 ng/sample (1.12 µg/m3) for mXDA and pXDA respectively. The recoveries at these levels are 97.7% for mXDA and 105.1% for pXDA.
4.5 Precision (analytical method)
The precisions of the analytical procedure are defined as the pooled relative standard deviations (RSDP). Relative standard deviations were determined from six replicate injections of standards at 0.5, 0.75, 1, 1.5, and 2 times the target concentrations. After assuring that the RSDs satisfy the Cochran test for homogeneity at the 95% confidence level, the RSDP for each analyte was calculated to be 0.37% for mXDA and 0.93% for pXDA.
Table 4.5.1 Instrument Response to mXDA
× target concn
(µg/sample)0.5×
0.7430.75×
1.1151.0×
1.4871.5×
2.3302.0×
2.974
peak heights
(µV)27970
27689
27650
27725
27681
2760941402
41270
41533
41536
41462
4117855092
55000
55437
55117
54978
5512582428
82257
82444
82372
82203
82570109852
108624
109552
108964
109860
108987mean
SD
RSD (%)27721
128.2
0.4641397
145.7
0.3555125
164.8
0.3082379
133.4
0.16109306
519.5
0.48
The Cochran test for homogeneity:
g = largest RSD2
RSD20.5× + RSD20.75× + RSD21× + RSD21.5× + RSD22×= 0.339 The critical value of the g statistic at the 95% confidence level for five variances, each associated with six observations, is 0.5065. Because the g statistic does not exceed this value, the RSDs can be considered equal and they can be pooled (RSDP) to give an estimated RSD for the concentration range studied.
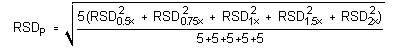
= 0.37% Table 4.5.2
Instrument Response to pXDA
× target concn
(µg/sample)0.5×
0.7510.75×
1.1261.0×
1.5021.5×
2.2532.0×
3.004
peak heights
(µV)30098
30197
30351
29540
30118
3038944767
46184
45999
45007
46063
4585461585
60967
60958
61248
61611
6162292635
92615
92565
92557
92898
91928123100
122820
122680
122980
123140
126150mean
SD
RSD (%)30116
306.1
1.0245646
602.0
1.3261332
318.2
0.5292533
321.9
0.35123478
1320
1.07
The Cochran test for homogeneity:
g = largest RSD2
RSD20.5× + RSD20.75× + RSD21× + RSD21.5× + RSD22×= 0.403 The critical value of the g statistic at the 95% confidence level for five variances, each associated with six observations, is 0.5065. Because the g statistic does not exceed this value, the RSDs can be considered equal and they can be pooled (RSDP) to give an estimated RSD for the concentration range studied.
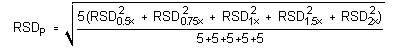
= 0.93% 4.6 Precision (overall procedure)
The precision of the overall procedure is determined from the storage data in Section 4.7. The determination of the standard error of estimate (SEER) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEER is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:

n = total no. of data points k = 2 for linear regression k = 3 for quadratic regression Yobs = observed % recovery at a given time Yest = estimated % recovery from the regression line at the same given time An additional 5% for pump error (SP) is added to the SEER by the addition of variances to obtain the total standard error of estimate.

The precision at the 95% confidence level is obtained by multiplying the standard error of estimate (with pump error included) by 1.96 (the z statistic from the standard normal distribution at the 95% confidence level). The 95% confidence intervals are drawn about their respective regression lines in the storage graphs, as shown in Figures 4.7.1.1., 4.7.1.2., 4.7.2.1. and 4.7.2.2. The precisions of the overall procedure of ±10.0% were obtained from Figures 4.7.1.2. and 4.7.2.2.
4.7 Storage test
Thirty-six storage samples were prepared by spiking sulfuric acid-treated glass fiber filters with 1.487 µg of mXDA and 1.502 µg of pXDA. The filters were then assembled in cassettes and 15 L of 80% RH air was drawn through the samplers at 1 L/min. Six samples were analyzed immediately after generation, fifteen were stored in a refrigerator at 0°C, and fifteen were stored in a closed drawer at ambient temperatures of 20-25°C. At three-day intervals, three samples were selected from each of the two storage sets and analyzed.
Table 4.7.1
Storage Test for mXDA
time
(days)refrigerated storage
recovery (%)ambient storage
recovery (%)
0
0
3
6
9
12
1597.6
95.6
96.7
95.4
95.5
96.9
97.097.7
96.8
96.3
96.3
95.8
97.2
96.896.1
96.1
96.6
95.9
95.5
96.8
96.297.6
95.6
97.0
96.2
96.0
94.9
94.597.7
96.8
97.7
95.8
95.5
94.1
95.996.1
96.1
97.6
97.1
97.0
94.0
93.9
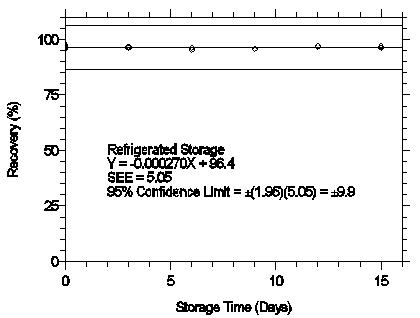
Figure 4.7.1.1. Refrigerated storage test for mXDA.
Figure 4.7.1.2. Ambient storage test for mXDA.Table 4.7.2
Storage Test for pXDA
time
(days)refrigerated storage
recovery (%)ambient storage
recovery (%)
0
0
3
6
9
12
1598.0
100.5
98.6
97.0
97.5
98.3
97.999.4
98.3
98.6
98.1
97.4
97.9
94.797.4
97.7
98.4
96.9
97.3
97.3
98.198.0
100.5
98.7
97.8
97.9
97.0
96.099.4
98.3
99.0
97.7
97.1
96.5
98.297.4
97.7
98.8
99.4
98.5
96.7
95.7

Figure 4.7.2.1. Refrigerated storage test for pXDA.
Figure 4.7.2.2. Ambient storage test for pXDA.4.8 Reproducibility
Six samples were prepared by injecting microliter quantities of standards onto sulfuric
acid-treated glass fiber filters, assembling the filters into cassettes, and drawing 15 L of 80% relative humidity air through the samplers at 1 L/min. The samples were submitted to an SLTC service branch and were analyzed nine days later. No sample result deviated greater than the precisions of the overall procedure determined in Section 4.7, which are ±10.0% for both mXDA and pXDA samples.Table 4.8.1
Reproducibility Data for mXDA
sample µg reported µg expected percent deviation
1
2
3
4
5
61.533
0.722
2.960
1.455
2.908
0.7231.487
0.743
2.974
1.487
2.974
0.743103.1
97.2
99.5
97.8
97.8
97.3+3.1
-2.8
-0.5
-2.2
-2.2
-2.7
Table 4.8.2
Reproducibility Data for pXDA
sample µg reported µg expected percent deviation
1
2
3
4
5
61.534
0.755
3.042
1.509
2.992
0.7291.502
0.751
3.004
1.502
3.004
0.751102.1
100.5
101.3
100.5
99.6
97.1+2.1
+0.5
+1.3
+0.5
-0.4
-2.9
4.9 Extraction efficiency and stability of extracted samples
4.9.1 Extraction efficiency
The extraction efficiencies (EE) for mXDA and pXDA were determined by injecting standards onto sulfuric acid treated filters with amounts equivalent to 0.05 to 2 times the target concentrations. These samples were stored overnight at ambient temperature and then extracted and analyzed. The average extraction efficiencies over the working range of 0.5 to 2 times the target concentrations are 98.8% and 98.6% for mXDA and pXDA respectively.
Table 4.9.1.1
Extraction Efficiency for mXDA
× target concn
mass spiked (µg)0.05×
0.07430.1×
0.14870.2×
0.29740.5×
0.74301.0×
1.4872.0×
2.974
EE (%) 100.1
95.2
96.2
96.6
97.4
94.197.5
102.2
99.5
98.2
97.5
94.198.5
98.2
97.5
96.2
97.8
91.898.8
102.0
104.3
99.1
98.3
98.796.8
97.8
101.4
98.0
98.5
97.697.1
96.7
98.0
98.2
97.8
99.2mean 96.6 98.2 96.7 100.2 98.4 97.8
Table 4.9.1.2
Extraction Efficiency for pXDA
× target concn
mass spiked (µg)0.05×
0.07510.1×
0.15020.2×
0.30040.5×
0.75101.0×
1.5022.0×
3.004
EE (%) 97.1
98.7
95.9
97.6
103.5
92.799.2
97.9
100.5
100.5
97.2
96.597.5
98.5
97.2
99.5
96.9
92.597.5
96.8
99.5
98.4
98.7
97.198.1
98.2
98.7
98.9
99.7
98.998.9
98.7
99.5
99.4
98.1
99.3mean 97.6 98.6 97.0 98.0 98.8 99.0
4.9.2 Stability of extracted samples
The stability of extracted samples was investigated by reanalyzing the target concentration samples 24 h after initial analysis. After the original analysis was performed three vials were recapped with new septa while the remaining three retained their punctured septa. The samples were reanalyzed with fresh standards. The average percent change was -1.0% and +0.3% for samples that were resealed with new septa, and +0.5% and +0.9% for those that retained their punctured septa for mXDA and pXDA respectively.
Table 4.9.2.1
Stability of Extracted mXDA Samples
punctured septa replaced punctured septa retained initial
EE
(%)EE after
one day
(%)difference initial
EE
(%)EE after
one day
(%)difference
96.8
97.8
101.4
98.796.8
97.5
98.6
(averages)
97.60.0
-0.3
-2.8
-1.098.0
98.5
97.6
98.098.6
98.6
98.3
(averages)
98.5+0.6
+0.1
+0.7
+0.5
Table 4.9.2.2
Stability of Extracted pXDA Samples
punctured septa replaced punctured septa retained initial
EE
(%)EE after
one day
(%)difference initial
EE
(%)EE after
one day
(%)difference
98.1
98.2
98.7
98.398.3
98.5
99.0
(averages)
98.6+0.2
+0.3
+0.3
+0.398.9
99.7
98.9
99.2100.3
100.3
99.7
(averages)
100.1+1.4
+0.6
+0.8
+0.9
4.10 Qualitative analysis
UV spectra for both analytes were obtained from a Waters 990 Photodiode Array Detector by injecting a standard using the same conditions given in Section 3.5.1.
Figure 4.10.1. UV spectra of mXDA.
Figure 4.10.2. UV spectra of pXDA.
4.1 Determination of detection limits
Detection limits (DL), in general, are defined as the amount (or concentration) of analyte that gives a response (YDL) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
YDL - YBR = 3(SDBR)
The direct measurment of YBR and SDBR in chromatographic methods is typically inconvenient and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of analytical standards or samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for DL:
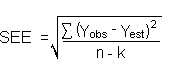
| Yobs | = observed response |
| Yest | = estimated response from regression curve |
| n | = total no. of data points |
| k | = 2 for a linear regression curve |
At point YDL on the regression curve
therefore
| DL = | (YDL - YBR)
A |
Substituting 3(SEE) + YBR for YDL gives
| DL = | 3(SEE)
A |
4.2 Detection limit of the analytical procedure (DLAP)
The DLAP is measured as the mass of analyte introduced into the chromatographic column. Ten analytical standards were prepared in equal descending increments with the highest standard containing 14.16 and 14.31 ng/mL of mXDA and pXDA respectively. These concentrations produce peaks approximately 10 times the baseline noise of a reagent blank. These standards, plus a solvent blank, were analyzed and the data obtained were used to determine the required parameters (A and SEE) for the calculation of the DLAPs. Values of 5.78 and 6.84 for A and 47.0 and 69.9 for SEE were obtained for mXDA and pXDA respectively. DLAPs were calculated to be 24.4 and 30.7 pg for mXDA and pXDA respectively.
| Table 4.2.1 | ||
| DLAP for mXDA | ||
|
| ||
| concentration | mass on column | peak height |
| (ng/mL) | (pg) | (µV) |
|
| ||
| 0.00 | 0.00 | 0 |
| 1.416 | 35.4 | 296 |
| 2.832 | 70.8 | 497 |
| 4.248 | 106.2 | 648 |
| 5.664 | 141.6 | 845 |
| 7.080 | 177.0 | 995 |
| 8.495 | 212.4 | 1226 |
| 9.910 | 247.8 | 1494 |
| 11.33 | 283.2 | 1729 |
| 12.74 | 318.5 | 18.31 |
| 14.16 | 354.0 | 2118 |
|
| ||
| Table 4.2.2 | ||
| DLAP for pXDA | ||
|
| ||
| concentration | mass on column | peak height |
| (ng/mL) | (pg) | (µV) |
|
| ||
| 0.00 | 0.00 | 0 |
| 1.430 | 35.8 | 350 |
| 2.861 | 71.5 | 60.9 |
| 4.292 | 107.3 | 867 |
| 5.722 | 143.0 | 1068 |
| 7.152 | 178.8 | 1287 |
| 8.583 | 214.6 | 1451 |
| 10.01 | 250.3 | 17.19 |
| 11.44 | 286.1 | 2164 |
| 12.87 | 321.9 | 2281 |
| 14.30 | 357.6 | 2487 |
|
| ||
5. References
5.1. OSHA Analytical Methods Manual; Vol. 3, Publ. #4542, U.S. Department of Labor, Occupational Safety and Health Administration; OSHA Salt Lake Technical Center: Salt Lake City, UT, 1990; Method 57: 4,4'-Methylenedianiline; American Conference of Governmental Industrial Hygienists (ACGIH): Cincinnati, OH.
5.2. ibid. Method 65: Benzidine, 3,3'-Dichlorobenzidine, 2,4- and 2,6-Toluenediamine.
5.3. ibid. Method 71: o-Dianisidine, 4,4'-Methylenebis(o-chloroaniline), o-Tolidine.
5.4. ibid. Method 73: o-, m-, and p-Toluidine.
5.5. ibid. Method 78: Diphenylamine, N-Isopropylaniline.
5.6. ibid., Vol. 4, Method No. 87; m-, o-, and p-Phenylenediamine.
5.7. ibid. Method No. 93; 4-Aminobiphenyl, 1-Naphthylamine and 2-Naphthylamine.
5.8. "American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values"; 5th ed., p. 638, Cincinnati, OH (1986).
5.9. Material Safety Data Sheet: m-xylylenediamine, Aldrich Chemical Co., Milwaukee, WI, June 1989.
5.10. Material Safety Data Sheet: p-xylylenediamine, Aldrich Chemical Co., Milwaukee, WI, November 1990.
5.11. Lewis, R.J., Sr., Ed. "Sax's Dangerous Properties of Industrial Materials", 8th ed., vol 3; Van Nostrand Reinhold Co.: New York, NY, 1992.
5.12 Bell, A.; Smith, J.G.; Kibler, C.J. J. Polym. Sci. A1, 19 (1965) in "Polyamide Fibers" in Encyclopedia of Chemical Technology 3rd ed., Vol. 18, p. 400, by J. H. Saunders, Monsanto Company.