![]()
| Method number: | 102 |
| Matrix: | Air |
| Target concentration: OSHA PEL: ACGIH TLV: |
5 ppm (20 mg/m3) 5 ppm (20 mg/m3) TWA 5 ppm (20 mg/m3) ceiling |
| Procedure: | Samples are collected open face on glass fiber filters coated
with veratrylamine and |
| Recommended air volume and sampling rate: |
7.5 L at 0.5 L/min ceiling 7.5 L at 0.05 L/min TWA |
| Reliable quantitation limit: | 0.094 ppm (0.39 mg/m3) |
| Standard error of estimate at the target concentration: |
6.4% |
| Special caution: | Ketene and acetyl chloride produce the same derivative as acetic anhydride. Coated filters should be used within a month of preparation. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1993 | Chemist: Yihlin Chan |
OSHA Salt Lake Technical Center
Salt Lake City, UT 84165-0200
1. General Discussion
- 1.1 Background
- 1.1.1 History
In OSHA Method 82, acetic anhydride is collected on a glass fiber
filter impregnated with

The acetic
1.1.2 Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The following is quoted from the ACGIH Documentation of TLV:
Smyth et al reported an oral LD50 in rats as 1.78 g/kg. Henderson and Haggard mentioned eye, nose and throat irritation and suggested that bronchial and lung injury were likely to occur from inhalation of acetic [an]hydride vapor. Fairhall considered acetic anhydride a marked lachrymator and found systemic effects unlikely. McLaughlin discussed serious corneal injury from the liquid in industry. Smyth found rats inhaling 1000 ppm for four hours survived, but 2000 ppm was fatal. The liquid causes skin burns. No cumulative effects are known. The value of 5 ppm, as a ceiling limit, is recommended by analogy with acetic acid and to prevent undue irritation. (Ref. 5.6)
The OSHA PEL for acetic anhydride is 5 ppm (20 mg/m3) TWA. (Ref. 5.5)
1.1.3 Workplace exposure
Exposure to acetic anhydride may occur in the following operations: manufacture of cellulose esters, fibers, plastics, lacquers, protective coating solution, photographic films, cigarette filters, magnetic tape, and thermoplastic molding compositions; manufacture of pharmaceuticals and pharmaceutical intermediates; use in organic synthesis as an acetylating agent, bleaching agent, and dehydrating agent; synthesis of perfume chemicals, explosives, and weed killers; use in acetylation of animal and vegetable oils; use as an acetylating and dehydrating agent in textile dyeing, chemical treatment of paper, and chemical analysis. (Ref. 5.7) Of these, by far the greatest single application for acetic anhydride is in the manufacture of cellulose esters. It is estimated that 95% of the total U.S. production is used for this purpose. (Ref. 5.8)
1.1.4 Physical properties and other descriptive information (Ref. 5.9)
| CAS no.: | 108-24-7 |
| synonyms: | acetic acid, anhydride; acetic oxide; acetyl anhydride; acetyl ether; acetyl oxide; ethanoic anhydrate |
| structural formula: | |
| molecular wt: | 102.10 |
| boiling point: | 139°C |
| melting point: | -73°C |
| appearance: | colorless liquid |
| odor: | strong acetic odor |
| vapor pressure: | 0.67 kPa (5 mmHg) at 25°C |
| flash point: | 49°C (closed-cup) |
| solubility: | slowly soluble in water, forming acetic acid; forms ethyl acetate with ethyl alcohol; soluble in chloroform, ether. |
| The analyte air concentrations throughout this method are based on the recommended sampling and analytical parameters. Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg). The analyte amounts and concentrations are listed as those of acetic anhydride even though the derivative is the actual species analyzed. |
- 1.2 Limit defining parameters
- 1.2.1 Detection limit of the analytical procedure
The detection limit of the analytical procedure is 6.1 pg. This is the amount of analyte that will give a response that is significantly different from the background response of a reagent blank. (Sections 4.1 and 4.2)
1.2.2 Detection limit of the overall procedure
The detection limit of the overall procedure is 0.88 µg per sample (0.028 ppm or 0.12 mg/m3). This is the amount of analyte spiked on the sampler that will give a response that is significantly different from the background response of a sampler blank. (Sections 4.1 and 4.3)
1.2.3 Reliable quantitation limit
The reliable quantitation limit is 2.94 µg per sample (0.094 ppm or 0.39 mg/m3). This is the amount of analyte spiked on a sampler that will give a signal that is considered the lower limit for precise quantitative measurements. (Section 4.4)
1.2.4 Precision (analytical procedure)
The precision of the analytical procedure, measured as the pooled relative standard deviation over a concentration range equivalent to 0.5 to 2 times the target concentration, is 0.53%. (Section 4.5)
1.2.5 Precision (overall procedure)
The precision of the overall procedure at the 95% confidence
level for the ambient temperature
1.2.6 Recovery
The recovery of AcVA from samples used in a 15-day storage test remained above 96.1% when the samples were stored at ambient temperature. (Section 4.7)
1.2.7 Reproducibility
Six samples collected from a controlled test atmosphere, with a draft copy of this procedure, were submitted to an SLTC service branch for analysis. The samples were analyzed after 2 days of storage at 5°C. No individual sample result deviated from its theoretical value by more than the precision reported in Section 1.2.5. (Section 4.8)
2. Sampling Procedure
- 2.1 Apparatus
- 2.1.1 Samples are collected using a personal sampling pump
calibrated, with the sampling device attached, to ±5% of the
recommended flow rate.
 2.1.2 Samples are collected with a
2.1.2 Samples are collected with a
2.1.3 The treated filters are prepared as follows: To make
40 coated filters, weigh 0.4 g of veratrylamine and 0.4 g of
2.2 Reagents
None required.
2.3 Technique
- 2.3.1 Prepare the sampler for open-face sampling by removing the
top piece and the end plug from the bottom piece. Attach the sampler
to the sampling pump with a piece of flexible tubing and place it in
the worker's breathing zone with the open face of the cassette
facing down.
2.3.2 Replace the top piece and the end plug after sampling. Wrap
each sample with a Form
2.3.3 Submit at least one blank with each set of samples. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.4 Record sample air volume for each sample.
2.3.5 List any compounds that could be considered potential interferences.
2.4 Sampler capacity
Sampling capacity is determined by measuring how much air can be sampled before breakthrough of analyte through the sampler occurs. Breakthrough is considered to occur when the effluent from the sampler contains a concentration of analyte that is 5% of the upstream concentration (5% breakthrough). The sampler capacity was determined to be over 30 L at a sampling rate of 0.5 L/min with an acetic anhydride concentration of 40 mg/m3 (2 times the target concentration). At a sampling rate of 0.05 L/min, 5% breakthrough point was not reached in 18 L. (Section 4.9)
2.5 Extraction efficiency
- 2.5.1 The average extraction efficiency for AcVA from the
treated glass fiber filter over the range of 0.5 to 2.0 times the
target concentration was 99.8%. (Section 4.10.1)
2.5.2 The extraction efficiency at 0.2, 0.1, and 0.05 times the target concentration was found to be 100.9%, 107.8%, and 108.0% respectively. (Section 4.10.1)
2.5.3 Extracted samples remain stable for at least 24 h. (Section 4.10.2)
2.6 Recommended air volume and sampling rate
- 2.6.1. For TWA samples the recommended air volume is 7.5 L at
0.05 L/min.
2.6.2. For short-term samples the recommended air volume is 7.5 L at 0.5 L/min.
2.7 Interferences (sampling)
- 2.7.1 Acetyl chloride and ketene react with veratrylamine to
form AcVA, causing positive interference. But in most industrial
applications they are rarely used together with acetic anhydride.
Other compounds that react with veratrylamine, such as isocyanates,
acyl halides, and other anhydrides, may interfere by consuming part
of the derivatizing agent.
2.7.2 Suspected interferences should be reported to the laboratory with submitted samples.
2.8 Safety precautions (sampling)
- 2.8.1 The sampling equipment should be attached to the worker in
such a manner that it will not interfere with work performance or
safety.
2.8.2 All safety practices that apply to the work area being sampled should be followed.
3. Analytical Procedure
- 3.1 Apparatus
- 3.1.1 A GC equipped with an NPD. A Hewlett-Packard 5890 GC
equipped with an NPD and a 7673 autosampler were used in this
evaluation.
3.1.2 A GC column capable of separating AcVA, benzalazine, and
any interferences. A
3.1.3 An electronic integrator or other suitable means of measuring detector response. A Waters 860 Networking Computer System was used in this evaluation.
3.1.4 Scintillation vials, 20-mL glass, with poly(tetrafluoroethylene)-lined caps.
3.1.5 A dispenser capable of delivering 5.0 mL of extraction solvent.
3.2 Reagents
- 3.2.1 Acetic anhydride. Acetic anhydride, ACS reagent grade, was
obtained from Aldrich Chemical.
3.2.2 Veratrylamine. Veratrylamine, 97%, was obtained from Aldrich.
3.2.3 Benzalazine. Benzalazine (95-99%) from ICN was used in this evaluation.
3.2.4 Toluene. Toluene, Optima grade, was obtained from Fisher.
3.2.5 2-Propanol. 2-Propanol, Optima grade, was obtained from Fisher.
3.2.6 Extraction solvent with internal standard. Dissolve 30 mg
of benzalazine in 1 L of
3.3 Standard preparation
- 3.3.1 Synthesis of AcVA:
With constant stirring, slowly add a solution of 5.18 g of
veratrylamine in 25 mL of toluene to a solution of 3.23 g of acetic
anhydride in 25 mL of toluene. Continue stirring for 10 more
minutes. Isolate the product by distillation; b.p.
3.3.2 Prepare stock standards by weighing about 10 mg of AcVA in 10-mL volumetric flasks and diluting to volume with the extraction solution. Apply a factor of 0.4880 to the weight of AcVA to convert it to that of acetic anhydride. For example, 10 mg of AcVA dissolved in 10 mL will give a standard stock solution representing 488.0 µg/mL of acetic anhydride.
(MW acetic anhydride) / (MW AcVA) = 102.09 / 209.2 = 0.4880
3.3.3 Prepare analytical standards by diluting the stock standards with extraction solvent. A 30 µg/mL standard solution corresponds to the target concentration.
3.3.4 Prepare a sufficient number of analytical standards to generate a calibration curve. Analytical standard concentrations must bracket sample concentrations.
3.4 Sample preparation
- 3.4.1 Transfer the two filters to separate scintillation vials.
3.4.2 Add 5.0 mL of the extraction solvent to each vial.
3.4.3 Cap the vials and shake them on a mechanical shaker for 30 min.
3.5 Analysis
- 3.5.1 GC conditions
| column: | HP-1 (5-m, 0.53-mm i.d., 2.65-µm film) | |
| zone temp: | column | 150°C to 270°C at 10°C/min |
| injector | 270°C | |
| detector | 300°C | |
| gas flow: | column (He) | 1.83 mL/min |
| hydrogen | 3.84 mL/min | |
| auxiliary (N2) | 27.4 mL/min | |
| air | 110.5 mL/min | |
| split vent | 68 mL/min (split ratio 37:1) | |
| ret. times: | AcVA | 3.8 min |
| benzalazine | 4.5 min (ISTD) | |
 Figure 3.5.1.1. Chromatogram of a standard at the target concentration. Key: 1 = AcVA, 2 = benzalazine (internal standard). |
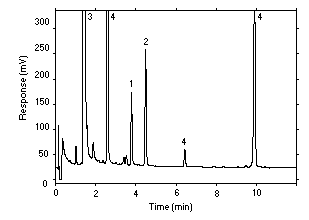 Figure 3.5.1.2. Chromatogram of an extracted sample at 0.75 times target concentration. Key: 1 = AcVA, 2 = benzalazine, 3 = veratrylamine, 4 = impurities. |
3.5.2 Peak areas are measured by an electronic integrator or other suitable means.
| 3.5.3 An internal standard (ISTD) calibration method is
used. A calibration curve is prepared by plotting micrograms
per milliliter versus |
 Figure 3.5.3. Calibration curve constructed from the data in Table 4.5. Equation for the line is Y = 0.203X - 0.574. |
3.6 Interferences (analytical)
- 3.6.1 Any compound that produces an NPD response and has a
similar retention time as benzalazine or AcVA is a potential
interference. If any potential interferences were reported, they
should be considered before samples are extracted. Generally,
chromatographic conditions can be altered to separate an
interference from the analyte.
3.6.2 When necessary, the identity or purity of an analyte peak may be confirmed with additional analytical data (Section 4.11).
3.7 Calculations
The amount of acetic anhydride per sample is obtained from the appropriate calibration curve in terms of micrograms uncorrected for extraction efficiency. The back filter is analyzed primarily to determine the extent of breakthrough. If any analyte is found on the back filter, it is added to the amount on the front filter. This total amount is then corrected by subtracting the total amount (if any) found on the blank. The air concentration is calculated using the following formulae.
| mg/m3 = | micrograms of acetic anydride per
sample
liters of air sampled × extraction efficiency |
| ppm = | 24.46 × mg/m3
molecular weight of acetic anhydride |
= (0.2396) (mg/m3) |
3.8 Safety precautions (analytical)
- 3.8.1 Adhere to the rules set down in your Chemical Hygiene
Plan.
3.8.2 Avoid skin contact and inhalation of all chemicals.
3.8.3 Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1 Determination of detection limits
Detection limits (DL), in general, are defined as the amount (or concentration) of analyte that gives a response (YDL) that is significantly different (three standard deviations (SDBR)) from the background response (YBR).
YDL - YBR = 3(SDBR)
The direct measurement of YBR and SDBR in chromatographic methods is typically inconvenient and difficult because YBR is usually extremely low. Estimates of these parameters can be made with data obtained from the analysis of a series of analytical standards or samples whose responses are in the vicinity of the background response. The regression curve obtained for a plot of instrument response versus concentration of analyte will usually be linear. Assuming SDBR and the precision of data about the curve are similar, the standard error of estimate (SEE) for the regression curve can be substituted for SDBR in the above equation. The following calculations derive a formula for DL:
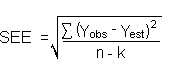
| Yobs = | observed response |
| Yest = | estimated response from regression curve |
| n = | total number of data points |
| k = | 2 for linear regression curve |
At point YDL on the regression curve
YDL = A(DL) + YBR A = analytical sensitivity (slope)
therefore
| DL = | (YDL - YBR)
A |
Substituting 3(SEE) + YBR for YDLgives
| DL = | 3(SEE)
A |
4.2 Detection limit of the analytical procedure (DLAP)
The DLAP is measured as the mass of analyte actually introduced into the chromatographic column. Ten analytical standards whose concentrations were equally spaced from 0 to 1.202 µg/mL were prepared. The standard containing 1.202 µg/mL represented approximately 10 times the baseline noise. The data obtained from analyzing these standards were used to determine the required parameters (A and SEE) for the calculation of the DLAP. Values of 0.0368 and 0.0751 were obtained for A and SEE respectively. DLAP was calculated to be 6.1 pg.
|
 Figure 4.2. Plot of data to determine the DLAP. | |||||||||||||||
4.3 Detection limit of the overall procedure (DLOP)
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based on the recommended sampling parameters. Ten treated filters were spiked with amounts of AcVA equally spaced from 0 to 6.01 µg/sample. The latter amount, when spiked on a sampler, would produce a peak approximately 10 times the baseline noise for a sample blank. These spiked filters were analyzed with the recommended analytical parameters, and the data obtained used to calculate the required parameters (A and SEE) for the calculation of the DLOP. Values of 1.35 and 0.3975 were obtained for A and SEE respectively. DLOP was calculated to be 0.88 µg/sample (0.028 ppm, 0.12 mg/m3).
|
 Figure 4.3. Plot of data used to determine the DLOP/RQL. | ||||||||||
4.4 Reliable quantitation limit
| The RQL is considered the lower limit for precise
quantitative measurements. It is determined from the regression
line data obtained for the calculation of the DLOP (Section
4.3), providing at least 75% of the analyte is recovered. The
RQL is defined as the amount of analyte that gives a response
(YRQL) such that YRQL - YBR = 10(SDBR) therefore
|
 Figure 4.4. chromatogram of the RQL. |
The RQL was calculated to be 2.94 µg per sample (0.094 ppm, 0.39 mg/m3). Recovery at this concentration is 103.0%.
4.5 Precision (analytical method)
The precision of the analytical procedure is defined as the pooled relative standard deviation (RSDP). Relative standard deviations were determined from six replicate injections of analytical standards at 0.5, 0.75, 1, 1.5, and 2 times the target concentration. After assuring that the RSDs satisfy the Cochran test for homogeneity at the 95% confidence level, RSDP was calculated to be 0.53%.
|
| |||||
| × target concn µg/mL |
0.5× 15.03 |
0.75× 22.55 |
1× 30.06 |
1.5× 45.09 |
2× 60.12 |
|
| |||||
| ISTD-adjusted response |
14.541 14.546 14.553 14.657 14.686 14.679 |
22.342 22.188 22.316 22.431 22.320 22.673 |
30.142 30.109 30.066 30.149 29.908 29.850 |
45.009 44.842 44.994 44.909 45.081 45.163 |
59.990 60.253 60.396 60.917 60.519 60.976 |
SD RSD (%) |
14.610 0.0705 0.483 |
22.378 0.1640 0.733 |
30.037 0.1274 0.424 |
45.000 0.1153 0.256 |
60.509 0.3827 0.632 |
|
| |||||
The Cochran test for homogeneity:
 |
= 0.3796 |
Since the g statistic does not exceed the critical value of 0.5065, the RSDs can be considered equal and they can be pooled (RSDP) to give an estimated RSD for the concentration range studied.
 |
= 0.53% |
4.6 Precision (overall procedure)
The precision of the overall procedure is determined from the storage data in Section 4.7. The determination of the standard error of estimate (SEER) for a regression line plotted through the graphed storage data allows the inclusion of storage time as one of the factors affecting overall precision. The SEER is similar to the standard deviation, except it is a measure of dispersion of data about a regression line instead of about a mean. It is determined with the following equation:

| n =
k = k = Yobs = Yest = |
total no. of data points 2 for linear regression 3 for quadratic regression observed % recovery at a given time estimated % recovery form the regression line at the same given time |
An additional 5% for pump error (SP) is added to the SEER by the addition of variances to obtain the total standard error of estimate.

The precision at the 95% confidence level is obtained by
multiplying the standard error of estimate (with pump error included)
by 1.96 (the
4.7 Storage test
Storage samples for AcVA were prepared from a controlled test
atmosphere of acetic anhydride.
|
| |||||||
| time (days) | percent recovery (ambient) |
percent
recovery (refrigerated) | |||||
|
| |||||||
| 0 0 3 7 10 13 15 |
102.2 98.8 95.0 103.0 97.8 92.3 94.2 |
103.4 98.0 91.8 106.5 99.2 96.5 93.0 |
93.2 104.3 101.6 99.9 97.9 98.3 97.6 |
102.2 98.8 111.0 90.9 111.4 105.9 96.5 |
103.4 98.0 96.7 107.0 104.1 100.8 98.1 |
93.2 104.3 98.8 109.7 111.1 102.4 94.5 | |
|
| |||||||
 Figure 4.7.1. Ambient storage test for AcVA. |
 Figure 4.7.2. Refrigerated storage test for AcVA. |
4.8 Reproducibility
Six samples were prepared by collecting them from a controlled test atmosphere similar to that which was used in the collection of the storage samples. The samples were submitted to an SLTC service branch for analysis. The samples were analyzed after being stored for 2 days at 5°C. No sample result had a deviation greater than the precision of the overall procedure determined in Section 4.7, which is ±12.5%
|
| |||
| mg/m3 expected | mg/m3 found | percent found | percent deviation |
|
| |||
| 33.58 33.58 33.58 33.58 33.58 33.58 |
32.20 32.63 32.14 30.11 29.71 31.31 |
95.9 97.2 95.7 89.7 88.5 93.2 |
-4.1 -2.8 -4.3 -10.3 -11.5 -6.8 |
|
| |||
4.9 Sampler capacity
The sampler capacity was assessed by sampling from a dynamically
generated test atmosphere of acetic anhydride at 2 times the target
concentration and at 25°C and 80% RH. The test atmosphere of acetic
anhydride was generated by pumping an ethyl acetate solution of acetic
anhydride at a rate of 9.9 mg/min (11 mg/mL × 0.9 mL/min) through a
TSI Model 3076 atomizer where it was dispersed with an air stream of
3.5 L/min. The aerosol passed through an electrostatic charge
neutralizer and was mixed with a dilution air stream of 47 L/min. The
test atmosphere was drawn through a sampler consisting of two filters
in series (separated by a spacer ring) at 0.5 L/min. Three samplers
were used in each of the two experiments. At
Additionally, two experiments with four samplers were conducted with vapor generator system where acetic anhydride in ethyl acetate was simply evaporated directly into an air stream. The breakthrough data obtained with acetic anhydride vapor were plotted in Figure 4.9.2. The data obtained from the two experiments were not very consistent but both showed that the 5% breakthrough point is over 30 L. The recommended air volume of 7.5 L provides an ample margin of safety against exceeding sampler capacity.
When the OSHA PEL was changed from 20 mg/m3 ceiling to 20 mg/m3 TWA, a breakthrough curve was also determined at a flow rate of 0.05 L/min. First, it was determined that sampling at flow rates of 0.5, 0.1, and 0.05 L/min resulted in the same concentration of acetic anhydride in the test atmosphere. This indicated that all three flow rates were suitable for collecting acetic anhydride atmosphere. The flow rate of 0.05 L/min was selected in order to accommodate a longer sampling time for TWA sampling. The breakthrough data with 0.05 L/min were plotted in Figure 4.9.3. The 5% breakthrough point was not reached in 18 L.
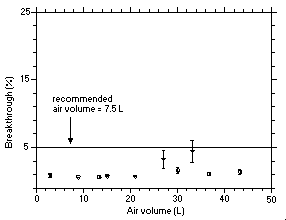 Figure 4.9.1. Capacity test for acetic anhydride at a flow rate of 0.5 L/min (aerosol). |
 Figure 4.9.2. Breakthrough curve for acetic anhydride at a flow rate of 0.5 L/min (vapor). |

Figure 4.9.3. Capacity test for acetic anhydride
at
a flow rate of 0.05 L/min (aerosol).
4.10 Extraction efficiency and stability of extracted samples
- 4.10.1 Extraction efficiency
The extraction efficiencies (EE) of AcVA were determined by liquid-spiking the treated glass fiber filters with AcVA at 0.05 to 2 times the target concentrations. These samples were stored overnight at ambient temperature and then extracted and analyzed. The average extraction efficiency over the working range of 0.5 to 2 times the target concentration was 99.8%.
|
| ||||||
| × target conc (µg) |
0.05× 7.46 |
0.1× 14.92 |
0.2× 29.83 |
0.5× 74.58 |
1.0× 149.2 |
2.0× 298.3 |
|
| ||||||
| EE (%) | 113.0 87.4 109.3 106.5 120.4 111.5 |
102.3 98.1 103.6 106.0 132.6 104.0 |
100.4 98.7 101.9 102.5 104.4 97.3 |
98.2 95.4 104.3 98.0 96.8 101.6 |
100.6 102.5 99.2 101.0 104.4 101.7 |
96.9 98.1 98.4 101.3 99.2 97.4 |
| 108.0 | 107.8 | 100.9 | 99.1 | 101.6 | 98.6 | |
|
| ||||||
4.10.2 Stability of extracted samples
The stability of extracted samples was investigated by reanalyzing the target concentration samples 24 h after initial analysis. After the original analysis was performed three vials were recapped with new septa while the remaining three retained their punctured septa. The samples were reanalyzed with fresh standards. The average percent change was -4.8% for samples that were resealed with new septa, and -2.0% for those that retained their punctured septa.
|
| |||||
| punctured septa replaced | punctured septa retained | ||||
| initial | EE after | initial | EE after | ||
| EE | one day | difference | EE | one day | difference |
| (%) | (%) | (%) | (%) | ||
|
| |||||
| 100.6 102.5 99.2 100.8 |
94.3 98.2 95.5 (averages) 96.0 |
-6.3 -4.3 -3.7 -4.8 |
101.0 104.4 101.7 102.4 |
98.9 103.5 98.8 (averages) 100.4 |
-2.1 -0.9 -2.9 -2.0 |
|
| |||||
4.11 Qualitative analysis
| The GC/MS of AcVA can easily be obtained by using GC
conditions similar to those given in Section 3.5. A
|
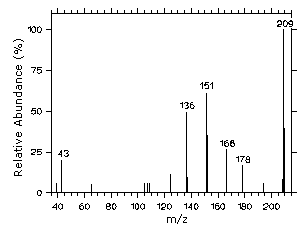 Figure 4.11. Mass spectrum of AcVA. |
5. References
- 5.1 Chan, Y. "OSHA Method No. 82; Acetic Anhydride", OSHA Salt
Lake Technical Center, unpublished, Salt Lake City, UT 84165, April
1990.
5.2 Chan, Y. "OSHA Method No. 86; Maleic Anhydride", OSHA Salt Lake Technical Center, unpublished, Salt Lake City, UT 84165, December 1990.
5.3 Chan, Y. "OSHA Method No. 90; Phthalic Anhydride", OSHA Salt Lake Technical Center, unpublished, Salt Lake City, UT 84165, October 1991.
5.4 Chan, Y. "OSHA Method No. 98; Trimellitic Anhydride", OSHA Salt Lake Technical Center, unpublished, Salt Lake City, UT 84165, November 1992.
5.5 Code of Federal Regulations, Title 29, 1910.1000, Table
5.6 Documentation of the Threshold Limit Values and Biological Exposure Indices, 5th ed., American Conference of Governmental Industrial Hygienists Inc., Cincinnati, OH, 1986.
5.7 "NIOSH/OSHA Occupational Health Guidelines for Chemical
Hazards", U.S. Department of Health and Human Services, Government
Printing Office, DHHS(NIOSH) Publication No.
5.8 Grayson, M., Ed., "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd edition, Volume 1, p. 151, Interscience Publishers, New York, NY, 1984.
5.9 Sweet, D.V., Ed., "Registry of Toxic Effects of Chemical
Substances",