DIETHYLENETRIAMINE (DETA)
TRIETHYLENETETRAMINE (TETA)
| Method no.: | 60 | |||||||||
| Matrix: | Air | |||||||||
| Procedure: | Samples are collected by drawing known volumes of air
through sampling tubes containing XAD-2 resin coated with 10%
| |||||||||
| Recommended air volume and sampling rate: |
10 L at 0.1 L/min | |||||||||
| ||||||||||
| Target
concentrations: ppm(mg/m3) |
| |||||||||
| Reliable quantitation limit: ppm(mg/m3) |
| |||||||||
| Standard error of estimate at the target concentration: (Section 4.4.) |
| |||||||||
| ||||||||||
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | |||||||||
| Date: September 1986 | Chemist: Carl J. Elskamp | |||||||||
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
Previous to this evaluation, OSHA industrial hygienists have been collecting EDA and DETA on silica gel sampling tubes. Immediately after sampling, the silica gel sections were transferred to vials and desorbed with 0.2 N HCl in a methanol/water solution in order to stabilize the collected amines until analyzed. OSHA has previously not had a recommended sampling procedure for TETA. (Ref. 5.1.) The analysis of free amines from an aqueous solution is difficult and field desorptions are a nuisance. Thus an improved method for determining occupational exposures to EDA, DETA, and TETA vapors was needed.
An NBD chloride derivatizing procedure used for certain low
molecular weight aliphatic monoamines (Ref. 5.2.) was evaluated for
these three polyamines without success. A method for determining EDA
in air (Ref. 5.3.), which is based on derivatization with
1.1.2. Toxic effects (This section is for information only and should not be used as the basis of OSHA policy.)
In general, the vapors of these amines are painful and irritating to the eyes, nose, throat, and respiratory system. The liquids can cause severe damage to the eye and serious burns to the skin. Hypersensitivity can be produced in some people which results in contact dermatitis or an asthmatic respiratory response, or both. The LD50 values for white rats for a single oral dose are 1.2, 1.4, and 2.5 g/kg body weight for EDA, DETA, and TETA respectively. (Ref. 5.5.)
There is currently an OSHA PEL of 10 ppm for EDA. There have been no PEL values yet set for DETA and TETA. ACGIH has adopted a TLV of 10 ppm for EDA and 1 ppm for DETA.
1.1.3. Workplace exposure
Aliphatic polyamines are versatile chemical intermediates having a broad spectrum of industrial applications. Some of the areas where exposures could possibly occur include production of fungicides (ethylenebisdithiocarbamates, imidazolines, EDA-copper sulfate complex), chelating agents (EDTA, pentasodium diethylenetriaminepentaacetic acid, trisodium N-hydroxyethylethylenediaminetriacetic acid), wet-strength resins (cationic urea-formaldehyde resins, modified melamine-formaldehyde resins, epichlorohydrin-modified resins, anionic polyamide resins), epoxy curing agents, polyamide resins, surfactants, softeners, corrosion inhibitors, lubricating oil and fuel additives, and asphalt emulsifiers. (Ref. 5.5.)
1.1.4. Physical properties (Ref. 5.5.)
|
| |||
| EDA | DETA | TETA | |
|
| |||
| CAS no. | 107-15-3 | 111-40-0 | 112-24-3 |
| molecular weight: | 60.1 | 103.2 | 146.2 |
| boiling point, °C: (at 760 mm Hg) |
117.0 | 206.7 | 277.4 |
| freezing point, °C: | 10.8 | -35 | -39 |
| color:(Ref. 5.6.) | colorless | yellow | yellow |
| specific gravity: (20/20°C)(Ref. 5.6.) |
0.8995 | 0.9542 | 0.9818 |
| vapor pressure, mm Hg: (at 20°C)(Ref. 5.6.) |
10.7 | 0.37 | |
| flash point, °C: (closed cup) |
40 | 98 | 118 |
| odor: | all have strong ammoniacal odor | ||
| molecular formulae: | H2N(CH2)2NH2 H2N(CH2)2NH(CH2)2NH2 H2N(CH2CH2NH)2CH2CH2NH2 |
(EDA) (DETA) (TETA) | |
- 1.2. Limit defining parameters (The analyte air concentrations
listed throughout this method are based on an air volume of 10 L and a
solvent desorption volume of 2.0 mL. Air concentrations listed in ppm
are referenced to 25°C and 760 mm Hg. Although the derivatives of the
amines are analyzed, the equivalent mass of the amines is listed
throughout the method.)
- 1.2.1. Detection limit of the analytical procedure
The detection limits of the analytical procedure are 4.6, 0.8, and 1.3 ng per injection for EDA, DETA, and TETA respectively. This amount of EDA gives a measurable response with the amounts of interferences present in an EDA standard. These amounts of DETA and TETA give peaks whose heights are approximately 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limits of the overall procedure are 3.7, 0.16, and 0.26 µg per sample for EDA, DETA, and TETA respectively. This is the amount of EDA which when spiked onto a sample tube and then desorbed gives a measurable response in the presence of trace interferences. These are the amounts of DETA and TETA which when spiked onto a sampling tube allow recovery of amounts equivalent to the detection limits of the analytical procedure. These detection limits correspond to air concentrations of 0.15 ppm (0.37 mg/m3), 0.004 ppm (0.016 mg/m3), and 0.004 ppm (0.026 mg/m3) for EDA, DETA, and TETA respectively. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limits are the same as the detection limits of the overall procedure since the desorption efficiencies are essentially 100% at these levels. These are the smallest amounts of analytes which can be quantitated within the requirements of recoveries of at least 75% and precisions (±1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limits and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amounts of analytes. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Instrument response to the analyte
The instrument response over the concentration ranges of 0.5 to 2 times the target concentrations is linear for all three analytes. (Section 4.3.)
1.2.5. Recovery
The recovery of EDA, DETA, and TETA from samples used in 15-day storage tests remained above 92, 87, and 89% respectively. (Section 4.4.) The storage samples were stored in a closed drawer at ambient temperatures. The recovery of analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical method only)
The pooled coefficients of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentrations are 0.013, 0.007, and 0.018 for EDA, DETA, and TETA respectively. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precisions at the 95% confidence level for the 15-day storage tests are ±10.7, ±11.5, and ±10.9% for EDA, DETA, and TETA respectively. (Section 4.4.) These include an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples for each analyte, prepared by liquid injection of standards onto the coated resin, and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after refrigerated storage for 15 days. No individual result deviated from its theoretical value by more than the precision reported in Section 1.2.7. (Section 4.5.)
- 1.3. Advantages
- 1.3.1. The solid sorbent tube provides a convenient method for
sampling.
1.3.2. The analysis is rapid, sensitive and precise.
1.3.3. The method involves analysis of derivatives, which is much more convenient than analyzing free amines.
- 1.4. Disadvantage
Sampling tubes are not commercially available at this time.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated within ±5% of the recommended flow rate with
the sampling tube in line.
2.1.2. Samples are collected on solid sorbent sampling tubes containing XAD-2 resin coated with 10% NITC by weight. A sampling tube consists of two sections of coated XAD-2 resin separated by a Teflon wool (Alltech) plug. The front section contains 80 mg of coated sorbent and the back section 40 mg. The sections are held in place with Teflon wool plugs in a glass tube 4-mm i.d. × 70-mm length.
The adsorbent is prepared by coating commercially purified 16/50 mesh XAD-2 (Supelco) with 10% NITC by weight using methylene chloride as a solvent. The solvent is removed by rotary evaporation.
2.1.3. Lengths of flexible tubing are needed to connect the sampling tubes to the sampling pumps.
2.1.4. Two plastic caps and an OSHA Form 21 are needed to seal each sampling tube after sampling.
- 2.2. Reagents
No sampling reagents are required.
2.3. Technique
- 2.3.1. Connect the sampling tube to the sampling pump with
flexible tubing. Air should pass through the 80-mg section first and
should not pass through any hose or tubing before entering the
sampling tube.
2.3.2. Place the sampling tube vertically in the worker's breathing zone.
2.3.3. After sampling, seal the tubes immediately with plastic caps and wrap lengthwise with OSHA Form 21.
2.3.4. Submit at least one blank for each sample set. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.5. Record sample volumes (in liters of air) for each sample, along with any potential interferences.
2.3.6. Ship any bulk sample(s) in a container separate from the air samples.
- 2.4. Sampler capacity
- 2.4.1. The front sections of the sampling tubes contain 8 mg of
NITC. This amount of NITC could theoretically collect 1.3 mg of EDA,
1.5 mg of DETA, or 1.6 mg of TETA. For a 10-L air sample, these
amounts correspond to 53 ppm EDA, 36 ppm DETA, or 27 ppm TETA.
2.4.2. Due to the reactivity, corrosive nature, and high boiling points of the analytes, it was impossible to prepare test atmospheres using the generating equipment available. Thus, a number of vapor-spiking experiments were done by drawing humidified air through a Teflon wool plug, that had been spiked with the pure amine of interest, which was positioned ahead of a sampling tube. In all cases there was either no or only a small amount of amine derivative found on the backup section. (Section 4.6.)
- 2.5. Desorption efficiency
- 2.5.1. The average desorption efficiencies from the lot of
coated resin used in this evaluation for EDA, DETA, and TETA are
99.2, 99.0, and 99.8% respectively over the range of 0.5 to 2 times
the target concentrations. (Section 4.7.)
2.5.2. Desorbed samples remained stable for at least 24 h. (Section 4.8.)
2.5.3. Desorption efficiencies must be determined for the lot of sampling tubes used for samples.
- 2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.1 L/min.
- 2.7. Interferences (sampling)
- 2.7.1. The presence of other primary or secondary amines could
reduce the capacity of the sampler.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
- 2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker so that it
will not interfere with work performance or safety.
2.8.2. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. An HPLC equipped with an ultraviolet detector. For this
evaluation a Waters
3.1.2. An HPLC column capable of separating the amine derivative of interest from DMF, NITC, and interferences. A Waters Radial Compression Separation System consisting of an RCM-100 module and a 10-µm Radial CN column was used in this evaluation.
3.1.3. An electronic integrator or some other means of measuring detector response in terms of peak areas or heights.
3.1.4. Small resealable vials with Teflon-lined caps or septa capable of holding at least 3 mL. WISP vials were used in this evaluation.
3.1.5. A dispenser or pipet capable of delivering 2.0 mL DMF.
3.1.6. Volumetric flasks and pipets for preparation of standards.
3.1.7. An analytical balance capable of weighing to the nearest 0.01 mg.
- 3.2. Reagents
- 3.2.1. Ethylenediamine (EDA), diethylenetriamine (DETA), and
triethylenetetramine (TETA) of known purity. EDA, DETA, and TETA
from Aldrich Chemical Company were used.
3.2.2. Dimethylformamide (DMF), LC grade. Burdick and Jackson 'Distilled in Glass' DMF was used.
3.2.3. 1-Naphthylisothiocyanate (NITC), reagent grade. NITC from Aldrich Chemical Company was used.
3.2.4. Isooctane and isopropanol, HPLC grade.
- 3.3. Standard preparation
- 3.3.1. Individual concentrated amine derivative standards are
prepared by adding an excess amount of NITC to a known amount of
amine that had been weighed in a volumetric flask. The neat reaction
is allowed to proceed at room temperature for at least 1 h. The
derivative and excess NITC are then dissolved with DMF. The solution
is brought up to the mark with DMF. Stock standards are stable for
at least a month when stored in brown bottles at room temperature.
Example: 89.18 mg of EDA are weighed in a 50-mL flask. Since one molecule of derivative is formed from two molecules of NITC (MW 185.25) and one molecule of EDA (MW 60.1), an excess of NITC would be an amount greater than 550 mg. In this case about 600 mg of NITC are added. The resulting concentration of this stock standard is 1.784 mg of EDA per milliliter.
(Note: It takes three moles of NITC per one mole of DETA and four moles of NITC per one mole of TETA to form one mole of each derivative.)
3.3.2. Working standards are prepared by diluting stock standards with DMF. Since samples are desorbed with 2.0 mL of DMF it is convenient to express the concentration of working standards in terms of µg of amine per sample. For the above example if 2.0 mL of the stock were diluted to 25.0 mL with DMF the resulting concentration would be 142.7 µg of EDA per mL or 285.4 µg of EDA per sample.
- 3.4. Sample preparation
- 3.4.1. The two adsorbent sections of each sample are transferred
to separate WISP vials.
3.4.2. The samples are desorbed by adding 2.0 mL of DMF to each vial.
3.4.3. The samples are allowed to desorb for at least 30 min with occasional shaking.
- 3.5. Analysis
- 3.5.1. HPLC conditions
| column: | 10-µm Radial CN, 100-mm × 8-mm i.d. |
| mobile phase: | 80:20 (v/v) isooctane/isopropanol, for EDA and 50:50 for DETA and TETA |
| flow rate: | 3 mL/min |
| injection size: | 10 µL |
| detector: | UV at 254 nm |
| chromatograms: | Section 4.9. |
3.5.2. Peak areas or heights are measured by an integrator or other suitable means.
3.5.3. A calibration curve is constructed by plotting detector response of standard injections versus µg of amine per sample. Sample concentrations must be bracketed by standards.
- 3.6. Interferences (analytical)
- 3.6.1. Any compound that gives a detector response and has the
same general retention time as the amine derivative of interest is a
potential interference. Suspected interferences reported to the
laboratory with submitted samples by the industrial hygienist must
be considered before samples are desorbed.
3.6.2. Chromatographic parameters may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Analyte identity should be confirmed by mass spectrometry if possible.
- 3.7. Calculations
The analyte concentration for samples is obtained from the calibration curve in terms of µg of amine per sample. If any amine is found on the backup section it is added to the amount found on the front section. This total amount is corrected by subtracting any amount found on the blank. The air concentrations are calculated using the following formulae:
| mg/m3 = | (micrograms of amine per sample)
(liters of air sampled) (desorption efficiency) |
| ppm = | (mg/m3) (24.46)
(molecular weight of analyte) |
| where | 24.46 | = | molar volume (liters) at 25°C and 760 mm Hg |
| MW | = | molecular weight (EDA, 60.1; DETA, 103.2; TETA, 146.2) |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood when possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
An injection size of 10 µL was used in the determination of the detection limits of the analytical procedure. The detection limits of 4.6 ng of EDA, 0.80 ng of DETA, and 1.3 ng of TETA were determined by making injections of 0.46 ng/µL, 0.08 ng/µL, and 0.13 ng/µL standards respectively. Chromatograms of such injections are shown in Figures 4.1.1. and 4.1.2.
4.2. Detection limit of the overall procedure and reliable quantitation limit
Six samples were prepared for each analyte by injecting 3.68 µg of EDA, 0.16 µg of DETA, and 0.26 µg of TETA into separate sampling tubes. Since there are more analytical interferences for EDA found in samples than standards, a larger amount of EDA had to be spiked onto the sample tubes in order to obtain a measurable amount of derivative. The samples were analyzed the next day to determine the amount recovered. Since the amounts recovered were nearly 100%, the detection limits of the overall procedure and the reliable quantitation limits (RQL) are taken to be 3.7, 0.16, and 0.26 µg per sample for EDA, DETA, and TETA respectively. These limits correspond to air concentrations of 0.15 ppm (0.37 mg/m3), 0.004 ppm (0.016 mg/m3), and 0.004 ppm (0.026 mg/m3) for EDA, DETA, and TETA respectively.
Detection Limit and RQL Data
|
| |||
| analyte | EDA | DETA | TETA |
| µg/sample | 3.68 | 0.16 | 0.26 |
|
| |||
| % recovery | 87.2 | 90.5 | 103.7 |
| 111.4 | 88.3 | 91.5 | |
| 93.2 | 92.6 | 85.4 | |
| 102.2 | 101.2 | 103.7 | |
| 93.2 | 103.3 | 97.6 | |
| 108.4 | 103.3 | 103.7 | |
| 99.3 | 96.5 | 97.6 | |
| SD | 9.6 | 6.8 | 7.7 |
| 1.96 SD | 18.8 | 13.3 | 15.1 |
|
| |||
4.3. Instrument Response and Precision (analytical method only)
The instrument response and precision of the analytical procedure were determined from multiple injections of analytical standards. These data are given in Tables 4.3.1. - 4.3.3. and Figures 4.3.1. and 4.3.2.
EDA Instrument Response and Precision Data
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 116.2 | 232.4 | 464.8 |
| ppm | 4.7 | 9.5 | 18.9 |
|
| |||
| area | 3775210 | 7606970 | 14609800 |
| counts | 3785800 | 7872510 | 14619200 |
| 3811990 | 7823330 | 14569700 | |
| 3873840 | 7570560 | 14433800 | |
| 3746570 | 7616510 | 14569700 | |
| 3769720 | 7545170 | 14641200 | |
| 3793855 | 7672508 | 14573900 | |
| SD | 44626 | 139131 | 74206 |
| CV | 0.012 | 0.018 | 0.005 |
| slope = 31700 area counts per µg/sample | |||
|
| |||
DETA Instrument Response and Precision Data
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 20.01 | 40.02 | 80.03 |
| ppm | 0.47 | 0.95 | 1.90 |
|
| |||
| area | 576229 | 1152670 | 2332620 |
| counts | 572171 | 1183290 | 2324940 |
| 573659 | 1169070 | 2323450 | |
| 581693 | 1182010 | 2314280 | |
| 578896 | 1169190 | 2314580 | |
| 579463 | 1167010 | 2327930 | |
| 577018 | 1170540 | 2322967 | |
| SD | 3654 | 11224 | 7318 |
| CV | 0.006 | 0.010 | 0.003 |
| slope = 29100 area counts per µg/sample | |||
|
| |||
TETA Instrument Response and Precision Data
|
| ||||||
| × target conc. | 0.5× | 1× | 2× | |||
| µg/sample | 29.62 | 59.23 | 118.5 | |||
| ppm | 0.50 | 0.99 | 1.98 | |||
|
| ||||||
| area | 725221 | 1386940 | 2769150 | |||
| counts | 723390 | 1421720 | 2709920 | |||
| 713792 | 1414790 | 2810150 | ||||
| 718708 | 1424570 | 2738470 | ||||
| 705824 | 1394920 | 2872080 | ||||
| 688488 | 1393210 | 2764600 | ||||
| 712570 | 1406025 | 2777395 | ||||
| SD | 13724 | 16241 | 57163 | |||
| CV | 0.019 | 0.012 | 0.021 | |||
| slope = 23500 area counts per µg/sample | ||||||
|
| ||||||
4.4. Storage
Thirty-six storage samples were prepared for each amine. Samplers were spiked with a known amount of amine and about 10 L of air at 80% relative humidity and ambient temperature of 20 to 26°C were then drawn through each of them. The EDA samples were spiked with 246 µg, the DETA samples with 43.2 µg, and the TETA samples with 58.1 µg. For a 10-L air sample, these amounts would be equivalent to 10.0 ppm EDA, 1.02 ppm DETA, and 0.97 ppm TETA. Six samples for each amine were analyzed immediately, fifteen were stored in a refrigerator at 2°C, and fifteen were stored in a closed drawer at ambient temperature. Six samples for each amine, three from refrigerated storage and three from ambient storage, were analyzed at intervals over a period of fifteen days. The results are given in Tables 4.4.1. - 4.4.3. and Figures 4.4.1. - 4.4.6.
Storage Data for EDA
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 91.7 | 92.9 | 90.8 | 91.7 | 92.9 | 90.8 | |
| 0 | 91.6 | 91.8 | 91.1 | 91.6 | 91.8 | 91.1 | |
| 2 | 95.1 | 97.2 | 94.2 | 95.9 | 94.1 | 93.1 | |
| 4 | 91.8 | 89.1 | 85.7 | 93.0 | 92.5 | 96.1 | |
| 6 | 98.2 | 97.7 | 95.2 | 95.9 | 94.0 | 93.1 | |
| 11 | 97.9 | 98.6 | 101.7 | 98.6 | 97.9 | 98.3 | |
| 15 | 95.3 | 96.2 | 94.6 | 90.0 | 94.9 | 98.4 | |
|
| |||||||
Storage Data for DETA
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 86.9 | 90.3 | 92.0 | 86.9 | 90.3 | 92.0 | |
| 0 | 91.7 | 90.9 | 90.6 | 91.7 | 90.9 | 90.6 | |
| 2 | 87.2 | 83.6 | 86.1 | 83.7 | 84.8 | 84.6 | |
| 4 | 86.2 | 92.4 | 91.7 | 92.3 | 84.7 | 85.7 | |
| 6 | 89.0 | 91.7 | 88.3 | 89.9 | 88.8 | 84.2 | |
| 11 | 88.0 | 88.2 | 88.1 | 86.1 | 86.6 | 82.2 | |
| 15 | 88.5 | 91.8 | 86.7 | 89.5 | 90.4 | 88.5 | |
|
| |||||||
Storage Data for TETA
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 91.0 | 91.6 | 92.4 | 91.0 | 91.6 | 92.4 | |
| 0 | 91.6 | 90.8 | 90.4 | 91.6 | 90.8 | 90.4 | |
| 2 | 89.5 | 87.2 | 90.7 | 90.8 | 88.8 | 89.6 | |
| 4 | 91.2 | 94.6 | 92.9 | 93.9 | 84.3 | 85.8 | |
| 6 | 88.8 | 91.4 | 87.0 | 89.1 | 88.2 | 86.0 | |
| 11 | 93.2 | 93.6 | 93.2 | 91.0 | 90.4 | 86.0 | |
| 15 | 89.2 | 94.3 | 87.5 | 91.6 | 91.9 | 89.8 | |
|
| |||||||
4.5. Reproducibility
Six samples for each amine were prepared by injecting µL amounts of amine standards onto the NITC-coated resin. The samples were analyzed by a chemist unassociated with this evaluation. The results are given in Tables 4.5.1. - 4.5.3.
Reproducibility Data for EDA
|
| |||
| sample no. | µg found | µg expected | % found |
|
| |||
| 1 | 223.8 | 241.9 | 92.5 |
| 2 | 223.4 | 241.9 | 92.4 |
| 3 | 227.3 | 241.9 | 94.0 |
| 4 | 227.2 | 241.9 | 93.9 |
| 5 | 227.9 | 241.9 | 94.2 |
| 6 | 226.4 | 241.9 | 93.6 |
|
| |||
Reproducibility Data for DETA
|
| |||
| sample no. | µg found | µg expected | % found |
|
| |||
| 1 | 40.93 | 41.98 | 97.5 |
| 2 | 41.80 | 41.98 | 99.6 |
| 3 | 40.96 | 41.98 | 97.6 |
| 4 | 40.45 | 41.98 | 96.4 |
| 5 | 41.03 | 41.98 | 97.7 |
| 6 | 41.84 | 41.98 | 99.7 |
|
| |||
Reproducibility Data for TETA
|
| |||
| sample no. | µg found | µg expected | % found |
|
| |||
| 1 | 57.05 | 59.19 | 96.4 |
| 2 | 58.45 | 59.19 | 98.7 |
| 3 | 57.22 | 59.19 | 96.7 |
| 4 | 56.68 | 59.19 | 95.8 |
| 5 | 57.42 | 59.19 | 97.0 |
| 6 | 58.65 | 59.19 | 99.1 |
|
| |||
4.6. Sampler capacity
A number of vapor-spiking experiments were done by drawing 10 L of air at 80% relative humidity through Teflon-wool plugs spiked with µL amounts of the pure amines. The plugs were positioned ahead of the samplers. Some of the results are given in Table 4.6.
Vapor-Spiking Experiments
|
| ||
| amine | µg found on | µg found on |
| spiked | 'A' section | 'B' section |
|
| ||
| EDA | 414 | 12.2 |
| EDA | 562 | 19.7 |
| DETA | 28.5 | None Detected |
| DETA | 57.2 | None Detected |
| TETA | 1.8 | None Detected |
| TETA | 32.5 | None Detected |
|
| ||
4.7. Desorption efficiency
The desorption efficiency for each analyte was determined by injecting known amounts of amine standards onto the front sections of NITC-coated resin tubes. The samples were stored in a refrigerator and analyzed the next day. The results are given in Tables 4.7.1. - 4.7.3.
Desorption Efficiency for EDA
|
| |||
| µg/sample | 123.1 | 246.2 | 492.5 |
| ppm | 5.0 | 10.0 | 20.0 |
|
| |||
| % desorption | 102.7 | 97.6 | 95.0 |
| 102.9 | 96.0 | 97.0 | |
| 106.3 | 97.9 | 97.1 | |
| 103.9 | 98.1 | 97.3 | |
| 105.0 | 99.7 | 95.0 | |
| 101.1 | 98.1 | 94.9 | |
| 103.6 | 97.9 | 96.0 | |
|
| |||
Desorption Efficiency for DETA
|
| |||
| µg/sample | 20.99 | 41.98 | 83.96 |
| ppm | 0.50 | 0.99 | 1.99 |
|
| |||
| % desorption | 98.6 | 98.3 | 99.2 |
| 98.8 | 98.7 | 99.9 | |
| 104.2 | 97.5 | 98.5 | |
| 98.9 | 98.9 | 99.3 | |
| 98.6 | 99.9 | 98.9 | |
| 97.1 | 97.9 | 98.9 | |
| 99.4 | 98.5 | 99.1 | |
|
| |||
Desorption Efficiency for TETA
|
| |||
| µg/sample | 29.59 | 59.19 | 118.4 |
| ppm | 0.50 | 0.99 | 1.98 |
|
| |||
| % desorption | 100.8 | 98.9 | 99.1 |
| 101.0 | 99.6 | 99.6 | |
| 106.4 | 98.0 | 98.4 | |
| 102.3 | 99.9 | 99.1 | |
| 100.5 | 100.5 | 98.3 | |
| 96.8 | 98.5 | 98.1 | |
| 101.3 | 99.2 | 98.8 | |
|
| |||
4.8. Stability of desorbed samples
The desorption efficiency samples at the target conentration for each amine were reanalyzed the next day.
Stability of Desorbed Samples
|
| |||
| analyte | EDA | DETA | TETA |
|
| |||
| % desorption | 96.5 | 99.9 | 98.6 |
| 94.3 | 100.2 | 99.2 | |
| 93.1 | 99.0 | 98.4 | |
| 97.4 | 99.2 | 98.9 | |
| 96.0 | 97.8 | 97.8 | |
| 97.2 | 95.8 | 97.0 | |
| 95.8 | 98.7 | 98.3 | |
|
| |||
4.9. Chromatogram
A chromatogram of a 232.4 µg/sample EDA standard is shown in Figure 4.9.1. and a chromatogram of a 40.01 µg/sample DETA standard and a 63.18 µg/sample TETA standard is shown in Figure 4.9.2.
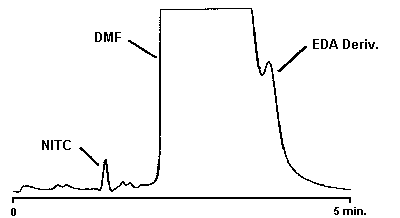
Figure 4.1.1. Detection limit chromatogram for EDA.
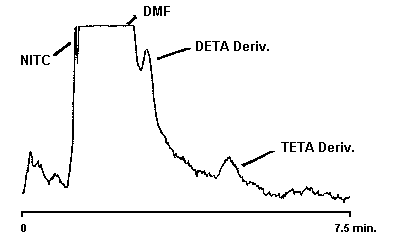
Figure 4.1.2. Detection limit chromatogram for DETA and TETA.
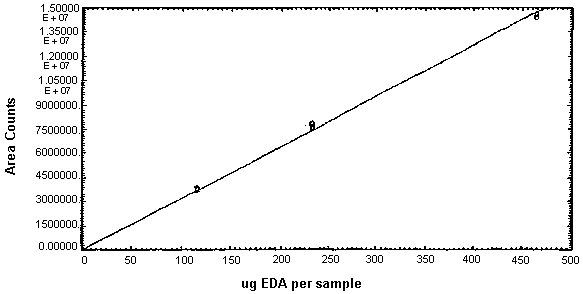
Figure 4.3.1. Calibration curve for EDA. 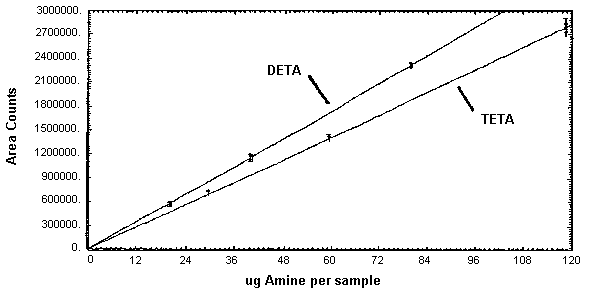
Figure 4.3.2. Calibration curves for DETA and TETA.
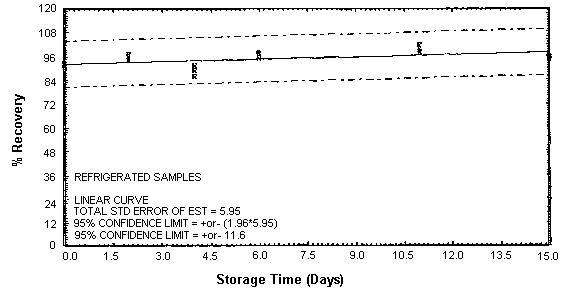
Figure 4.4.1. Refrigerated EDA storage samples. 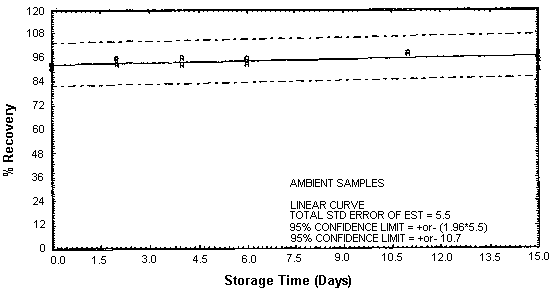
Figure 4.4.2. Ambient EDA storage samples. 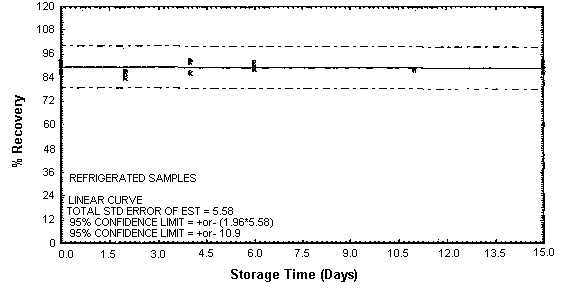
Figure 4.4.3. Refrigerated DETA storage samples. 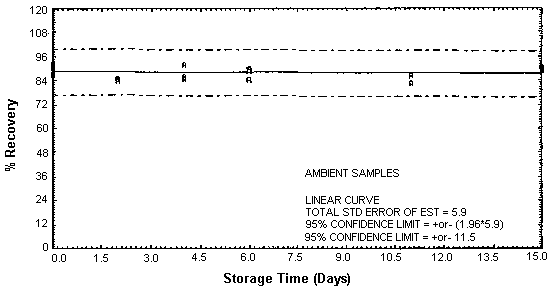
Figure 4.4.4. Ambient DETA storage samples. 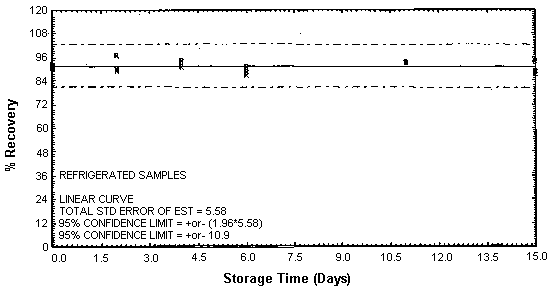
Figure 4.4.5. Refrigerated TETA storage samples. 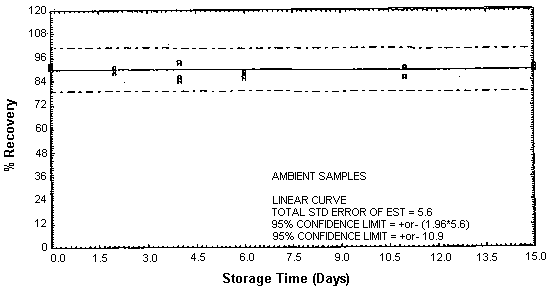
Figure 4.4.6. Ambient TETA storage samples. 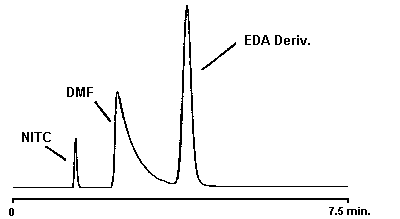
Figure 4.9.1. Chromatogram of an EDA standard. 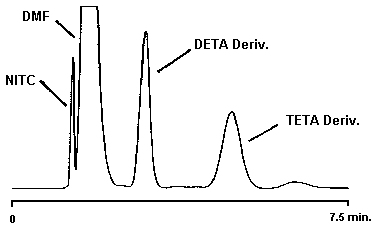
Figure 4.9.2. Chromatogram of a DETA and TETA standard.
5. References
- 5.1. "Chemical Information File", U.S. Department of Labor,
Occupational Safety & Health Administration, Directorate of
Technical Support, June 14, 1985.
5.2. Elskamp, C.J.; Schultz, G.R. Am. Ind. Hyg. Assoc. J. 1986, 47, 41-49.
5.3. Andersson, K.; Hallgren, C.; Leaven, J.; Nelson, C. Am. Ind. Hyg. Assoc. J. 1985, 46, 225-229.
5.4. Outer, C.M.; Moffett, E.W. J. Am. Chem. Soc. 1933, 55, 2497-2499.
5.5. Spitz, R.D. in "Kirk-Othmer Encyclopedia of Chemical Technology"; Vol. 7, 3rd ed., Part 7.; Grayson, M., Ed.; John Wiley & Sons: New York, N.Y., 1979; pp 580-602.
5.6. "The Condensed Chemical Dictionary", 8th ed.; Van Nostrand Reinhold Co.: New York, N.Y., 1971.