| 2-METHOXYETHANOL (METHYL CELLOSOLVE,
2ME) 2-METHOXYETHYL ACETATE (METHYL CELLOSOLVE ACETATE, 2MEA) 2-ETHOXYETHANOL (CELLOSOLVE, 2EE) 2-ETHOXYETHYL ACETATE (CELLOSOLVE ACETATE, 2EEA) |
| Method no.: | 53 | ||||||||||||
| Matrix: | Air | ||||||||||||
| Procedure: | Samples are collected by drawing known volumes of air through charcoal tubes. Samples are desorbed with 95/5 (v/v) methylene chloride/ methanol and analyzed by gas chromatography using a flame ionization detector. | ||||||||||||
| Recommended air volume and sampling rate: |
10 L at 0.1 L/min | ||||||||||||
| |||||||||||||
| OSHA PEL*
ppm(mg/m3) Target conc. ppm(mg/m3) Reliable quantitation limits: ppm(mg/m3) Standard error of estimates at target concentrations: |
| ||||||||||||
| (Section 4.4.) |
| ||||||||||||
| *All have "Skin" notation. | |||||||||||||
| Special requirements: | Samples for 2MEA and 2EEA should be refrigerated upon receipt by the laboratory to minimize hydrolysis. | ||||||||||||
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. | ||||||||||||
| Date: January 1985 | Chemist: Carl J. Elskamp | ||||||||||||
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The procedures used by OSHA to determine airborne concentrations of 2ME, 2MEA, 2EE, and 2EEA are based on NIOSH methods S79(Ref. 5.1.), S39(Ref. 5.2.), S361(Ref. 5.3.), and S41(Ref. 5.4.). All four of these methods specify collection on activated charcoal sampling tubes and analysis by gas chromatography (GC) using flame ionization detection. The methods for 2ME and 2EE specify 95/5(v/v) methylene chloride/methanol for the desorption solvent, while the methods for 2MEA and 2EEA utilize carbon disulfide. NIOSH validated these methods over the approximate ranges of 10 to 50 ppm 2ME, 10 to 50 ppm 2MEA, 100 to 400 ppm 2EE, and 50 to 200 ppm 2EEA.
ACGIH has proposed to reduce the time-weighted TLVs for these four analytes to 5 ppm (Refs. 5.5.-5.8.), thus target concentrations at this level were chosen for this evaluation. In NIOSH's evaluation of the 2MEA method, it was reported that the desorption efficiency dropped off with mass of analyte collected. (Ref. 5.9.) At a loading of 4.83 mg the average desorption efficiency was 81.9%. at 2.42 mg it was 80.9%, and at 1.21 mg it was 72.3%. For 10-L air samples, this range equals 100 down to 25 ppm. Similar results were reported for 2EEA. (Ref. 5.10.) At a loading of 10.7 mg the average desorption was 84.2%, at 5.26 mg it was 83.9%, and at 2.53 mg it was 68.0%. For 10-L air samples, this range equals 200 down to 50 ppm. Since the selected target concentration of 5 ppm is much lower than these two ranges, it was anticipated that the desorption efficiency at this level would be unacceptably low, thus a better desorption solvent was needed for 2MEA and 2EEA.
An average desorption efficiency of 97.6% was reported by NIOSH for the 2ME method over the range of 65 to 260 ppm for 10-L air samples with no apparent decrease with decreasing concentration. (Ref. 5.11.) Thus a methylene chloride/methanol mixture was considered for a desorption solvent for all four analytes.
Initial tests were done to determine if the recommended 95/5 (v/v) mixture was the optimum ratio of methylene chloride to methanol. Desorption studies were done on all four analytes using various mixtures if the two solvents ranging from 99/1 (v/v) methylene chloride/methanol to pure methanol. The 95/5 and 90/10 mixtures gave essentially the same efficiencies of around 100% for all four analytes, while the efficiencies for 2ME and 2EE were lower for a 99/1 mixture and the efficiencies for 2MEA and 2EEA were lower for a 50/50 mixture. The desorption efficiencies for all four analytes were much lower when pure methanol was used compared to the 95/5 and 90/10 mixtures. To maintain consistency with the NIOSH methods for 2ME and 2EE, a 95/5 mixture was chosen.
Many GC column packings that are used for analysis of solvent samples are suitable for glycol ethers. These include 20% SP-2100/0.1% CW1500 on 80/100 Supelcoport, 10% SP-1000 on 80/100 Supelcoport, and 0.1% SP-1000 on Carbopack C. Another packing that was found to work particularly well was 0.2% CW1500 on 80/100 Carbopack C. This was used throughout the evaluation. For convenience in packing, a glass column was used. It is believed that a stainless steel column will perform just as well.
Breakthrough studies were performed as discussed in Section 2.4. The breakthrough volumes are much higher than the recommended sample volume, but to maintain consistency with the many other solvent methods that specify charcoal sampling tubes, 10 L was chosen. This also provides a safety margin when other solvents are present in the sampled atmosphere. The presence of other solvent vapors will diminish the total capacity of the charcoal for the analytes.
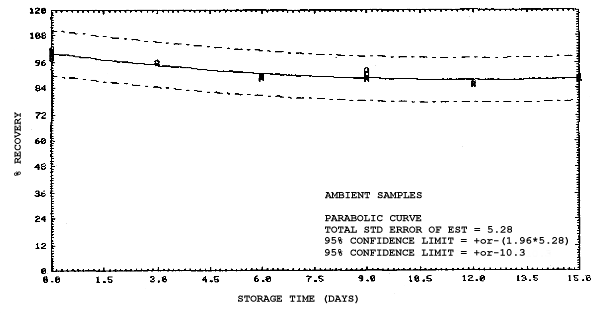
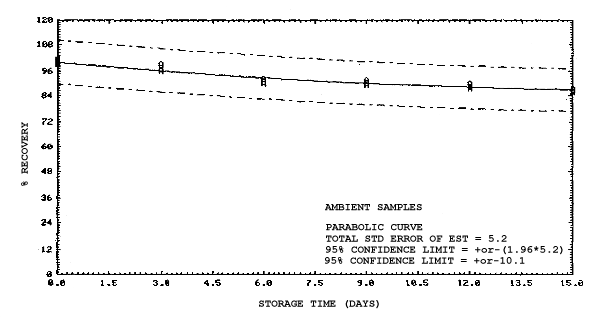
All four analytes were found to be stable on charcoal except when 2MEA and 2EEA were stored at ambient temperatures. Under this condition there is some loss of these two analytes through hydrolysis. This was confirmed by analyzing some of the 2MEA samples for 2ME, and 2EEA samples for 2EE, that had been stored at ambient temperatures for 15 days. The amounts of 2ME and 2EE found accounted for the corresponding loss of 2MEA and 2EEA. Although the loss is only on the order of 10 to 15% after 15 days compared to refrigerated samples, it is recommended that samples for 2MEA and 2EEA be refrigerated until analyzed as a precautionary measure.
All four analytes were successfully evaluated by collection on charcoal, desorption with 95/5 (v/v) methylene chloride/methanol, and analysis by GC using flame ionization detection.
1.1.2. Toxic effects (This section is for information only and should not be taken as basis for OSHA policy.)
As reported in the Documentation of Threshold Limit Values (Refs. 5.5. - 5.8.), all four analytes were investigated by Nagano et al (Ref. 5.12.) in terms of potency for testicular effects. They concluded that on an equimolar basis, the respective acetate esters were about as potent as 2ME and 2EE in producing testicular atrophy and leukopenia (an abnormally low number of white blood cells) in mice. Based on this study and because 2MEA and 2EEA hydrolyze to 2ME and 2EE respectively in the body, ACGIH suggests lowering the time-weighted TLVs for all four analytes to 5 ppm.
The following is quoted from NIOSH Current Intelligence Bulletin 39. (Ref. 5.13.)
- The National Institute for Occupational Safety and Health
(NIOSH) recommends that 2-methoxyethanol (2ME) and 2-ethoxyethanol
(2EE) be regarded in the workplace as having the potential to
cause adverse reproductive effects in male and female workers.
These recommendations are based on the results of several recent
studies that have demonstrated dose-related embryotoxicity and
other reproductive effects in several species of animals exposed
by different routes of administration. Of particular concern are
those studies in which exposure of pregnant animals to
concentrations of 2ME or 2EE at or below their respective
Occupational Safety and Health Administration (OSHA) Permissible
Exposure Limits (PELs) led to increased incidences of embryonic
death, teratogenesis, or growth retardation. Exposure of male
animals resulted in testicular atrophy and sterility. In each case
the animals had been exposed to 2ME or 2EE at concentrations at or
below their respective OSHA PELs. Therefore, appropriate controls
should be instituted to minimize worker exposure to both
compounds.
1.1.3. Workplace exposure
2ME- It is used as a solvent for many purposes: cellulose esters, dyes, resins, lacquers, varnishes, and stains; and as a perfume fixative and jet fuel deicing additive. (Ref. 5.5.)
2MEA- It is used in photographic films, lacquers, textile printing, and as a solvent for waxes, oils, various gums and resins, cellulose acetate, and nitrocellulose. (Ref. 5.6.)
2EE- It is used as a solvent for nitrocellulose, natural and synthetic resins, and as a mutual solvent for the formulation of soluble oils. It is also used in lacquers, in the dyeing and printing of textiles, in varnish removers, cleaning solutions, in products for the treatment of leather, and as an anti-icing additive for aviation fuels. (Ref. 5.7.)
2EEA- It is used as a blush retardant in lacquers; as a solvent for nitrocellulose, oils and resins; in wood stains, varnish removers, and in products for the treatment of textiles and leathers. (Ref. 5.8.)
1.1.4. Physical properties (Refs. 5.5.-5.8.)
|
| ||||
| analyte | 2ME | 2MEA | 2EE | 2EEA |
|
| ||||
| mol wt: | 76.09 | 118.13 | 90.11 | 132.16 |
| bp (°C): | 124.5 | 145 | 135.6 | 156.4 |
| color: | all are colorless liquids | |||
| sp gr: | 0.9663 | 1.005 | 0.931 | 0.975 |
| vp: (mm Hg at 20°C) |
6 | 2 | 3.7 | 2 |
| flash pt.: (°C, closed cup) |
43 | 49 | 40 | 49 |
| odor: (Ref. 5.14.) |
mild non-residual |
mild, ether-like |
sweetish | mild, non-residual |
| explosive limits, % (Ref. 5.14.) lower: upper: |
2.5 19.8 |
1.1 8.2 |
1.8 14 |
1.7 ? |
|
| ||||
synonyms: (Ref. 5.14.)
2ME - Methyl Cellosolve; Glycol monomethyl ether; Ethylene glycol monomethyl ether; Methyl oxitol; Ektasolve; Jeffersol EM
2MEA - Methyl Cellosolve Acetate; Glycol monomethyl ether acetate; Ethylene glycol monomethyl; ether acetate
2EE - Cellosolve solvent; Ethylene glycol monoethyl ether
2EEA - Cellosolve acetate; Glycol monoethyl ether acetate; Ethylene glycol monoethyl ether acetate
chemical formulae:
| 2ME- CH3OCH2CH2OH | 2MEA- CH3OCH2CH2OOCCH3 |
| 2EE- CH3CH2OCH2CH2OH | 2EEA- CH3CH2OCH2CH2OOCCH3 |
1.2. Limit Defining Parameters (The analyte air concentrations listed throughout this method are based on an air volume of 10 L and a solvent desorption volume of 1.0 mL. Air concentrations listed in ppm are referenced to 25°C and 760 mm Hg.)
- 1.2.1. Detection limits of the analytical procedure
The detection limits of the analytical procedure are 2.6, 0.40, 0.47, and 0.46 ng per injection for 2ME, 2MEA, 2EE, and 2EEA respectively. These are the amounts of analytes which give measurable responses with the amounts of interferences present in standards. (Section 4.1.)
1.2.2. Detection limits of the overall procedure
The detection limits of the overall procedure are 3.1, 0.48, 0.56, and 0.55 µg per sample for 2ME, 2MEA, 2EE, and 2EEA respectively. These are the amounts of analytes spiked on the sampling device which allow recovery of amounts of analytes equivalent to the detection limits of the analytical procedure. These detection limits correspond to air concentrations of 0.1 ppm(0.3 mg/m3), 0.01 ppm(0.05 mg/m3), 0.02 ppm(0.06 mg/m3), and 0.01 ppm(0.05 mg/m3) for 2ME, 2MEA, 2EE, and 2EEA respectively. (Section 4.2.)
1.2.3. Reliable quantitation limits
The reliable quantitation limits are the same as the detection limits of the overall procedure since the desorption efficiencies are essentially 100% at these levels. These are the smallest amounts of analytes which can be quantitated within the requirements of recoveries of at least 75% and precisions (±1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limits and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amounts of analytes. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
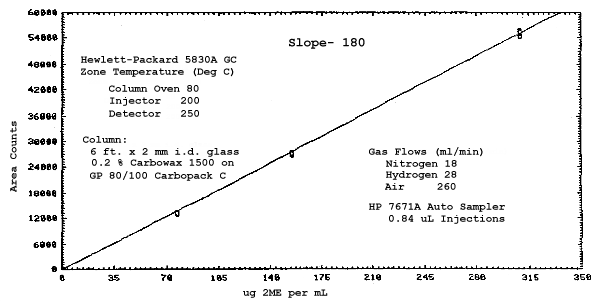
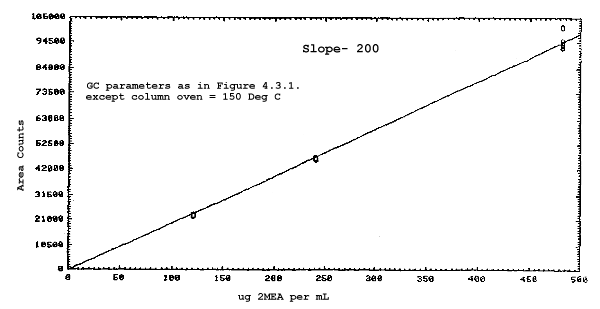
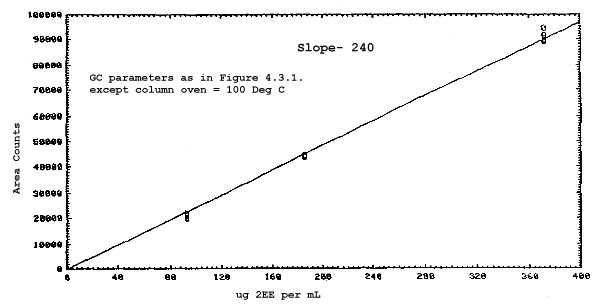
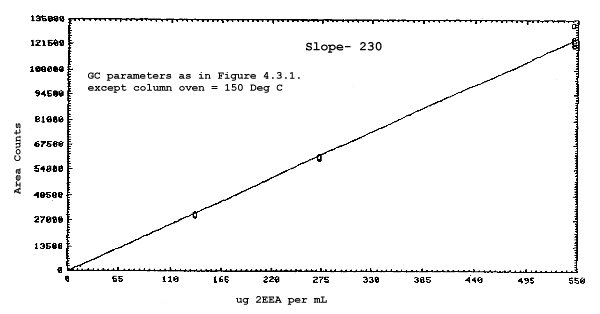
The sensitivities of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentrations based on the recommended air volume are 180, 200, 240, and 230 area units per µg/mL for 2ME, 2MEA, 2EE, and 2EEA respectively. These are determined by the slopes of the calibration curves. (Section 4.3.) The sensitivity will vary with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of 2ME, 2MEA, 2EE, and 2EEA from samples used in a 15-day storage test remained above 97, 97, 99, and 99% respectively. The 2ME and 2EE samples were stored in a closed drawer at ambient temperatures of 20 to 25°C and the 2MEA and 2EEA samples were stored in a refrigerator at 0°C. (Section 4.4.) The recovery of analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical method only)
The pooled coefficients of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentrations are 0.009, 0.019, 0.021, and 0.019 for 2ME, 2MEA, 2EE, and 2EEA respectively. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precisions at the 95% confidence level for the 15-day storage tests are ±10.5, ±10.1, ±10.5, and ±10.0% for 2ME, 2MEA, 2EE, and 2EEA respectively. (Section 4.4.) These include an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Six samples for each analyte collected from controlled test
atmospheres and a draft copy of this procedure were given to a
chemist unassociated with this evaluation. The samples for 2ME,
2MEA, 2EE, and 2EEA were analyzed after refrigerated storage for 24,
24, 16, and 18 days respectively. The average recoveries were 105%
(SD = 7.3%), 98.8%
1.3. Advantages
- 1.3.1. The solid sorbent tube provides a convenient method for
sampling.
1.3.2. The analysis is rapid, sensitive, and precise.
1.4. Disadvantages
- 1.4.1. Samples for 2MEA and 2EEA should be refrigerated until
analyzed to minimize hydrolysis.
1.4.2. It may not be possible to analyze co-collected solvents using this method. Most of the other common solvents are desorbed with carbon disulfide.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Samples are collected by use of a personal sampling pump
that can be calibrated within ±5% of the recommended flow rate with
the sampling tube in line.
2.1.2. Samples are collected on solid sorbent sampling tubes containing coconut shell charcoal. Each tube consists of two sections of charcoal separated by a urethane foam plug. The front section contains 100 mg of sorbent and the back section 50 mg. The sections are held in place with glass wool plugs in a glass tube 4-mm i.d. × 70-mm length. For this evaluation, SKC Inc. sorbent tubes (catalog number 226-01, lot 120) were used.
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Immediately before sampling, break off the ends of the
charcoal tube. All tubes should be from the same lot.
2.3.2. Connect the sampling tube to the sampling pump with flexible tubing. Position the tube so that sampled air first passes through the 100-mg section.
2.3.3. Air being sampled should not pass through any hose or tubing before entering the sampling tube.
2.3.4. Place the sampling tube vertically (to avoid channeling) in the employee's breathing zone.
2.3.5. After sampling, seal the tubes immediately with plastic caps and wrap lengthwise with OSHA Form 21.
2.3.6. Submit at least one blank sampling tube with each sample set. Blanks should be handled in the same manner as samples, except no air is drawn through them.
2.3.7. Record sample volumes (in liters of air) for each sample, along with any potential interferences.
2.3.8. Ship any bulk sample(s) in a container separate from the air samples.
2.4. Breakthrough
- 2.4.1. Individual breakthrough studies were performed on each of
the four analytes by monitoring the effluent from sampling tubes
containing only the 100-mg section of charcoal while sampling at 0.2
L/min from atmospheres containing 10 ppm analyte. The atmospheres
were at approximately 80% relative humidity and 20-25°C. No
breakthrough was detected in any of the studies after sampling for
at least 6 h (>70 L).
2.4.2. A similar study as in 2.4.1. was done while sampling an atmosphere containing 10 ppm of all four analytes. The atmosphere was sampled for more than 5 h (>60 L) with no breakthrough detected.
2.5. Desorption efficiency
- 2.5.1. The average desorption efficiencies of 2ME, 2MEA, 2EE,
and 2EEA are 97.9, 100.2, 99.0, and 101.6% respectively over the
range of 0.5 to 2 times the target concentrations. (Section 4.6.)
2.5.2. Desorption efficiencies must be determined for the lot of charcoal used for samples.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.1 L/min.
2.7. Interferences (sampling)
- 2.7.1. It is not known if any compound(s) will severely
interfere with the collection of any of the four analytes on
charcoal. In general, the presence of any other contaminant vapors
in the air will reduce the capacity of charcoal to collect the
analytes.
2.7.2. Suspected interferences should be reported to the laboratory with submitted samples.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the employee so that it
will not interfere with work performance or safety.
2.8.2. Wear eye protection when breaking the ends of the charcoal tubes.
2.8.3. Follow all safety procedures that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A GC equipped with a flame ionization detector. For this
evaluation, a Hewlett-Packard 5830A GC was used with a 7671A
Automatic Sampler.
3.1.2. A GC column capable of separating the analyte of interest from the desorption solvent and any interferences. A 6-ft × 2-mm i.d. glass column packed with 0.2% Carbowax 1500 on GP 80/100 Carbopack C was used in this evaluation.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas or heights.
3.1.4. Small vials with Teflon-lined caps capable of holding 2 mL.
3.1.5. A dispenser capable of delivering 1.0 mL to prepare standards and samples. If a dispenser is not available, a 1-mL pipet may be used.
3.1.6. Syringes such as 5-µL for preparation of standards and 1-µL for injection of samples and standards into a GC.
3.1.7. Volumetric flasks and pipets to dilute the pure analytes in preparation of standards.
3.2. Reagents
- 3.2.1. 2-Methoxyethanol, 2-methoxyethyl acetate,
2-ethoxyethanol, and 2-ethoxyethyl acetate, reagent grade.
3.2.2. Methylene chloride, chromatographic grade.
3.2.3. Methanol, chromatographic grade.
3.2.4. A suitable internal standard such as butanol or hexanol, reagent grade.
3.2.5. The desorption solvent consists of methylene chloride/methanol, 95/5 (v/v) containing an internal standard at a concentration of approximately 0.2 µL per mL.
3.2.6. GC grade nitrogen, air, and hydrogen.
3.3. Standard preparation
- 3.3.1. Concentrated stock standards are prepared by diluting the
pure analytes with methanol. Working standards are prepared by
injecting microliter amounts of concentrated stock standards into
vials containing 1.0 mL of desorption solvent delivered from a
dispenser.
3.3.2. Working standard concentrations should bracket sample concentrations. If samples fall outside of the concentration range of prepared standards, additional standards should be prepared and analyzed to ascertain the linearity of response.
3.4. Sample preparation
- 3.4.1. Transfer each section of the samples to separate vials.
The glass tubes and plugs are discarded.
3.4.2. Add 1.0 mL of desorption solvent to each vial using the same dispenser as used for preparation of standards.
3.4.3. The vials are immediately capped and shaken periodically for 30 min before analysis.
3.5. Analysis
- 3.5.1. GC conditions
| zone temperatures (°C): | column - | 80 for 2ME 150 for 2MEA 100 for 2EE 150 for 2EEA | ||||||||
| injector - | 200 | |||||||||
| detector - | 250 | |||||||||
| gas flows (mL/min): | nitrogen - | 18 | ||||||||
| hydrogen - | 28 | |||||||||
| air - | 240 | |||||||||
| injection volume: | 0.84 µL | |||||||||
| column: | 6-ft × 2-mm i.d. glass, 0.2% Carbowax 1500 on GP 80/100 Carbopack C | |||||||||
| retention times (min): |
| |||||||||
| chromatograms: | Section 4.7. | |||||||||
3.5.2. Peak areas (or heights) are measured by an integrator or other suitable means.
3.5.3. A calibration curve is constructed by plotting response (areas or heights) of standard injections versus microliters of analyte per sample. Sample concentrations must be bracketed by standards.
3.6. Interferences (analytical)
- 3.6.1. Any compound that responds on a flame ionization detector
and has the same general retention time of the analyte or internal
standard is a potential interference. Possible interferences should
be reported to the laboratory with submitted samples by the
industrial hygienist. These interferences should be considered
before samples are desorbed.
3.6.2. GC parameters (i.e. column and column temperature) may be changed to possibly circumvent interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Analyte identity should be confirmed by GC/mass spectrometer if possible.
3.7. Calculations
The analyte concentration for samples is obtained from the appropriate calibration curve in terms of micrograms per sample, uncorrected for desorption efficiency. The air concentration is calculated using the following formulae. If any analyte is found on the backup section, it is added to the amount found on the front section. This total amount is then corrected by subtracting the total amount (if any) found on the blank.
| mg/m3 = | (micrograms of analyte per
sample)
(liters of air sampled) (desorption efficiency) |
| ppm = | (mg/m3) (24.46)
(molecular weight of analyte) |
- where 24.46 = molar volume (liters) at 760 mm Hg and 25°C
| molecular weight of analyte = | 76.09 for 2ME, 118.13 for 2MEA, 90.11 for 2EE, 132.16 for 2EEA |
3.8. Safety precautions (analytical)
- 3.8.1. Avoid skin contact and inhalation of all chemicals.
3.8.2. Restrict the use of all chemicals to a fume hood if possible.
3.8.3. Wear safety glasses and a lab coat at all times while in the lab area.
4. Backup Data
- 4.1. Detection limits of the analytical procedure
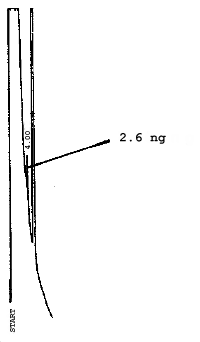
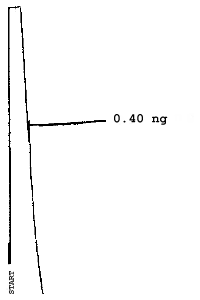
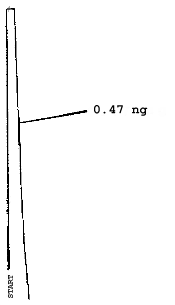
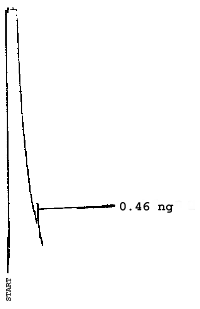
The injection size recommended in the analytical procedure (0.84 µL) was used in the determination of the detection limits of the analytical procedure. The detection limits of 2.6 ng of 2ME, 0.40 ng of 2MEA, 0.47 ng of 2EE, and 0.46 ng of 2EEA were determined by making 0.84-µL injections of 3.09 ng/µL, 0.48 ng/µL, 0.56 ng/µL, and 0.55 ng/µL standards respectively. Chromatograms of such injections are shown in Figures 4.1.1.-4.1.4.
4.2. Detection limits of the overall procedure and reliable quantitation limits
Six samples for each analyte were prepared by injecting 3.09 µg of 2ME, 0.48 µg of 2MEA, 0.56 µg of 2EE, and 0.55 µg of 2EEA into separate charcoal tubes. The samples were refrigerated and analyzed the next day to determine the amount recovered. Since the amounts recovered were high and approximately equal to the detection limits of the analytical procedure, the detection limits of the overall procedure and the reliable quantitation limits are taken to be 3.1, 0.48, 0.56, and 0.55 µg per sample for 2ME, 2MEA, 2EE, and 2EEA respectively. These limits correspond to air concentrations of 0.1 ppm (0.3 mg/m3), 0.01 ppm (0.05 mg/m3), 0.02 ppm (0.06 mg/m3), and 0.01 ppm (0.05 mg/m3) for 2ME, 2MEA, 2EE, and 2EEA respectively.
Detection Limit Data
|
| ||||
| analyte µg/sample |
2ME 3.1 |
2MEA 0.48 |
2EE 0.56 |
2EEA 0.55 |
|
| ||||
| % recovered SD 1.96 SD |
96.1 94.8 97.1 97.1 93.5 95.5 95.7 1.4 2.7 |
102 95.9 97.1 90.5 94.8 107 97.9 5.8 11.4 |
96.4 100 98.2 105 98.2 98.2 99.4 3.2 6.3 |
105 97.1 103 --- 95.4 95.4 99.2 4.5 8.8 |
|
| ||||
4.3. Sensitivity and Precision (analytical method only)
The sensitivity and precision of the analytical procedure were determined from multiple injections of analytical standards. The sensitivities are the slopes of the calibration curves. These data are given in Tables 4.3.1. and 4.3.2. and in Figures 4.3.1.-4.3.4.
Sensitivity and Precision Data for 2ME and 2MEA
|
| |||||||
| analyte | 2 ME | 2 MEA | |||||
| × target conc. µg/mL |
0.5× 77.3 |
1× 154.6 |
2× 309.2 |
0.5× 120.6 |
1× 241.2 |
2× 482.4 | |
|
| |||||||
| area counts SD CV |
13050 13080 13330 13390 13330 13330 13252 146.8 0.011 |
27290 27410 27080 27130 26950 27150 27168 161.5 0.006 0.009 |
55740 55570 55550 54490 55090 55370 55302 454.8 0.008 |
23410 22910 23210 23270 23090 23370 23210 186.3 0.008 |
46560 46850 47160 46890 46460 46630 46758 257.6 0.006 0.019 |
93860 94140 101000 95000 93960 92600 95093 2994 0.031 | |
|
| |||||||
Sensitivity and Precision Data for 2EE and 2EEA
|
| |||||||
| analyte | 2 EE | 2 EEA | |||||
| × target conc. µg/mL |
0.5× 93.1 |
1× 186.2 |
2× 372.4 |
0.5× 136.5 |
1× 273.0 |
2× 546.0 | |
|
| |||||||
| area counts SD CV |
21140 20310 21800 21420 21310 21870 21308 564.7 0.027 |
44390 44150 43780 43790 44720 44580 44235 397.6 0.009 0.021 |
94640 91580 90160 90160 89500 89460 90917 1979 0.022 |
30470 29890 30280 30350 30120 30510 30270 232.8 0.008 |
60830 31100 61480 61070 60600 60770 60975 310.9 0.005 0.019 |
123000 123300 132000 124100 122800 121300 124417 3826 0.031 | |
|
| |||||||
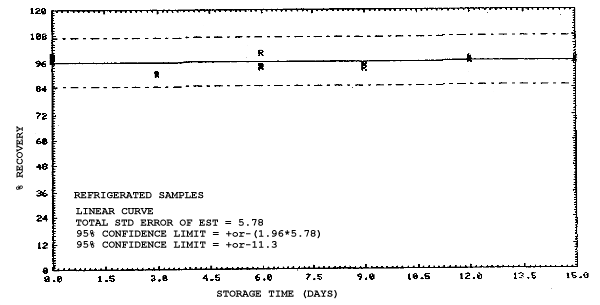
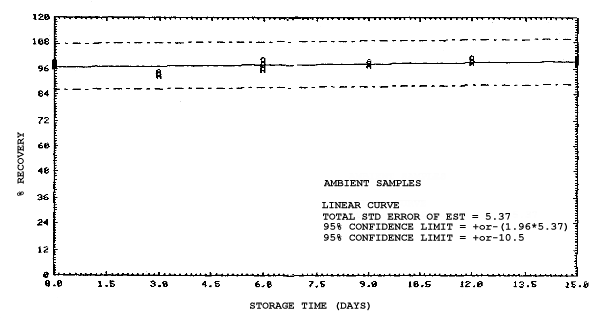
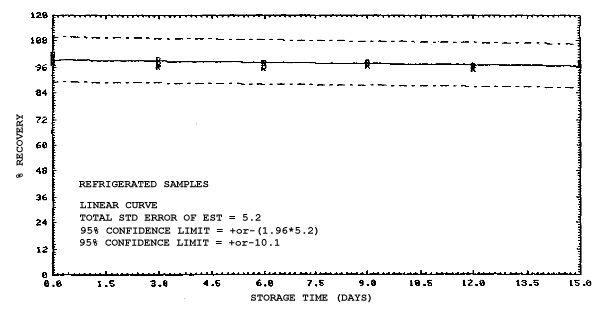
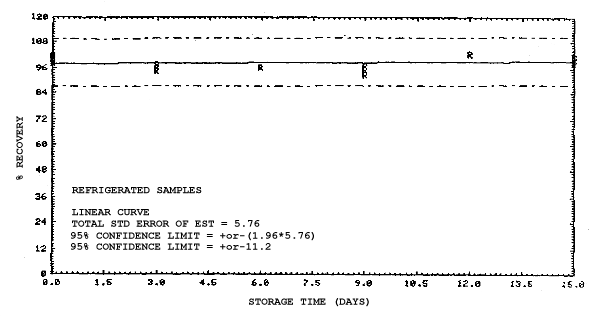
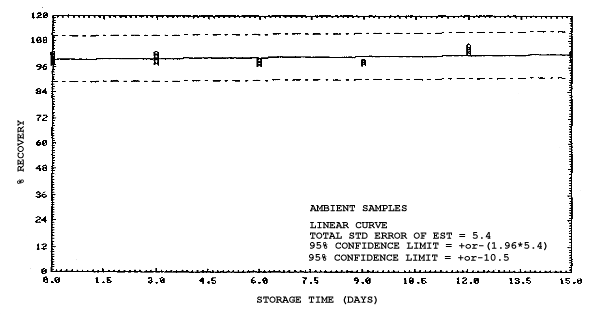
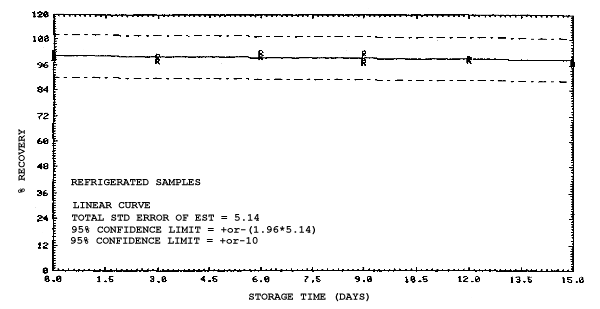
4.4. Recovery data (storage)
Storage samples were generated from test atmospheres (air at
approximately 80% relative humidity and ambient pressures and
temperatures) containing the analytes near the target concentration of
5 ppm. All four analytes were done separately. The samples were
generated by drawing approximately 10 L of test atmospheres through
charcoal tubes at about 0.2 L/min for 50 min. For each set of 36
samples generated for each analyte, six samples were analyzed
immediately after generation, fifteen were stored in a refrigerator at
0°C and fifteen were stored in a closed drawer at ambient temperatures
of 20-25°C. Six samples, three from refrigerated and three from
ambient storage, were analyzed in three-day intervals over a period of
fifteen days. The results are given in Tables 4.4.1.-4.4.4. and shown
graphically in Figures
Storage Data for 2ME
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
97.2 98.2 90.7 100.4 94.9 97.2 98.4 |
96.8 99.1 90.7 93.7 95.4 98.0 96.7 |
99.0 98.0 91.0 94.7 93.4 96.9 98.5 |
97.2 98.2 93.2 95.9 97.8 99.3 100.7 |
96.8 99.1 94.4 99.9 99.2 98.8 98.8 |
99.0 98.0 93.1 98.9 98.4 101.0 98.0 | |
|
| |||||||
Storage Data for 2MEA
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
98.0 100.8 97.5 95.7 97.0 95.2 97.2 |
98.4 102.1 99.6 98.3 98.6 95.9 97.9 |
100.2 100.7 96.6 98.2 97.9 96.9 98.4 |
98.0 100.8 96.0 88.6 88.4 86.4 89.2 |
98.4 102.1 96.3 89.8 92.2 86.4 89.5 |
100.2 100.7 95.8 88.9 90.1 85.5 88.2 | |
|
| |||||||
Storage Data for 2EE
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
98.2 100.5 94.4 96.1 93.1 102.3 98.4 |
98.6 101.9 97.8 96.5 95.4 102.3 100.4 |
99.1 101.4 96.2 96.6 97.4 103.0 101.5 |
98.2 100.5 98.4 97.8 98.2 103.4 100.5 |
98.6 101.9 101.0 99.3 99.0 105.5 102.9 |
99.1 101.4 102.1 98.8 98.7 104.1 101.5 | |
|
| |||||||
Storage Data for 2EEA
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 0 3 6 9 12 15 |
99.1 100.2 99.9 100.1 99.5 99.3 99.4 |
99.4 101.7 98.2 100.0 97.4 98.5 96.8 |
99.5 99.9 100.1 101.8 101.8 98.9 97.4 |
99.1 100.2 96.0 90.1 89.6 87.7 86.3 |
99.4 101.7 99.3 92.4 91.9 90.2 87.4 |
99.5 99.9 96.4 90.2 90.7 88.3 86.0 | |
|
| |||||||
4.5. Reproducibility data
Six samples for each analyte, collected from controlled test
atmospheres (about 80% R.H.,
Reproducibility Data for 2ME and 2MEA
|
| |||||||
| 2ME | 2MEA | ||||||
|
|
| ||||||
| sample no. |
ppm found |
ppm theoretical |
% found |
ppm found |
ppm theoretical |
% found | |
|
| |||||||
| 1 2 3 4 5 6 SD | 6.53 5.67 5.66 5.45 5.69 5.47 |
5.46 5.46 5.46 5.46 5.46 5.46 |
119.6 103.8 103.7 99.8 104.2 100.2 105.0 7.3 |
5.84 5.78 5.84 5.81 5.85 5.80 |
5.89 5.89 5.89 5.89 5.89 5.89 |
99.2 98.1 99.2 98.6 99.3 98.5 98.8 0.5 | |
|
| |||||||
Reproducibility Data for 2EE and 2EEA
|
| |||||||
| 2ME | 2MEA | ||||||
|
|
| ||||||
| sample no. |
ppm found |
ppm theoretical |
% found |
ppm found |
ppm theoretical |
% found | |
|
| |||||||
| 1 2 3 4 5 6 SD | 6.29 6.29 6.44 6.39 6.30 6.38 |
6.39 6.39 6.39 6.39 6.39 6.39 |
98.4 98.4 100.8 100.0 98.6 99.8 99.3 1.0 |
6.13 6.06 6.13 5.71 6.10 6.25 |
6.21 6.21 6.21 6.21 6.21 6.21 |
98.7 97.6 98.7 91.9 98.2 100.6 97.6 3.0 | |
|
| |||||||
4.6. Desorption efficiency
The desorption efficiency for each analyte was determined by injecting a known amount of analyte onto the front section of a charcoal tube. Eighteen samples were prepared for each analyte, six samples for each concentration listed below. The samples were stored in a refrigerator and analyzed the next day.
Desorption Efficiency Data for 2ME and 2MEA
|
| |||||||
| analyte | 2 ME | 2 MEA | |||||
| µg/sample ppm |
77.3 2.48 |
154.6 4.97 |
309.2 9.94 |
120.6 2.50 |
241.2 4.99 |
482.4 9.98 | |
|
| |||||||
| desorption efficiency, % |
97.2 96.8 97.3 96.1 97.2 99.5 97.4 |
96.5 98.1 95.8 97.6 96.7 97.5 97.0 97.9 |
98.7 98.6 99.1 98.4 103.0 98.8 99.4 |
99.7 103.7 100.8 103.7 102.6 101.5 102.0 |
99.3 100.3 100.2 99.5 100.3 99.5 99.9 100.2 |
98.6 97.4 99.6 98.8 101.4 97.5 98.9 | |
|
| |||||||
Desorption Efficiency Data for 2EE and 2EEA
|
| |||||||
| analyte | 2 EE | 2 EEA | |||||
| µg/sample ppm |
93.1 2.53 |
186.2 5.05 |
372.4 10.1 |
136.5 2.53 |
273.0 5.05 |
546.0 10.1 | |
|
| |||||||
| desorption efficiency, % |
98.6 99.1 98.7 98.4 98.6 98.6 98.7 |
99.0 99.4 99.0 98.2 99.7 99.0 99.0 99.0 |
98.2 99.2 99.9 99.7 99.7 99.7 99.4 |
101.0 104.5 103.1 101.2 104.2 102.9 102.8 |
101.2 102.5 102.1 102.0 99.7 103.8 101.9 101.6 |
99.6 100.2 101.2 100.5 99.5 99.6 100.1 | |
|
| |||||||
4.7. Chromatograms of the four analytes are shown in Figures 4.8.1.-4.8.3. The chromatograms represent 0.84-µL injections of standards equivalent to 5-ppm air samples.
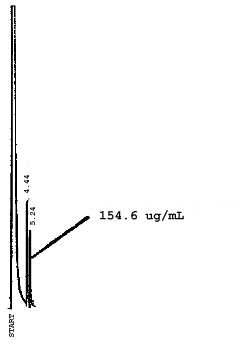
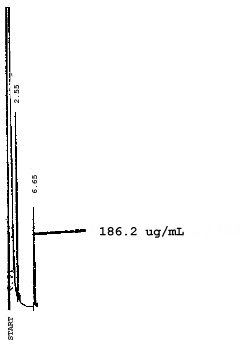
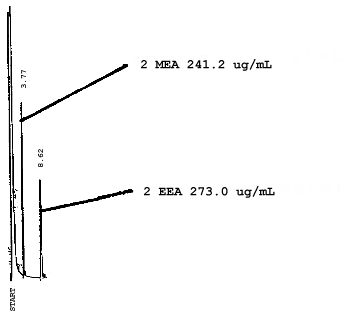
5. References
- 5.1. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of
Health, Education and Welfare, National Institute for Occupational
Safety and Health: Cincinnati, OH, 1977; Vol. 2, Method No. S79;
DHEW(NIOSH) Publ.(U.S.), No. 77-157-B.
5.2. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Vol. 2, Method No. S39; DHEW(NIOSH) Publ.(U.S.), No. 77-157-B.
5.3. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Vol. 5, Method No. S361; DHEW(NIOSH) Publ.(U.S.), No. 79-141.
5.4. "NIOSH Manual of Analytical Methods", 2nd ed.; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Vol. 2, Method No. S41; DHEW(NIOSH) Publ.(U.S.), No. 77-157-B.
5.5. "American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values, Supplemental Documentation for 1982", pp. 259-260, Cincinnati, OH (1982).
5.6. "American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values, Supplemental Documentation for 1982", p. 260, Cincinnati, OH (1982).
5.7. "American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values, Supplemental Documentation for 1982", p. 171, Cincinnati, OH (1982).
5.8. "American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values, Supplemental Documentation for 1982", p. 172, Cincinnati, OH (1982).
5.9. "Documentation of the NIOSH Validation Tests"; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Method No. S39; DHEW(NIOSH) Publ.(U.S.), No. 77-185.
5.10. "Documentation of the NIOSH Validation Tests"; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Method No. S41; DHEW(NIOSH) Publ.(U.S.), No. 77-185.
5.11. "Documentation of the NIOSH Validation Tests"; Department of Health, Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1977; Method No. S79; DHEW(NIOSH) Publ.(U.S.), No. 77-185.
5.12. Nagano, K.; Nakayama, E.; Koyano, M.; Oobayaski, H.; Adachi, H.; Yamada, T. Jap. J. Ind. Health 1979, 21, 29-35.
5.13. "Current Intelligence Bulletin 39, Glycol Ethers"; May 2, 1983, U.S. Department of Health and Human Services, Public Health Service, Center for Disease Control, NIOSH.
5.14. "Pocket Guide to Chemical Hazards", NIOSH/OSHA, Sept. 1978, DHEW (NIOSH) Publ. No. 78-210.