| Method no.: | 51 |
| Matrix: | Air |
| Target concentration: | 15 mg/m3 (4 ppm) |
| Procedure: | Samples are collected by drawing known volumes of air through Ambersorb XE-347 sampling tubes. Following desorption with 1 mL of 95/5 (v/v) methylene chloride/methanol the samples are analyzed by gas chromatography with flame ionization detection (FID). |
| Recommended air volume and sampling rate: |
24 L at 0.1 L/min |
| Reliable quantitation limit: | 0.04 mg/m3 (0.01 ppm) |
| Standard error of estimate: | 5.8% |
| (Figure 4.5.2.) | |
| Special requirements: | Upon receipt at the laboratory the sampling tubes should be stored at refrigerated temperature until analyzed. |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: March 1985 | Chemist: Kevin J. Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The current NIOSH sampling and analytical method for vinyl acetate uses an over-sized stainless steel sampling tube containing Chromosorb 107 adsorbent. The sample is analyzed by gas chromatography with FID detection using thermal desorption (Ref. 5.1.). An alternative sampling procedure was explored since this sampling tube is not readily available and thermal desorption is not routinely employed as an analytical technique at the OSHA Laboratory. Additionally, it was observed that Chromosorb 107 did not have a high capacity for the adsorption of vinyl acetate, especially in the presence of high humidity.
A variety of different adsorbents were tested to improve the sampling method for vinyl acetate. The porous polymer adsorbents such as XAD-2 and XAD-7 did not exhibit adequate capacity and appeared to be similar to Chromosorb 107 in this respect. Both coconut and petroleum based charcoal sampling tubes from SKC, Inc. exhibited high capacity for vinyl acetate with high initial recoveries; however, poor sample stability was observed upon storage. The recovery of vinyl acetate from charcoal using either liquid-spiked samples or with samples collected from a test atmosphere was approximately 100% upon analysis the same day. Recoveries after a week's storage fell to approximately 50%. Para-methoxyhydroquinone, an inhibitor of free radical polymerization, coated onto petroleum based charcoal did not improve sample recovery upon storage. The only adsorbents tested which were found to effectively collect vinyl acetate and result in a high recovery upon storage were Carbosphere and Ambersorb XE-347. Both of these adsorbents are carbonaceous porous polymers. Ambersorb XE-340 and 348, which are similar, were not evaluated.
Ambersorb XE-347 was selected for evaluation since it is commercially available in packed sampling tubes and exhibits a high capacity for vinyl acetate with moderately good sample stability upon storage. Analysis of the samples is performed by gas chromatography with FID detection using a glass column packed with 0.1% Carbowax 1500 on Carbopack C. The samples are desorbed with 1 mL of 95/5 (v/v) methylene chloride/methanol. Recoveries of 88% are observed upon storage for 18 days at reduced temperature. Although the recommended sampling volume is 24 L, a broad range of air volumes can be used in the field for monitoring exposures. This is because the analysis is relatively sensitive, and because the Ambersorb adsorbent has a high capacity for vinyl acetate.
1.1.2. Toxic effects. (This section is for information only and should not be taken as a basis of OSHA policy. Reference 5.2. is the source for this information.)
The available evidence indicates that vinyl acetate exposures have produced primarily respiratory irritation effects. Respiratory irritation is reported to occur at the 10- to 20-ppm level for humans. Inhalation studies with beagle dogs indicate no observable effect at 106 ppm, but blinking and reddening of the eyes is observed at 240 ppm. The odor threshold for humans is 5 ppm or less depending upon the individual. Olfactory fatigue is reported to occur in a matter of minutes at 20 ppm. Vinyl acetate is not considered to be a serious skin irritant, nor is it thought to induce dermatitis. Serious eye injuries are thought to be avoidable with prompt flushing of the affected area.
Vinyl acetate exhibits moderate acute systemic toxicity. Four-hour exposures to a 4000 ppm atmosphere produce death in 50% of exposed rats (LC50). LC50s of 1550 ppm and 2500 ppm respectively are observed for mice and rabbits in the same study. Repeated exposures of rats to a 100 ppm atmosphere for 6 h/day, 5 days/week for 3 weeks produced no sign of adverse effects. Vinyl acetate is moderately toxic upon ingestion. An LD50 of 2.5 g/kg is reported for rats.
NIOSH reports that there exists no available evidence to indicate that vinyl acetate is either a mutagen, a teratogen, or a carcinogen. Since it is structurally related to a number of known mutagens and carcinogens such as the vinyl halides, which are thought to be activated to their mutagenic form by means of epoxide formation, it is suggested that the metabolism of vinyl acetate proceeds by a different mechanism. Metabolic studies suggest that vinyl acetate is rapidly hydrolyzed to acetic acid and vinyl alcohol. The vinyl alcohol rapidly tautomerizes to acetaldehyde before epoxide formation can occur.
1.1.3. Workplace exposure
Vinyl acetate is used primarily in polymerization processes to produce polymers and copolymers such as polyvinyl acetate, polyvinyl alcohol, and vinyl chloride-vinyl acetate. These polymers are used to produce adhesives, paints, paper coatings, and textile finishes. In 1977, 1.6 million pounds of vinyl acetate were produced in the United States. NIOSH estimates that approximately 70,000 workers are potentially exposed to vinyl acetate in the United States (Ref. 5.2.).
1.1.4. Physical properties (Ref. 5.2.)
| CAS no.: | 108-05-4 |
| molecular weight: | 86.09 |
| boiling point: | 72.7°C |
| appearance: | clear, colorless liquid |
| specific gravity: | 0.9338 at 20°C |
| vapor pressure: | 100 mm Hg at 21.5°C |
| solubility: | 2.5 g/100 mL H2O, soluble in most organic solvents |
| odor: | pleasant, sweet to sharp, irritating |
| flashpoint (open cup): | -5.5°C |
| explosive limits in air: | 2.6-13.4% by volume |
| molecular formula: | CH3CO2CH=CH2 |
| synonyms: | acetic acid, vinyl ester; acetic acid, ethenyl ester; vinyl a monomer; ethenyl ethanoate; and Vy Ac |
1.2. Limit Defining Parameters (The analyte air concentrations listed throughout this method are based on a 24-L air sample unless otherwise noted. Air concentrations listed in ppm are referenced to 25°C and 760 mm Hg.)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.0 ng per injection. This is the amount of analyte which will give a measurable response with the amounts of interferences present in a standard. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1.0 µg per sample (0.04 mg/m3 or 0.01 ppm). This is the amount of analyte spiked on the sampling device which allows recovery approximately equal to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 1.0 µg per sample (0.04 mg/m3 or 0.01 ppm). This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
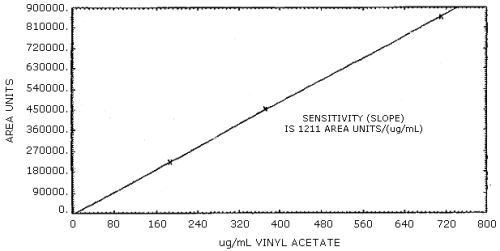
The sensitivity of the analytical procedure over the concentration range representing 0.5 to 2 times the target concentration based on a 24-L air sample is 1211 area units per µg/mL. This is determined by the slope of the calibration curve. The sensitivity may vary with the particular instrument used in the analysis. (Section 4.4.)
1.2.5. Recovery
The recovery of vinyl acetate from samples used in a 18-day storage test remained above 88% when the samples were stored at 5°C. This was determined from the regression line calculated from the tabulated storage data. (Section 4.5.) The recovery of analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0073. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 18-day storage test is ±11% for the refrigerated samples (Section 4.5.). This includes an additional ±5% for sampling error. The overall procedure must provide results that are ±25% or better at the 95% confidence level. (Section 4.5.)
1.2.8. Reproducibility
Six samples spiked with vinyl acetate in a methanol solution and a draft copy of this procedure were given to a chemist unassociated with this evaluation. The samples were analyzed after 26 days of storage at 5°C. The average recovery was 87.4% with a standard deviation of 1.3%. (Section 4.6.)
1.3. Advantages
- 1.3.1. The Ambersorb sampling tube can be used to sample a
larger air volume since it has a higher capacity for vinyl acetate
than does the NIOSH-recommended Chromosorb 107 tube.
1.3.2. Samples can be conveniently reanalyzed at a later date since the analytical method uses liquid desorption rather than thermal desorption.
1.4. Disadvantages
- 1.4.1. Ambersorb XE-347 adsorbent is not currently being
manufactured, although it is still available from SKC, Inc.
(Eighty-Four, PA).
1.4.2. Collected vinyl acetate samples should be refrigerated until analysis to minimize decomposition.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A constant flow personal sampling pump is used which can
be calibrated to within ±5% of the recommended 0.1 L/min flow rate
while the sampling tube is in line.
2.1.2. Standard size SKC, Inc. sampling tubes containing 160 mg and 80 mg of Ambersorb XE-347 adsorbent in the front and back sections, respectively (cat. no. 226-62-347) were used in this study.
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Properly label the sampling tube before sampling.
2.3.2. Attach the sampling tube to the pump using a section of flexible, plastic tubing such that the large, front section of the sample tube is exposed directly to the atmosphere. Do not place any tubing ahead of the sampling tube. The sampling tube should be attached in the worker's breathing zone in a vertical manner such that it does not impede work performance.
2.3.3. After sampling for the appropriate time, remove the sampling tube from the pump, and then cap and seal the tube with an official OSHA seal (Form 21).
2.3.4. Include at least one blank for each sampling set. The blank should be handled in the same manner as the samples with the exception that air is not drawn through it.
2.3.5. List any potential interferences on the sample data sheet.
2.4. Breakthrough
Breakthrough studies were performed by sampling a 150 mg/m3 (10 × target concentration) atmosphere of vinyl acetate at approximately 70% R.H. and ambient temperature, at a flow rate of 0.19 L/min with the front section of the sampling tube. A total hydrocarbon analyzer was used to monitor downstream from the sampling tube and to determine the breakthrough volume. The 5% breakthrough volume (the volume of air sampled that results in a concentration of vinyl acetate downstream from the front section of the sampling device which is 5% of the upstream concentration) is about 58 L.
2.5. Desorption efficiency
The average percent recovery of vinyl acetate from sample tubes spiked with amounts of vinyl acetate in methanol equivalent to 0.5, 1, and 2 times the target concentration for a 24-L air sample is 95.3%. (Section 4.7.)
2.6. Recommended air volume and sampling rate
A 24-L air sample obtained by sampling for 4 h at 0.1 L/min is recommended. The sensitivity of the method will permit shorter sampling periods. The analytical method is sensitive enough to monitor a 15 mg/m3 atmosphere with a 15-min air sample taken at 0.05 L/min (0.75-L air volume).
2.7. Interferences
Any substance collected with vinyl acetate that is capable of reacting with it is a potential interference. Acids, bases, free radical initiators, etc., are all capable of reacting with vinyl acetate during and after air sampling.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph equipped with a flame ionization
detector is needed for the analysis of vinyl acetate. A
Hewlett-Packard Model 5730A was used in this evaluation.
3.1.2. An electronic integrator or other suitable means of measuring detector response is needed. The Hewlett-Packard 3357 data system was used in this evaluation.
3.1.3. Sample vials fitted with septa are needed for the analysis. Four-milliliter, screw-cap vials (Waters, Inc. WISP-type vials) obtained from Sun Brokers, Inc. (Wilmington, NC) were used in this evaluation. Crimp-cap vials for use with Hewlett-Packard autosamplers should also be suitable.
3.1.4. Precision 1- and 10-µL syringes are needed for preparation of standards and for the analysis.
3.2. Reagents
- 3.2.1. Methylene chloride, Burdick and Jackson (Muskegon, MI).
3.2.2. Methanol, Burdick and Jackson.
3.2.3. Vinyl acetate, practical grade, inhibited with 275-325 ppm diphenylamine, Eastman-Kodak (Rochester, NY).
3.3. Standard preparation
A stock solution of vinyl acetate in methanol is prepared by pipetting 2 mL of vinyl acetate into a 25-mL volumetric flask and diluting to volume with methanol. Working standards are prepared by spiking 2.5, 5.0, and 10.0 µL of this stock solution into separate septum-capped vials containing 1 mL of 95/5 (v/v) methylene chloride/methanol.
3.4. Sample preparation
The front adsorbent section along with the glass wool plug and the back adsorbent section minus the foam plugs are placed in separate vials and desorbed with 1 mL of 95/5 (v/v) methylene chloride/ methanol. The vials are capped, shaken vigorously for several seconds and then analyzed as described in Section 3.5.
3.5. Analysis
- 3.5.1. This evaluation was performed using an HP 5730A gas
chromatograph which was not equipped with an auto sampler. Minor
modifications of this method for adaptation to a gas chromatograph
equipped with an autosampler should not affect the results.
3.5.2. Gas chromatographic conditions
| column: | 6-ft × 2-mm i.d. glass column packed with 0.2% Carbowax 1500 on Carbopack C. | |
| zone temperatures: | inlet oven detector |
150°C 90°C 250°C |
| carrier gas: | nitrogen | 20 mL/min |
| detector gases: | hydrogen air |
20 mL/min 250 mL/min |
| injection volume: | 1 µL | |
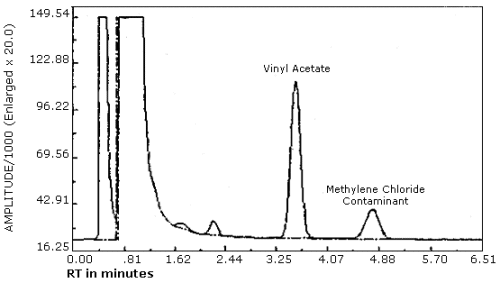
3.5.3. A chromatogram of a vinyl acetate standard is shown in Figure 3.5.3. The peak eluting after vinyl acetate is a contaminant present in the methylene chloride. Other brands of methylene chloride contained a different contaminant in varying levels which eluted before vinyl acetate. In most cases these solvents could also have been used for the analysis. It is recommended that the chromatography be examined before desorption of samples to ensure that the methylene chloride contaminants do not cause significant interferences with the vinyl acetate peak.
3.6. Interferences
There are no known interferences to this analytical method. Any substance that has the same retention time as vinyl acetate under the existing analytical conditions is a potential interference. It may be necessary to modify the analytical conditions in order to circumvent an interference. Confirmation of the samples by GC/MS is recommended as a useful means of identification.
3.7. Calculations
- 3.7.1. A calibration curve is prepared by plotting µg of vinyl
acetate per sample versus area response. A linear least squares fit
is used to determine the amount of vinyl acetate present in the
samples.
3.7.2. The total amount of vinyl acetate found on both the front and back sections, corrected for the amount found on the blank, is used along with the air volume sampled to report sample results in ppm (760 mm Hg, 25°C) using the following formula:
| ppm = | µg vinyl acetate × 24.46 × 1
L of air sampled 86.09 (DE) |
| where | 24.46 | = | molar volume of an ideal gas at 760 mm Hg and 25°C. |
| 86.09 | = | molecular weight of vinyl acetate. | |
| DE | = | desorption efficiency for vinyl acetate. |
3.8. Safety precautions
- 3.8.1. Minimize exposure to all reagents and solvents by
performing all sample and standard preparations in a well-ventilated
hood.
3.8.2. Avoid skin contact with all solvents and reagents.
3.8.3. Wear safety glasses in the laboratory at all times.
4. Backup Data
- 4.1. Detection limit
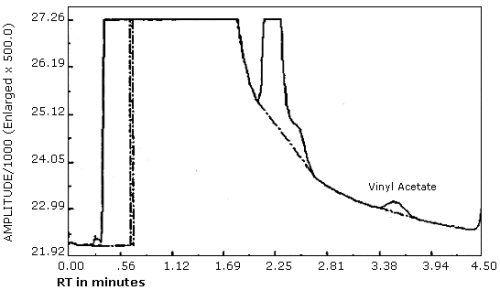
The detection limit for the analytical procedure is 1 ng per injection. This is based on a 1-µL injection of a 1.0 ng/µL working standard. This is the amount of analyte which will give a measurable response with the amounts of interferences present in a standard. (Figure 4.1.)
4.2. Detection limit of overall procedure and reliable quantitation limit
The reliable quantitation limit and the detection limit of the overall procedure for this method is 1.00 µg per sample (0.04 mg/m3 or 0.01 ppm based on a 24-L air sample). Six vials, each containing the front section of an Ambersorb XE-347 sampling tube, were spiked with 1.34 µL of a 0.748 µg/µL vinyl acetate standard in methanol and then analyzed several hours later.
Reliable Quantitation Limit Data
|
| |||
| % recovery | statistics | ||
|
| |||
| 96.2 | |||
| 95.5 | |||
| 91.4 | = | 94.3 | |
| 97.9 | SD | = | 2.7 |
| 91.6 | 1.96 SD | = | 5.2 |
| 93.0 | |||
|
| |||
4.3. Precision of the analytical method
The pooled coefficient of variation for vinyl acetate over a range of 0.5 to 2 times the target concentration is 0.0073. This value was determined from six injections each of three working standards which correspond to 186.9, 373.8, and 710.2 µg of vinyl acetate per sample, respectively.
Precision of the Analytical Method
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 186.9 | 373.8 | 710.2 |
|
| |||
| area | 221725 | 451666 | 860566 |
| response | 221475 | 451530 | 860159 |
| 222323 | 448789 | 861885 | |
| 222322 | 456926 | 846813 | |
| 222903 | 455627 | 850597 | |
| 222601 | 461323 | 860899 | |
| 222225 | 454310 | 856820 | |
| SD | 535 | 4538 | 6424 |
| CV | 0.0024 | 0.0100 | 0.0075 |
|
| |||
4.4. Sensitivity
The slope of the calibration curve over the range of 0.5 to 2.0 times the target concentration represents the sensitivity for the method. The sensitivity is 1211 area units per µg of vinyl acetate per mL. (Figure 4.4.)
4.5. Storage
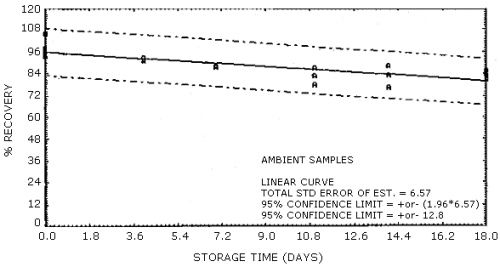
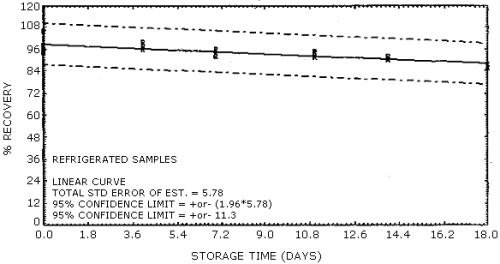
Storage of samples over an 18-day period was performed at both ambient and refrigerated temperatures. The samples were prepared by sampling a 30 mg/m3 test atmosphere of vinyl acetate at 80% R.H. and ambient temperature for 50 min at 0.22 L/min. This sample load is approximately equivalent to a 4-h, 15 mg/m3 exposure, sampled at 0.1 L/min. A total of 36 samples were generated for this evaluation in one day using the vapor generation system. Six of the samples were analyzed the same day and the remaining 30 samples were randomly split into two groups of 15 samples each for storage either at ambient or at reduced temperature in a refrigerator. At three- to four-day intervals over the next 18 days, three samples from each group were selected at random and analyzed. The percent recovery (uncorrected for desorption efficiency) for each of the samples is reported in Table 4.5. These results are depicted graphically for both the ambient and the refrigerated samples in Figures 4.5.1. and 4.5.2. respectively.
Some loss in recovery is observed with time for both the ambient and the refrigerated stored samples. The loss in recovery is more severe for samples stored at ambient temperature; therefore, it is recommended that samples be stored at reduced temperature following collection to minimize losses. Special shipping requirements are not considered necessary.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (ambient) | (refrigerated) | |||||
|
| |||||||
| 0 | 98.1 | 95.5 | 94.3 | 98.1 | 95.5 | 94.3 | |
| 0 | 94.6 | 106 | ---- | 94.6 | 106 | ---- | |
| 4 | 92.9 | 90.8 | 91.2 | 97.8 | 96.1 | 100 | |
| 7 | 87.3 | 88.0 | 87.8 | 92.0 | 94.1 | 96.2 | |
| 11 | 82.7 | 77.8 | 86.9 | 94.8 | 94.7 | 91.9 | |
| 14 | 83.0 | 76.2 | 88.1 | 90.5 | 90.2 | 92.1 | |
| 18 | 80.8 | 83.1 | 85.7 | 88.8 | 85.8 | 85.7 | |
|
| |||||||
4.6. Reproducibility
Six sample tubes were each spiked with 381 µg of vinyl acetate from a stock solution in methanol and the samples were stored at 5°C until analysis. The samples were analyzed after 26 days storage by a chemist unassociated with this method.
Reproducibility
|
| |||
| % recovery | statistics | ||
|
| |||
| 88.7 | |||
| 88.8 | |||
| 86.4 | = | 87.4 | |
| 87.4 | SD | = | 1.3 |
| 85.4 | |||
| 88.0 | |||
|
| |||
4.7. Desorption efficiency
The percent recovery of vinyl acetate spiked onto the front section of a sampling tube and then stored overnight before analysis was determined for sample loads equivalent to 0.5, 1.0, and 2.0 times the target concentration. Three sets of six sample tubes were each spiked with either 2.5, 5.0, or 9.5 µL of a 74.76 µg/µL solution of vinyl acetate in methanol. The average percent recovery over the 0.5 to 2.0 times target concentration is 95.3%.
Desorption Efficiency
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 187 | 374 | 710 |
|
| |||
| desorption | 95.8 | 95.7 | 95.2 |
| efficiency, | 94.1 | 96.3 | 95.4 |
| % | 95.4 | 95.7 | 94.6 |
| 94.5 | 95.1 | 95.2 | |
| 95.2 | 94.7 | 95.2 | |
| 95.7 | 96.3 | 94.9 | |
| 95.1 | 95.6 | 95.1 | |
|
| |||
5. References
- 5.1. NIOSH Manual of Analytical Methods, 2nd ed., Dept. of Health,
Education and Welfare, National Institute for Occupational Safety and
Health, Cincinnati, OH, 1977, Vol. 4, Method No. P&CAM 278, DHEW
(NIOSH) Publication No. 77-157-A.
5.2. Occupational Exposure to Vinyl Acetate, NIOSH Criteria Document, National Institute for Occupational Safety and Health, Sept. 1978, U.S. Dept. of Health, Education, and Welfare, Public Health Service, DHEW (NIOSH) Publication No. 78-205.