| Method no.: | 45 |
| Matrix: | Air |
| Target concentration: | 0.5 mg/m3 |
| Procedure: | Samples are collected by drawing a known volume of
air through a sampling device consisting of two specially prepared
|
| Recommended air volume and sampling rate: |
48 L at 0.2 L/min |
| Reliable quantitation limit: | 0.003 mg/m3 |
| Standard error of estimate: (Figure 4.7.1.) |
6.65% |
| Special requirements: | The special sampling device as represented in Figure
4.2. must be obtained from the laboratory. It contains two
|
| Status of method: | A sampling and analytical method which has been subjected to established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1983 | Chemist: Kevin Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
This air sampling and analytical procedure for 2,3,4,6-tetrachlorophenol (TCP) is essentially the same as OSHA Method 39 for pentachlorophenol (PCP) and is designed for the simultaneous collection and analysis of both of these analytes since they are used together in the wood industry (Ref. 5.1.). Only minor changes in sample tube design and in the analytical conditions have been made from the PCP method. Although there are three isomers of tetrachlorophenol, the industrial production of TCP from the chlorination of phenol produces primarily the 2,3,4,6-isomer with PCP as a contaminant since phenol is an ortho-para-director. (Ref. 5.2.) All evaluations in this method were performed with the 2,3,4,6-isomer, both in the presence and absence of PCP. For future reference, TCP will refer to the 2,3,4,6-isomer unless otherwise indicated.
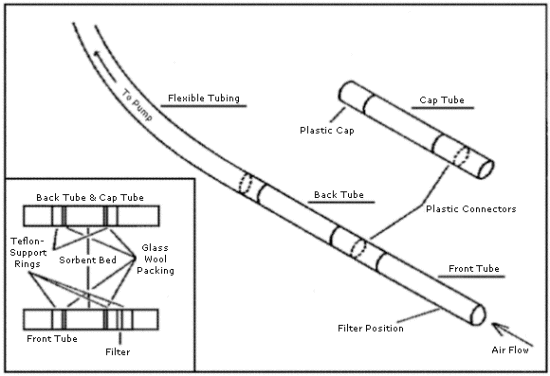
The sampling tubes for PCP and TCP as represented in Figure 4.2.
consist of two laboratory prepared
The air sampling evaluations for TCP were performed with an aerosol generation system described in Section 4.5. Technical grade TCP containing 15-20% PCP was used for these studies although a purified TCP standard was prepared for use as an analytical standard. A majority of the atmosphere generated by the aerosol system was in the vapor phase because of the high volatility of both TCP and PCP; however, a measurable aerosol component was also produced.
The effectiveness of this sampling device in sampling an
atmosphere of TCP/PCP was demonstrated by collecting side-by-side
samples of
A large number of GC methods have been published for the analysis of chlorophenols; however, many of these methods require precolumn derivatization (Ref. 5.3.). Several methods have been recently published for the direct analysis of TCP by HPLC (Refs. 5.1., 5.4. - 5.8.) and one by GC (Ref. 5.9.).
The HPLC analytical conditions employed for this analysis using a UV detector at 210 nm and a Zorbax ODS reverse phase column do not differ greatly from many of the previous HPLC methods. These conditions differ somewhat from those described previously for the PCP method; however, the analysis can be performed adequately using either procedure.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy)
Although the toxic effects of TCP have not been as well studied as PCP, based on LD50 data, TCP has a comparable acute toxicity. For the rat, LD50's range from 130 mg/kg to 210 mg/kg depending on the mode of administration (Ref. 5.10.). Oral LD50's for each tetrachlorophenol isomer and for PCP have been determined by one laboratory for rats. LD50 values for 2,3,5,6-; 2,3,4,6-; 2,3,4,5-TCP, and PCP of 109, 131, 400, and 74 mg/kg respectively were reported (Ref. 5.11.).
Acute exposure to TCP in animals produces symptoms common to the lower chlorinated phenols and some symptoms common to PCP. Convulsant activity, a characteristic of exposure to the lower chlorinated phenols, and signs of inhibition of oxidative phosphorylation which are characteristic of PCP exposure, are observed (Ref. 5.12.). Accelerated respiration, elevated blood pressure and hyperpyrexia (elevated body temperature), which are all symptoms of oxidative phosphorylation inhibition can be anticipated upon acute exposure to TCP.
TCP is a strong irritant and can produce skin and eye irritation upon contact. Like PCP, TCP is readily absorbed through the skin and can produce systemic effects. Although a literature search did not reveal any human cases of acute exposure to TCP, it is widely used in the wood industry.
Exposure to TCP as a contaminant of PCP also occurs since technical grade PCP contains from 5-12% TCP (Ref. 5.13.). Widespread exposure of the general population to TCP is evidenced by the analysis of urine samples from the general population. Low ppb levels of 2,3,4,6-TCP which are approximately 1/5 to 1/3 the PCP levels are reported for twelve samples from the general population (Ref. 5.14.). Low ppm levels of both TCP and PCP are reported in the urine of exposed Finnish workers (Ref. 5.3.). Since skin exposure is a significant route of exposure for both TCP and PCP, biological monitoring through urine analysis is desirable.
Although no reports of a mutagenic or a teratogenic effect from TCP were found in the literature, the possibility of such an effect from polychlorinated dioxin and polychlorinated dibenzofuran contaminants must be considered. Trace levels of these contaminants in the blood of exposed workers have been measured (Ref. 5.15.).
1.1.3. Potential workplace exposure
TCP is used almost exclusively in the wood industry to treat wood. A dilute aqueous solution of mainly sodium tetrachlorophenate with lesser amounts of the pentachlorophenate salt is sprayed on newly milled wood surfaces to prevent the wood from darkening during the aging process (Ref. 5.4.). The pressure treatment of lumber with PCP dissolved in oil is also a potential source of exposure since TCP is a major contaminant. It is not known if TCP alone is used in this manner.
1.1.4. Physical properties (Ref. 5.16.)
| CAS no.: | 58-90-2 |
| molecular weight: | 231.89 |
| melting point: | 70°C |
| boiling point: | 150°C (15 mm Hg) |
| soluble in: | alcohol, benzene, chloroform, petroleum ether |
| physical state: | white to tan crystalline solid |
| synonyms and trade names: | TCP, Dowicide 6 |
1.2. Limit defining parameters (The analyte air concentrations listed throughout this method are based on an air volume of 48 L and a solvent desorption volume of 2 mL)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.5 ng per injection. This is the amount of the analyte which will give a peak whose height is approximately 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.15 µg per sample (0.003 mg/m3) for TCP. This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.15 µg per sample (0.003 mg/m3) for TCP. This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
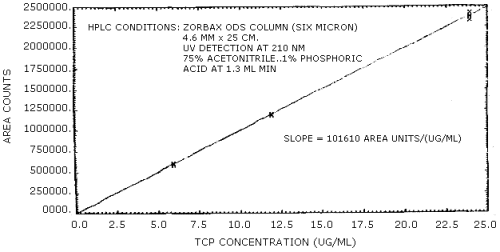
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 101,610 area units per µg/mL. The sensitivity is determined from the slope of the calibration curve. The sensitivity may vary with different instruments or instrumental conditions. (Section 4.4.)
1.2.5. Recovery
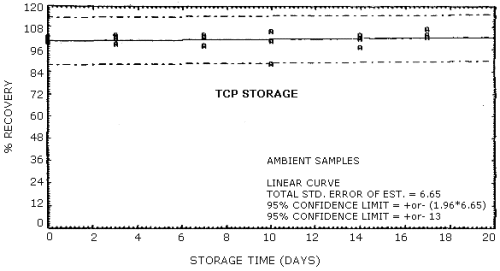
The recovery of TCP from samples used in a 17-day storage test was 102% when the samples were stored at ambient conditions in the dark. This is the percent recovery at 17 days determined from the linear least squares line from the storage data. The recovery of the analyte from the collection medium during storage must be 75% or greater. (Section 4.7.)
1.2.6. Precision (analytical procedure only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.011. (Section 4.3.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 17-day storage test is ±13% for TCP. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level. (Section 4.7.)
1.2.8. Reproducibility
Six liquid-spiked samples and a draft copy of this method were submitted to the OSHA laboratory for analysis by a chemist unassociated with this evaluation. The samples were analyzed approximately two weeks after preparation. The average recovery for the six samples was 94.0% with a percent standard deviation of 3.9%. (Section 4.9.)
1.3. Advantages
- 1.3.1. The two solid sorbent sampling tubes in series represent
a convenient method for sampling both TCP and PCP.
1.3.2. The analysis is rapid, sensitive, and precise.
1.4. Disadvantages
- 1.4.1. The method has not been field tested.
1.4.2. The sampling tubes are not commercially available.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A constant flow personal sampling pump is used which can
be calibrated to within ±5% of the recommended 0.2 L/min flow rate
while the sampling train is in line.
2.1.2. The sampling tubes, as represented in Figure. 4.2.,
consist of two 50-mm by
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Properly label all three sections of the sampling device
prior to sampling.
2.3.2. Before sampling, remove and save the front glass tube
section containing
2.3.3. Attach the sampling tubes to the pump using a section of flexible, plastic tubing so that the adsorbent tube containing the glass fiber filter serves as the front sampling section. Do not place any tubing ahead of the sampling device. Attach the sampling device in the workers breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the sampling device from the pump, cap the front end of the device with the resin-filled glass tube and cap the back end of the device with a plastic cap. Insure that the caps are well fitted and label the sampling tubes with OSHA seals (Form 21).
2.3.5. Include at least one blank for each sampling set. The blank should be handled in the same manner as the samples with the exception that air is not drawn through it.
2.3.6. Any bulk samples submitted for analysis must be shipped in separate containers to avoid contamination of the air samples.
2.3.7. List any potential interferences on the sample data sheet.
2.4. Breakthrough
Since
In the first study, an
In the second study, the ability of a
2.5. Desorption efficiency
The average desorption efficiency over the range of 0.5 to 2 times the target concentration was 95.4% for TCP and 99.8% for PCP. The percent recovery ranged from 91.2% for 2 times the target concentration to 99.7% for the 0.5 times target concentration for TCP. Similarly, PCP recoveries ranged from 95.5% to 105% over the same range. The variability in desorption efficiency with amount loaded on sample is not understood but may be a function of the spiking technique.
In the course of this evaluation, it was also observed that the
desorption efficiency from
The desorption efficiency of the resin that was dried at 105°C under vacuum and of the resin similar to the one used in this study was not affected by humidity.
These variations in desorption efficiency for
2.6. Recommended air volume and sampling rate
A 48-L air sample obtained by sampling at 0.2 L/min for 4 h is recommended for TCP. If necessary, the sensitivity of the analytical method will permit a sampling period as short as 15 min at 0.2 L/min for determination of the analyte at the target concentration. Higher flow rates can also be employed if necessary.
2.7. Interference
There are no known interferences to the sampling procedure.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A high performance liquid chromatograph equipped with
sample injector, ODS bonded phase HPLC column, UV detector, and
chart recorder are needed for the analysis. A Waters 6000A pump, a
Waters WISP 710 auto sampler, a Dupont UV detector and a Zorbax
25-cm × 4.6-mm i.d. ODS-bonded phase column were used in this study.
3.1.2. An electronic integrator or other suitable means of measuring detector response is required. The Hewlett-Packard 3354 data system was used in this study.
3.1.3. Various sizes of volumetric glassware and pipettes are needed for sample and standard preparations.
3.1.4. Three-milliliter (or larger) screw-cap or crimp-type vials
are needed for desorbing the
3.1.5. Small brown glass bottles fitted with inert cap liners are needed to store standard solutions.
3.1.6. A repetitive dispenser capable of accurately delivering the desorption solution is needed.
3.2. Reagents
- 3.2.1. HPLC grade methanol and acetonitrile.
3.2.2. Reagent grade phosphoric acid.
3.2.3. HPLC grade water. Water obtained from a Milli-Q reagent grade water system (Millipore, Inc. Bedford, Mass.) was used in this study.
3.2.4. Eighty-five percent pure 2,3,4,6-TCP containing PCP
contaminant was purchased from Fluka Chemical (Hauppauge, NY, USA)
for preparation of a purified standard. A semi-preparative HPLC
technique which utilized a (
3.3. Standard preparation
Prepare a stock solution of TCP by accurately weighing approximately 32 mg of the standard in a 100-mL volumetric flask and diluting to volume with methanol. Prepare 1/50, 1/25, and 2/25 dilutions of this stock solution to obtain standards which correspond to approximately 0.5, 1 and 2 times the target concentration for the recommended sampling conditions.
3.4. Sample preparation
Prepare samples for analysis by transferring the entire contents of
the sampling tube including the Teflon-support rings, both glass wool
plugs, the
3.5. Analysis
- 3.5.1. Prepare a high performance liquid chromatograph for
sample analysis using the HPLC conditions listed below:
| column: | Zorbax 25 cm × 4.6-mm i.d. ODS bonded phase |
| mobile phase: | 25/75 (v/v) acetonitrile/water containing approximately 0.1% by volume of phosphoric acid |
| flow rate: | 1.3 mL/min |
| UV detector: | 210 nm |
| injection volume: | 20 µL |
| retention time: | 4.6 min |
3.5.2. Analyze the front and back sampling tubes and the sample cap tube separately. Verify that the sample responses lie within the range of the responses observed for the standards.
3.5.3. Since column to column variations do occur, it is important to ensure that TCP is separated from PCP. The injection of a TCP/PCP mixture should produce baseline separation if the analytical conditions are properly selected.
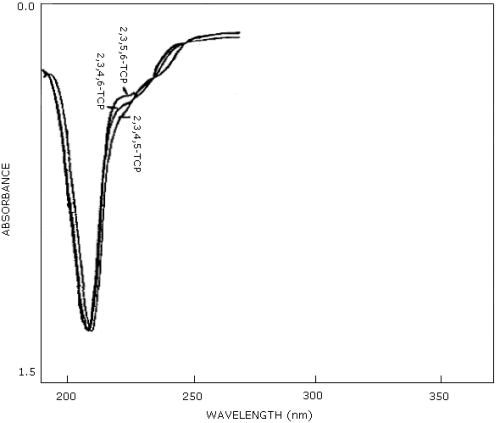
3.6. Interferences
Any compound which has the same retention time as 2,3,4,6-TCP is a potential interference. Under the analytical conditions outlined, 2,3,5,6-TCP and 2,3,4,6-TCP are not resolved. However, since only the 2,3,4,6 isomer of TCP is used industrially, and the response factors for these two isomers are very similar at 210 nm, no significant error would be introduced in determining TCP content under these conditions (Figure 4.10.). A complete separation of the three isomers of TCP in 30 min has been performed using gradient reverse phase HPLC conditions (Ref. 5.2.). A normal phase separation of the isomers using a silica column and a GC separation have also been reported in the literature (Ref. 5.8.). These alternative methods are useful for sample confirmation. GC/Mass spectrometry may also be a useful method of sample confirmation.
3.7. Calculations
Prepare a standard calibration curve of area response versus concentration for TCP by determining the least squares fit equation for the curve. Calculate the amount of analyte (µg/mL) in the samples, preferably by entering their response values into the equation and solving for the sample concentration. Add the results from the backup and cap tubes to that of the front tube. Sample air concentrations are calculated as follows:
mg/m3 = (µg/mL)(2 mL)(1 mg/1000 µg)/(air vol. m3)(desorp. effic.)
To convert to ppm at 760 mm and 25°C:
| ppm = (mg/m3)(24.46)/(MW) | where | 24.46 MW |
= = |
the molar volume 231.89 |
3.8. Safety precautions
- 3.8.1. Minimize exposure to TCP by performing standard
preparations in a well ventilated hood.
3.8.2. Avoid all skin contact with TCP.
3.8.3. Restrict the use of solvents to hoods which provide adequate ventilation.
3.8.4. Wear safety glasses in laboratory areas at all times.
4. Backup Data
- 4.1. Detection limit for analytical procedure
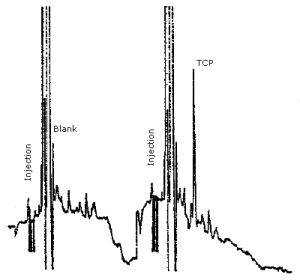
The detection limit for the analytical procedure is 1.5 ng for TCP. This is based on a 20-µL injection of a 0.075 µg/µL standard, and represents approximately 5 times the baseline noise (Figure 4.1.).
4.2. Detection limit of the overall procedure and reliable quantitation limit
The detection limit of the overall procedure and the reliable quantitation limit are both 0.15 µg per sample (0.003 mg/m3) for TCP.
Six
Detection Limit Data
|
| |||
| % recovery | statistics | ||
|
| |||
| 98.8 98.8 99.8 95.2 98.8 98.8 |
SD 1.96 SD |
= = = |
98.4 1.60 3.14 |
|
| |||
4.3. Precision of the analytical method
The pooled coefficient of variation for TCP is 0.011 over a range of 0.5 to 2 times the target concentration. This value was determined from multiple injections of three standard solutions. The results are listed in Table 4.3.
Precision Data
|
| |||
| × target conc. µg/mL |
0.5× 5.98 |
1× 11.97 |
2× 23.94 |
|
| |||
| area
counts SD CV |
599574 600337 600090 612430 614097 612310 606473 7123 0.0117 |
1198240 1193360 1189100 1207610 1208830 1208540 1200947 8596 0.00716 |
2355190 2333730 2410920 2380600 2411150 2371280 2377145 30693 0.0129 |
|
| |||
4.4. Sensitivity
The slope of the calibration curve over the range of 0.5 to 2 times the target concentration for the analysis represents the sensitivity of the method. The sensitivity determined in this manner is 101,610 area units per µg/mL for TCP (Figure 4.4.).
4.5. Breakthrough
Breakthrough studies were performed using a sub-micron aerosol generation system consisting of a TSI (St. Paul, MN) atomizer used in the non-recirculating mode, an aerosol electrostatic neutralizer, a sampling chamber, and a TSI Model 3203 Particle Mass Monitor.
Submicron aerosols of TCP/PCP, less than 0.3 microns in diameter, were generated by pumping a 1.5 mg/mL isopropanol solution of technical grade 2,3,4,6-TCP containing PCP into the atomizer with a Waters (Milford, MA) Model 6000A pump at a 0.7 mL/min flow rate. A fine aerosol spray is produced in the atomizer as the TCP solution passes into a high velocity jet air stream which consists of dry laboratory air supplied at a flow of 3.5 L/min. The large droplets of the aerosol, which produce large particles, impact on the wall of the atomizer and are drained to a waste container and do not enter the air stream. The submicron aerosol that is produced by the atomizer is then passed through an electrostatic neutralizer before it is diluted with 25 L/min of dry laboratory air and drawn into the sampling chamber.
The sampling chamber consists of a 9 in. by 20 in. clear acrylic plastic cylinder equipped with a diffuser plate at the top, and at the bottom are mounted 3.75-in. outlet lines attached to a flow-controlled vacuum pump that is used to maintain a 20 L/min air flow through the chamber. A separate vacuum pump attached to a manifold is used in conjunction with critical orifices to sample from six sampling ports positioned at the base of the chamber. Attached to a seventh sampling port in the base is a TSI particle mass monitor for measuring the total aerosol concentration of the chamber.
A simple experiment demonstrating the importance of the glass fiber
filter in sampling a submicron aerosol was performed with the
described apparatus. A glass fiber filter mounted in a glass sampling
tube and placed ahead of the particle mass monitor reduced a 1.71
mg/m3 TCP/PCP aerosol to background levels
(0.034 mg/m3) indicating that the filter
alone was effective in trapping the aerosol. However, an
In other studies, breakthrough of TCP and PCP was observed upon analysis of backup sections from sampling tubes not equipped with a glass fiber filter which were taken from an aerosol test atmosphere.
These results differ from those observed for samples collected from 2.2 µm PCP aerosol generated by a TSI monodisperse generator. For these larger size aerosol particles, a sampling tube alone was completely effective in trapping the aerosol.
Although it is clear that TCP and PCP are quite volatile and exist largely as a vapor in test atmospheres, the potential for an aerosol component exists in the work environment. Penetration of a packed sampling tube by small aerosols, which has been demonstrated here and is reported in the literature can occur in the work atmosphere and does represent a potential source for loss of sample (Ref. 5.16.). Under these laboratory conditions, no sample breakthrough was observed for the sampling tube as designed.
Tests of the capacity of the
In order to test the effects of humidity on the sampling device, a
crude vapor generation system was devised. This system consisted of a
syringe drive pump which delivered 0.01 mL/min of 4 mg/mL technical
grade 2,3,4,6-TCP in methanol into one end of a glass tee which was
wrapped with heat tape and packed with silanized glass wool. A
Rheostat set at 20% of full scale was used to heat the sampling tee.
Air at 80% relative humidity and ambient temperature was drawn through
the tee at 1 L/min and into an
4.6. Desorption efficiency
Amberlite
Three sets of six sample tubes packed with this
Desorption Efficiency (TCP)
|
| |||
| × target conc. µg/sample |
0.5× 12.55 |
1× 25.1 |
2× 50.2 |
|
| |||
| desorption efficiency, % |
98.4 99.2 98.5 99.2 99.7 102.9 99.65 |
93.8 92.1 91.3 93.0 102.0 100.2 95.4 |
92.1 91.3 91.1 91.0 90.5 91.0 91.2 |
|
| |||
Desorption Efficiency (PCP)
|
| |||
| × target conc. µg/sample |
0.5× 12.52 |
1× 25.05 |
2× 50.1 |
|
| |||
| desorption efficiency, % |
104.0 105.6 103.6 104.4 105.4 107.0 105.0 |
99.1 98.0 98.7 99.4 100.2 98.5 99.0 |
95.5 98.0 95.5 94.8 94.3 94.7 95.5 |
|
| |||
In order to test the effect of elevated drying temperatures of the
adsorbent on desorption efficiency, a new portion of
Effect of Adsorbent Drying Temp. on Desorption Efficiency
|
| |||
| average % recovery | |||
| lot no. | treatment | TCP (% SD) | PCP (% SD) |
|
| |||
| 103A 103B 103C 103D 103E |
methanol extraction, to dryness with rotary evaporation 103A plus 12 h at 50°C 103B plus 12 h at 65°C 103C plus 12 h at 80°C 103D plus 12 h at 105°C |
105(2.2) 105(1.9) 104(2.6) 88.1(4.8) 87.4(0.8) |
110(1.6) 110(1.7) 109(2.5) 94.4(6.7) 92.6(0.5) |
|
| |||
The effects of humidity on the dried
Effect of Humid Air on Desorption Efficiency
|
| |||
| average % recovery | |||
| lot no. | treatment | TCP (% SD) | PCP (% SD) |
|
| |||
| 103E 103E 103C 103C 103C |
138 L of humid air (80% RH) sampled after spike control, no air sampled (four samples) 60 L of humid air (80% RH) sampled after spike control, no air sampled (four samples) 80 L of humid air (80% RH) sampled spike |
84.7(1.3) 87.0(0.9) 97.8(1.3) 102.(1.3) 99.1(1.4) 99.9(2.0) |
89.3(1.7) 90.7(1.0) 104(1.3) 107(1.3) 103(1.4) 103(1.8) |
|
| |||
4.7. Storage test
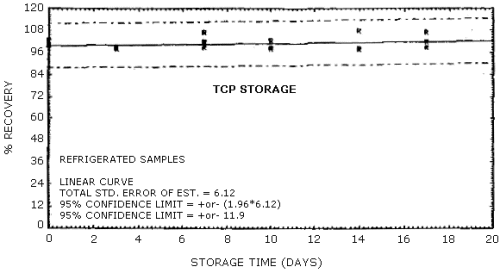
No storage problem was observed for either the ambient or the refrigerated storage samples which were generated with the submicron aerosol generation system described in Section 4.5. Total air concentrations of TCP in the 1.2 - 1.7 mg/m3 range were generated by using a 1.5 mg/mL solution of the technical grade TCP metered into the atomizer at a 0.7 mL/min flow rate. All other conditions for the aerosol system were the same as described in breakthrough. For storage, seven sets of six samples each were collected from the aerosol system at sampling rates ranging from 0.8 to 1.0 L/min. The individual sampling rates for each sample were determined both before and after collection with the sample tube in line. A 25-min collection period was used for all seven sets of storage samples. Since there was some variability in the air concentration of TCP generated in the aerosol chamber from one sample set to the next, one sample from each set was selected as a control and analyzed immediately. Of the remaining total of 35 samples generated for storage, three samples were discarded because of clogged sampling orifices, two were randomly selected for zero-day storage and analyzed immediately, and the remaining 30 samples were randomly split into equal-sized ambient and refrigerated groups for storage. All of the stored samples were capped and stored in the dark either at ambient conditions on a laboratory shelf or in a refrigerator at 5°C prior to analysis. Since variability in the air concentration of TCP occurred during the generation process, the percent recoveries reported below in Table 4.7. for each sample are determined relative to the concentration of TCP of the control sample for that set. A plot of percent recovery of TCP versus days stored for both ambient and refrigerated samples is shown in Figures 4.7.1. and 4.7.2.
Storage Samples
|
| |||||||||||||||||||||||
| (set 1) sample |
mg/m3 |
days stored |
% recovery |
(set 2) sample |
mg/m3 |
days stored |
% recovery | ||||||||||||||||
|
| |||||||||||||||||||||||
| 1 2 3 4 5 6 CV |
1.28 1.13 1.34 1.30 1.33 1.32 1.28 0.061 |
control 10 (A)1 17 (R)2 7 (R) 14 (A) 0 |
100 88.3 105 102 104 103 |
1 2 3 4 5 6 |
1.25 1.23 1.35 1.34 1.27 1.33 1.295 0.039 |
control 3 (R) 14 (R) 7 (R) 14 (A) 10 (A) |
100 98.4 108 107 102 106 | ||||||||||||||||
|
| |||||||||||||||||||||||
| (set 3) sample |
mg/m3 |
days stored |
% recovery |
(set 4) sample |
mg/m3 |
days stored |
% recovery | ||||||||||||||||
|
| |||||||||||||||||||||||
| 1 2 3 4 5 6 CV |
1.46 1.51 1.50 1.46 1.48 1.52 1.49 0.017 |
control 10 (R) 7 (A) 0 10 (A) 17 (A) |
100 103 103 100 101 104 |
1 2 3 4 5 6 |
1.46 1.51 1.50 1.42 1.56 1.52 1.50 0.033 |
control 3 (A) 17 (A) 14 (A) 17 (A) 7 (A) |
100 103 103 97.3 107 104 | ||||||||||||||||
|
| |||||||||||||||||||||||
| (set 5) sample |
mg/m3 |
days stored |
% recovery |
(set 6) sample |
mg/m3 |
days stored |
% recovery | ||||||||||||||||
|
| |||||||||||||||||||||||
| 1 2 3 4 5 CV |
1.64 1.62 1.61 1.63 1.61 1.62 0.008 |
control 7 (R) 10 (R) 3 (A) 3 (R) |
100 98.8 98.2 99.4 98.2 |
1 2 3 4 5 |
1.66 1.64 1.63 1.72 1.62 1.65 0.024 |
control 10 (R) 7 (A) 3 (A) 14 (R) |
100 98.8 98.2 104 97.6 | ||||||||||||||||
| |||||||||||||||||||||||
|
| |||||||||||||||||||||||
| 1 ambient conditions 2 refrigerated conditions | |||||||||||||||||||||||
4.8. Comparative sampling data
Comparative sampling of a TCP/PCP atmosphere was performed with
Four sets of six samples were collected from the test atmosphere
over several days using three
The overall average recovery of the
Comparison Sampling with IPA bubblers and
|
| |||||
| sample set 1 | mg/m3 (TCP) | mg/m3 (PCP) | |||
|
| |||||
IPA |
- 1 - 2 - 3 - 1 - 2 - 3 |
1.25 1.26 1.28 1.19 1.25 1.21 |
0.26 0.33 0.31 0.22 0.24 0.24 |
||
|
| |||||
| sample set 2 | mg/m3 (TCP) | mg/m3 (PCP) | |||
|
| |||||
IPA |
- 1 - 2 - 3 - 1 - 2 - 3 |
1.29 1.24 1.24 1.31 1.33 1.34 |
0.30 0.29 0.28 0.26 0.26 0.27 |
||
|
| |||||
| sample set 3 | mg/m3 (TCP) | mg/m3 (PCP) | |||
|
| |||||
IPA |
- 1 - 2 - 3 - 1 - 2 - 3 |
1.48 1.60 1.50 1.68 1.60 1.58 |
0.33 0.21 0.32 0.32 0.30 0.29 |
||
|
| |||||
| sample set 4 | mg/m3 (TCP) | mg/m3 (PCP) | |||
|
| |||||
IPA |
- 1 - 2 - 3 - 1 - 2 |
1.23 1.37 1.41 1.32 1.30 |
0.22 0.29 0.36 0.24 0.25 |
||
|
| |||||
For the comparative sampling with NaOH bubblers, four sets of six
samples were sampled from the test atmosphere over several days using
four
Comparison Sampling with NaOH Bubblers and
|
| ||||||
| sampling | TCP | PCP | (sampling | |||
| set 1 | rate (L/min) | (mg/m3) | (mg/m3) | time 93 min) | ||
|
| ||||||
NaOH |
- 1 - 2 - 3 - 4 - 1 - 2 |
0.920 0.213 0.197 1.02 0.480 0.490 |
0.63 0.62 0.56 0.68 0.73 0.67 |
0.17 0.16 -- 0.19 0.17 0.18 |
||
|
| ||||||
| sampling | TCP | PCP | (sampling | |||
| set 2 | rate (L/min) | (mg/m3) | (mg/m3) | time 93 min) | ||
|
| ||||||
NaOH |
- 1 - 2 - 3 - 4 - 1 - 2 |
0.902 0.212 0.196 0.980 0.475 0.470 |
0.70 0.67 0.66 0.67 0.78 0.66 |
0.15 0.17 0.16 0.15 0.18 0.17 |
||
|
| ||||||
| sampling | TCP | PCP | (sampling | |||
| set 3 | rate (L/min) | (mg/m3) | (mg/m3) | time 93 min) | ||
|
| ||||||
NaOH |
- 1 - 2 - 3 - 4 - 1 - 2 |
0.905 0.211 0.203 0.199 0.466 0.445 |
0.77 0.84 0.75 0.76 0.76 0.82 |
0.17 0.17 0.17 0.16 0.15 0.18 |
||
|
| ||||||
| sampling | TCP | PCP | (sampling | |||
| set 4 | rate (L/min) | (mg/m3) | (mg/m3) | time 93 min) | ||
|
| ||||||
NaOH |
- 1 - 2 - 3 - 4 - 1 - 2 |
0.896 0.211 0.196 0.201 0.448 0.63 |
0.70 0.67 0.70 0.65 0.80 0.78 |
0.17 0.14 0.16 0.15 0.18 0.15 |
||
|
| ||||||
4.9. Reproducibility
Six sample tubes, each spiked with 3 µL of a 10.75 mg/µL (79% pure) TCP standard in methanol. This resulted in a sample loading of 25.5 µg. The samples, along with a blank sample, were capped, labeled and submitted to an OSHA laboratory service branch, with a draft copy of this method, for analysis. The samples were analyzed approximately two weeks after preparation by a chemist not associated with this evaluation. The percent recoveries for the six samples were: 97.3, 99.9, 90.5, 93.5, 92.0, and 90.5. The average is 94.0 and the standard deviation is 3.9.

5. References
- 5.1. Cummins, K., Pentachlorophenol, (Method No. 39, Organic
Methods Evaluation, OSHA Laboratory, Salt Lake City, Utah),
unpublished (6-82).
5.2. J.D. Doedens in "Kirk-Othmer Encyclopedia of Chemical
Technology", Vol. 5, PP.
5.3. Pekari, K; Aitio, A., J. Chromatogr. (1982), 232, 129-36.
5.4. Daniels, C.R.; Swan, E.P., J. Chrom. Science (1979), 17, 628-30.
5.5. Ervin, H.E.; McGinnis, G.D., J. Chromatogr. (1980), 190, 203-07.
5.6. Ivanov, Z.; Magee, R.J., Microchemical Journal (1980), 25, 543-47.
5.7. Ugland, K; Lundanes, E; Greibrokk, T; Bjorseth, A., J.
Chromatogr. (1981), 213,
5.8. Mundy, D.E.; Machin, A.F., J. of Chromatogr. (1981), 216, 229-38.
5.9. Edgerton, T.R.; Moseman, R.F., J. Chrom. Science (1980), 18, 25-29.
5.10 "NIOSH Registry of the Toxic Effects of Chemical Substances", USDHEW, PHS, CDC, NIOSH, Washington, D.C., U.S. Government Printing Office (1977).
5.11. Ahlborg, U.G.; Larrsson, K., Arch. Toxicol. (1978), 40(1), 63-74.
5.12. Deichmann, W.; Keplinger, M.L., in "Patty's Industrial Hygiene and Toxicology", 3rd revised ed.; Clayton, G.D.; Clayton, F.E., Ed.; John Wiley & Sons, Inc., New York, 1981; Vol. IIA, Chapter 36.
5.13. Lamberton, J; Griffin, D.; Arbogast, B.; Inman, R.; Deinzer, M., Am. Ind. Hyg. Assoc. J. (1979), 40, 816-21.
5.14. Edgerton, T.R.; Moseman, R.F.; Lores, E.M.; Wright, L.H., Anal. Chem. (1980), 52, 1774-77.
5.15. Rappe, C.; Buses, H.; Rudolf, in "Chemical Hazards in the Workplace", Choudhary, Gangadhar, Ed.; American Chemical Society, 1981; ACS Series 149, Chapter 20.
5.16. Beast, R.C., "CRC Handbook of Chemistry and Physics", 62nd ed.; CRC Press Inc., Boca Raton, Florida; 1981-82.
5.17. Fairchild, C.I.; Tillery, M.I., Am. Ind. Hyg. Assoc. J. (1977), 38, 277-83.