| Method no.: | 39 |
| Matrix: | Air |
| Target concentration: | 0.5 mg/m3 (OSHA PEL/TWA) |
| Procedure: | Samples are collected by drawing a known volume of air through two specially prepared XAD-7 adsorbent tubes which are connected in series. Following desorption with methanol the samples are analyzed by high performance liquid chromatography with ultraviolet (UV) detection. |
| Recommended air volume and sampling rate: |
48 L at 0.2 L/min |
| Reliable quantitation limit: | 0.007 mg/m3 |
| Standard error of estimate: (Figure 4.7.1.) |
6.9% |
| Special requirements: | Sampling tubes must be obtained from the laboratory. Prior to sampling, remove the XAD-7 resin-filled front section of the sampling device and save it. This resin packed tube serves as a cap to trap any PCP which might volatilize from the filter after sampling. Immediately after the completion of sampling, re-attach this section onto the front section of the sampling device for shipment to the laboratory. (Section 2.) |
| Status of method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1982 | Chemist: Kevin Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
This air sampling procedure for pentachlorophenol (PCP) uses two laboratory prepared XAD-7 sampling tubes connected in series. A small glass fiber disc is placed ahead of the resin bed of the front tube to trap any aerosols present in the air. The second tube serves as a backup section in the unlikely event of breakthrough.
Unlike a previous NIOSH sampling method which employed ethylene glycol (Ref. 5.1.), or the frequently used aqueous sodium hydroxide solution, this method does not require the use of a cumbersome bubbler for sampling.
Previous evaluations of air sampling methods for phenol and cresol, and a preliminary study performed with a mixture of all of the chlorinated phenols indicate that XAD-7 resin will probably serve as a versatile sampling medium for a wide variety of phenolic compounds (Ref. 5.2.).
The majority of the air sampling evaluations performed with PCP in this study used an aerosol generation system which is described in Section 4.5. The effectiveness of XAD-7 as a sampling media was established by comparing the collection efficiency of both commercial (SKC Inc., Eighty-Four, PA.) and laboratory prepared XAD-7 sampling tubes with a variety of other samplers. Similar efficiencies were observed for XAD-7, silica gel, 0.1 N sodium hydroxide bubblers, isopropanol bubblers, and a glass fiber filter in a cassette followed by a XAD-7 sampling tube. The volatile nature of PCP was clearly demonstrated in aerosol studies. Approximately two thirds of the total PCP collected was observed to have passed through the glass fiber filter and was trapped on the backup XAD-7 tube. However, efficient collection of a sodium pentachlorophenate (NaPCP) aerosol was observed for the glass fiber filter alone. Since the recommended sampling device is a glass fiber filter placed ahead of the XAD-7 resin bed, this sampling device should effectively trap NaPCP. The HPLC analytical method, however, does not distinguish between PCP and NaPCP.
The high capacity of XAD-7 resin for PCP was clearly established in breakthrough studies performed with laboratory prepared XAD-7 sampling tubes. No breakthrough was observed after sampling a 0.82 mg/m3 PCP aerosol atmosphere for over 4.5 h at 1 L/min, or after sampling a 10 mg/m3 PCP vapor atmosphere for over 6 h at 1 L/min (Section 4.5.).
Laboratory prepared XAD-7 sampling tubes using 8-mm o.d., 6-mm i.d. glass tubing were selected for use in preference to the smaller commercial XAD-7 sampling tubes for a number of reasons: the large diameter opening of the laboratory-packed tube may improve aerosol sampling efficiency (Ref. 5.3.); the 6-mm diameter opening permits easy insertion of a glass fiber disc for trapping an aerosol in a one-unit system; and finally the Soxhlet extracted XAD-7 resin used in the laboratory prepared tubes, unlike the commercial XAD-7 resin, is free of a contaminant which will interfere with the HPLC analysis of the lower-molecular-weight chlorinated phenols.
In the course of this evaluation it was observed that low recoveries of PCP were obtained for samples which were spiked with a stock solution of PCP directly onto the glass fiber filter disc mounted ahead of the resin bed. No losses were observed, however, if the PCP was spiked into the resin bed of the sampling device. (Section 1.2.8.) The losses from the filter can most likely be attributed to volatilization since no adsorption on glass fiber filters has been observed. Since no differences in recovery have been observed for this sampling method versus other collection methods in sampling an aerosol generated atmosphere of PCP, the loss of PCP from the aerosol generated samples must be much less than for the glass fiber filter spiked samples. In actual work environments it is anticipated that the collection of PCP on the sampling device will more closely resemble the pattern observed for the laboratory generated aerosol samples. The analysis of individual components of the sampling tube of aerosol generated samples indicates that approximately two-thirds of the sample is collected on the resin bed while approximately one-third is found on the filter. Thus, only a portion of the sample is retained on the filter for possible loss. If this is the case, only minimal losses of PCP from the glass fiber filter disc can be expected. In order to ensure that no sample loss is experienced, an XAD-7 packed glass tube will be used to cap the sampling device and capture any PCP which might escape following sampling.
A number of reverse and normal phase high performance liquid chromatographic methods have been published for the analysis of PCP in a variety of matrices (Refs. 5.1 and 5.4 - 5.9.). Gas chromatographic analysis of PCP both by derivatization and by direct analysis is also reported (Refs. 5.8. - 5.9.). The GC methods, although generally considered to be more difficult to perform than HPLC, are more sensitive, and can be used to complement HPLC. Thin layer chromatography and colorimetry have also been used to analyze PCP (Ref. 5.6.)
In this method, PCP is analyzed by reverse-phase HPLC using a UV detector set at 220 nm. A cyano-alkyl bonded column is used with a water-acetonitrile mobile phase containing phosphoric acid to suppress the ionization of the acidic analyte. A Zorbax ODS column exhibited a higher capacity than the CN column for the chlorinated phenols, however, it offered little or no improvement in selectivity. Using the recommended conditions, PCP can be separated from all of the other chlorinated phenols in approximately 8 min.
As with many industrial chemicals which can be absorbed through the skin, the determination of PCP or NaPCP air concentration is not a total measure of occupational exposure. For example, significant exposure to NaPCP can occur through skin contact with lumber which has been sprayed with a dilute aqueous solution of NaPCP to prevent wood darkening. Elevated urinary PCP levels among lumber workers who handle NaPCP-treated lumber have been observed in the absence of significant air exposures. Urinary PCP analysis may represent a reliable means of measuring total exposure to PCP or NaPCP. In a preliminary study conducted at this laboratory, PCP recoveries of over 90% were obtained for urine spiked at the 1 ppm level. Following acidification, the urine was extracted using commercial octadecasilane extraction columns, and analyzed by HPLC/UV. Although UV detection is less sensitive than electrochemical or electron capture detection, this method can provide a means for rapidly screening for significant exposures to PCP or NaPCP.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The toxic properties of PCP and NaPCP have been well studied due to its toxic nature and wide industrial and commercial use. Dusts in excess of 1 mg/m3 produce eye, nose, and throat irritation among the unacclimated, although acclimation has been reported to occur at air concentrations as high as 2.4 mg/m3 (Ref. 5.10.). Prolonged skin exposure can result in an acne-like dermatitis. In addition to their contact irritant properties, both PCP and NaPCP can be absorbed through the skin to produce a systemic effect. The toxic action of PCP is believed to be the result of its ability to uncouple oxidative phosphorylation enzyme systems. (Ref. 5.11.) The resultant failure to generate sufficient stores of energy by normal metabolic pathways is manifested by elevated body temperatures (hyperpyrexia), and an increase in respiration and heart rate. Intoxication is characterized by weakness, loss of appetite (anorexia), weight loss, and profuse sweating (Ref. 5.10.). Headaches, dizziness, nausea, vomiting, shortness of breath (dyspnea), and chest pain may also be experienced. The risk of a toxic effect due to PCP exposure is more pronounced at high ambient temperatures or among persons with impaired liver or kidney function. The world literature reports 31 deaths among 50 reported poisonings resulting from PCP use (Ref. 5.12.). Included in this total is a death resulting from a 14 h exposure in a spraying operation. Fatalities from the skin absorption of PCP due to the uncontrolled use of a 1.5-3% PCP/NaPCP solution in fuel oil have also been reported. Acute gastric LD50s for mice and rats of 130 and 184 mg/kg respectively have been reported for PCP. A dermal LD50 of 96 mg/kg in rats has been reported (Ref. 5.12.).
Concern has been expressed about the presence of highly toxic dibenzo-p-dioxins and dibenzofurans which are contaminants in PCP formed from the condensation of chlorophenols in the production process (Refs. 5.13 and 5.14.). Although less than 0.05 ppm of the highly toxic 2,3,7,8-tetrachlorodibenzo-para-dioxin is reported in one analysis of PCP, parts-per-million levels of the toxic hexachlorodibenzo-para-dioxin, and parts-per-thousand levels of the less toxic octachlorodibenzo-para-dioxin were reported in the same sample (Ref. 5.14.).
Based on available information, the International Agency for Research on Cancer (IARC) does not make a judgment on the carcinogenicity of PCP (Ref. 5.14.). In one animal study an excess of hepatomas was observed in one of two strains of male mice upon subcutaneous injection of a single dose of PCP. Two other separate studies using mice and rats were negative. No human data was reported to be available for evaluation by IARC.
1.1.3. Potential workplace exposure.
In 1974 there were 23.4 million kg of PCP used in the United States (Ref. 5.14.). Over 80% of this total was used in the wood industry. Pentachlorophenol and its sodium salt are primarily used as antimicrobial and antifungal agents. PCP in a fuel oil mixture is used to treat and preserve posts, logs, and lumber in the lumber industry. PCP is also used in the construction industry to prevent mold formation on tiles, building surfaces and concrete blocks; in the leather industry to protect upper leather in shoes; and in the paint industry for the protection of protein-based latex paints (Ref. 5.15.).
The sodium salt of pentachlorophenol is used as an antimicrobial agent in starch or protein-based adhesives, and in the protection of leather and paints. The sodium salt is also used in the paper industry to protect stored pulp and fiberboard from mildew and rot; in the oil industry to prevent the growth of bacteria in drilling muds; in the textile industry to prevent mold formation on finished yarns and cloth in storage; and in water treatment to prevent the growth of algae, fungi and bacteria. The pentachlorophenol salt is primarily used in aqueous solutions. A dilute aqueous solution of the sodium salt is used to spray freshly milled lumber to prevent darkening of the wood by fungi.
1.1.4. Physical properties (Ref. 5.16. unless otherwise indicated)
| Pentachlorophenol | Sodium salt | |
| CAS no.: | 87-86-5 | -- |
| molecular weight: | 266.35 | 288.34 |
| melting point: | 191°C (anh) 184°C (1·H2O) |
-- -- |
| boiling point: | 293.08°C | -- |
| specific gravity: | 1.978 at 22°C | -- |
| solubility: | water - 35 ppm at 50°C. soluble in ether, alcohol, and benzene | water - 15% at 4°C, soluble in alcohol & acetone |
| vapor pressure at 15°C: | 0.00019 mm Hg | -- |
| dissociation constant at 25°C, (KA): | 1.2 × 10-5 (Ref. 5.15.) |
-- |
| synonyms and trade names: | ||
| pentachlorophenol: | Chem-Tol, Permacide, Penta, Santophen 20, Dowicide 7, Eurisan, Cuprinol, Penchloral, PCP, Pentachlorofenol | |
| sodium salt: | Santobrite, Dowicide G | |
| physical appear.: | PCP and NaPCP are white or tan solids with a distinct phenolic odor. | |
1.2. Limit defining parameters (The analyte air concentrations listed through this method are based on an air volume of 48 L and a solvent desorption volume of 2 mL)
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 4.1 ng per injection. This is the amount of the analyte which will give a peak whose height is approximately 5 times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.33 µg per sample (0.007 mg/m3) for pentachlorophenol. This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.33 µg per sample (0.007 mg/m3) for pentachlorophenol. This is the smallest amount of analyte which can be quantitated within the requirements of a recovery of at least 75% and a precision (1.96 SD) of ±25% or better. (Section 4.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
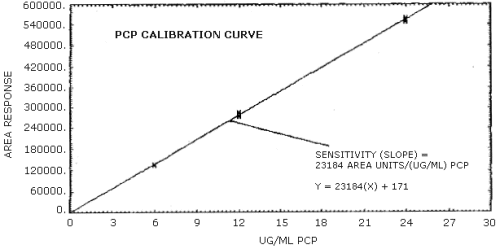
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 23,184 area units per µg/mL for pentachlorophenol. The sensitivity is determined from the slope of the calibration curve. The sensitivity may vary with different instruments or instrumental conditions. (Section 4.4.)
1.2.5. Recovery
The recovery of pentachlorophenol from samples used in a 19-day storage remained above 99% when the samples were stored at ambient conditions in the dark. (Section 4.8.) The recovery of the analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (Analytical procedure only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1 and 2 times the target concentration is 0.010 (Section 4.3.).
1.2.7. Precision (overall procedure)
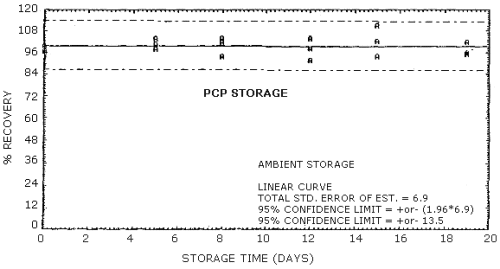
The precision at the 95% confidence level for the 19 day storage test is ±13.5% for pentachlorophenol. (Section 4.8.) The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.2.8. Reproducibility
Three spiked samples were prepared by injection of a stock standard of PCP in methanol into the resin bed of the sample tube and were given along with a draft copy of this method to a chemist unassociated with this evaluation for analysis. The samples were analyzed after 22 days storage at room temperature. The average recovery for the three samples loaded with 12.1, 24.3 and 40.5 µg of PCP respectively was 101%. The recovery ranged from 98 to 105%.
Three other samples were also prepared at the same time by spiking PCP directly onto the glass fiber filter disc which is placed ahead of the resin bed and these samples were analyzed in conjunction with the three resin-bed spiked samples. The average recovery for these three samples with the same loadings as the resin spiked samples was 80%. The range was from 67 to 89%. Similar results were also obtained by the chemist who developed this method upon analysis of an identical set of six samples which were prepared at the time of the above samples and analyzed at approximately the same time. The low recoveries obtained from samples spiked onto the glass fiber filter most probably can be attributed to volatilization losses since no adsorption of PCP onto glass fiber filters has been observed. The excellent recoveries obtained upon analysis by an independent chemist of samples spiked into the resin bed, although based on limited data, indicate good reproducibility. (Section 4.8.)
1.3. Advantages
- 1.3.1. The two solid sorbent sampling tubes in series represent
a more convenient method than the previous methods using bubbler
solutions.
1.3.2. The analysis for pentachlorophenol is rapid, sensitive, and precise.
1.4. Disadvantages
- 1.4.1. The method has not been field tested.
1.4.2. The sampling tubes are not commercially available.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A constant flow personal sampling pump is used which can
be calibrated to within ±5% of the recommended 0.2 L/min flow rate
while the sampling train is in line.
2.1.2. The sampling tubes consist of two 50-mm long by 8-mm o.d.,
6-mm i.d. glass tubes which are packed with approximately 175 mg
(15-mm bed length) of XAD-7 resin held in place with two silanized
glass wool plugs. The tubes are butted together using a connector
made from a 9/32-in. diameter plastic cap from which the closed end
has been removed. The first sampling tube in the series also
contains an 8-mm glass fiber filter disc as a precautionary measure
to trap any aerosols of the analyte. A number 4 cork borer is used
to cut out the discs from Gelman (Ann Arbor, Michigan, USA) Type A
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Properly label all three sections of the sampling device
prior to sampling.
2.3.2. Before sampling, remove and save the front glass tube section containing XAD-7 resin which will serve as a cap following completion of sampling.
2.3.3. Attach the sampling tubes to the pump using a section of flexible, plastic tubing so that the adsorbent tube containing the glass fiber filter serves as the front sampling section. Do not place any tubing ahead of the sampling device. Attach the sampling device in the workers breathing zone in such a manner that it does not impede work performance.
2.3.4. After sampling for the appropriate time, remove the sampling device from the pump, cap the front end of the device with the resin-filled glass tube and cap the back end of the device with a plastic cap. Insure that the caps are well fitted and label the sampling tubes with OSHA seals (Form 21).
2.3.5. Include at least one blank for each sampling set. The blank should be handled in the same manner as the samples with the exception that air is not drawn through it.
2.3.6. Any bulk samples submitted for analysis must be shipped in separate containers to avoid contamination of the air samples.
2.3.7. List any potential interferences on the sample data sheet.
2.4. Breakthrough
Since XAD-7 resin has a very high capacity for pentachlorophenol, the determination of the amount of analyte which can be collected from an atmosphere before breakthrough occurs was found to be experimentally difficult to determine and of little practical value. Two studies were performed, however, to demonstrate the high capacity of the resin for pentachlorophenol.
In the first study, an XAD-7 sampling tube without a glass fiber filter insert was used to sample an independently measured 0.82 mg/m3 PCP aerosol of 2.2 µm in diameter which was generated by an aerosol generation system. No breakthrough to the backup sampling tube was observed after 275 L of dry air was sampled at 1 L/min for 4.6 h. A more complete description of the experiment and the aerosol generation system is discussed in Section 4.5.
In the second study the ability of a XAD-7 sampling tube to collect PCP vapors was investigated under high humidity conditions. No breakthrough was observed after sampling 378 L of a 10 mg/m3 atmosphere of PCP at 1 L/min with an XAD-7 sampling tube containing a glass fiber filter. Details of this study are discussed in Section 4.5.
2.5. Desorption efficiency (solvent adsorption effect)
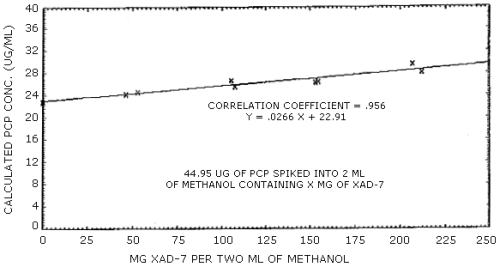
The values obtained for desorption efficiency of PCP over the range of 0.5 to 2 times the PEL averaged 118% for XAD-7 sampling tubes. This high value is probably the result of a change in the concentration of PCP in solution due to the preferential adsorption of methanol onto XAD-7 resin. This effect is proportional to the amount of XAD-7 used in the sampling tube and can best be illustrated by the linear graph obtained from plotting the observed PCP concentration versus the amount of XAD-7 added to 2 mL of methanol containing a known amount of PCP. (Figure 4.6.) The high correlation coefficient of 0.956 observed for the line strongly suggests that the observed response is a function of the amount of XAD-7 used. Since this effect is apparently not concentration dependent, a reliable correction for sample results can be made if a uniform weight of XAD-7 resin is used in all sampling tubes. (Section 4.6.)
2.6. Recommended air volume and sampling rate
A 48-L air sample obtained by sampling at 0.2 L/min for 4 h is recommended for pentachlorophenol. If necessary, the sensitivity of the analytical method will permit a sampling period as short as 15 min at 0.2 L/min for determination of the analyte at the target concentration.
2.7. Interferences
There are no known interferences to the sampling procedure.
2.8. Safety precautions
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A high performance liquid chromatograph equipped with
sample injector, a cyano bonded phase HPLC column, a variable
wavelength UV detector, and a chart recorder are needed for the
analysis. A Waters 6000A pump, a Waters WISP 710 auto sampler, a
Perkin-Elmer LC-55 UV-Visible detector and a Zorbax 25-cm × 4.6-mm
i.d. CN bonded phase column were used in this study.
3.1.2. An electronic integrator or other suitable means of measuring detector response is required. The Hewlett-Packard 3354 data system was used in this study.
3.1.3. Various sizes of volumetric glassware and pipettes are needed for sample and standard preparations.
3.1.4. Three-milliliter (or larger) screw-cap or crimp-type vials are needed for desorbing the XAD-7 sampling adsorbent. Four-milliliter Waters WISP vials were used in this study.
3.1.5. Small brown glass bottles fitted with inert cap liners are needed to store standard solutions.
3.1.6. A repetitive dispenser capable of accurately delivering the desorption solution is needed.
3.2. Reagents
- 3.2.1. HPLC grade methanol and acetonitrile.
3.2.2. Reagent grade phosphoric acid.
3.2.3. HPLC grade water. Water obtained from a Milli-Q reagent grade water system (Millipore, Inc., Bedford, Mass.) was used in this study.
3.2.4. A reagent grade standard of pentachlorophenol is required. Pentachlorophenol, Chem Service, Lot #PS-7, West Chester, PA was used in this study.
3.3. Standard preparation
- 3.3.1. Prepare a stock solution of pentachlorophenol by
accurately weighing approximately 200 mg of the standard and
transferring it to a 25-mL volumetric flask. Dilute to volume with
methanol. Dilute this stock solution 1/25 to yield an approximate
320 µg/mL solution in methanol. Prepare 1/50, 1/25 and 2/25
dilutions of the 320 µg/mL PCP solution to yield standards which
correspond approximately to 0.5, 1, and 2 times the PEL for the
recommended sampling conditions.
3.3.2. As an alternative method of preparing standards, spike appropriate quantities of the stock PCP standard into vials containing 2 mL of methanol and the same approximate weight of XAD-7 resin as was used in the sampling tube (175 mg). These spiked samples can be used as standards, or they can be used to determine desorption efficiencies with standard solutions as outlined above. No correction for the solvent adsorption effect will need to be made if the standards are prepared by spiking PCP into solutions containing 175 mg of XAD-7 resin.
3.4. Sample preparation
Prepare samples for analysis by transferring the entire contents of
the sampling tube including both glass wool plugs, the resin, and the
glass fiber disc into a 4-mL vial. Considerable care must be exercised
in transferring the samples to the vials to avoid sample loss from
static
3.5. Analysis
- 3.5.1. Prepare a high performance liquid chromatograph for
sample analysis using the HPLC conditions listed below.
| column: | Zorbax 25-cm × 4.6-mm i.d. CN bonded phase |
| mobile phase: | 40/60 (v/v) acetonitrile/water containing approximately 0.1% by volume of phosphoric acid. |
| flow rate: | 1.3 mL/min |
| UV detector: | 220 nm |
| injection volume: | 25 µL |
| retention time: | 8.1 min |
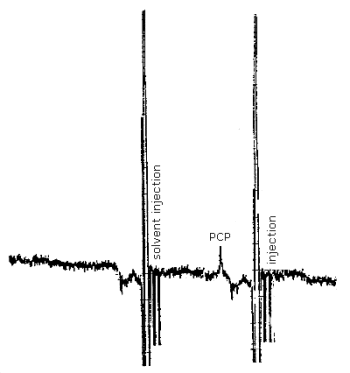
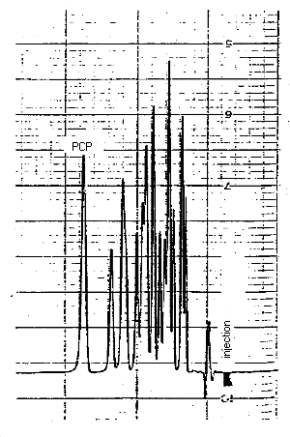
3.5.2. Analyze the front and back sampling tubes and the sample cap tube separately. Verify that the sample responses lie within the range of the responses observed for the standards. (See chromatogram, Figure 4.9.)
3.5.3. Since column-to-column variations do occur, it is important to ensure that PCP is being separated from the other chlorinated phenols. The injection of a tetra/pentachlorophenol standard mixture will produce baseline separation between pentachlorophenol and the tetrachlorophenol isomers if the analytical conditions are properly selected. Under these conditions the other chlorinated phenols are not interferences.
3.6. Interferences
- 3.6.1. Any compound which has the same retention time as
pentachlorophenol is a potential interference. Under the recommended
analytical conditions none of the mono-, di-, tri-, or
tetrachlorophenols interfered with the analysis. (See chromatogram,
Figure 4.10.)
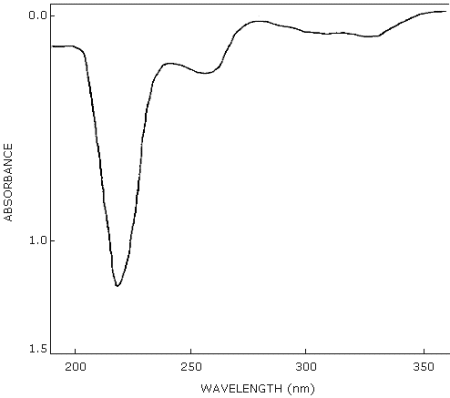
3.6.2. Comparison of peak height ratios of analyte response at two wavelengths for both samples and standards is a valuable confirmatory technique in HPLC. (See UV scan, Figure 4.11.) Normal phase HPLC methods and gas chromatographic methods may also be used for sample confirmation. GC/mass spectrometry is an additional confirmatory technique which may be used.
3.7. Calculations
- 3.7.1. Prepare a standard calibration curve of area response
versus concentration for PCP by determining the least squares fit
equation for the curve. Calculate the analyte concentration in the
samples by entering their response values into the equation and
solving for the sample concentration. A laboratory data system, or
one of many small hand-held calculators, can be used to perform
these calculations.
3.7.2. Combine the amount of analyte found on all sections including the sample tube cap. Express results in mg/m3 using the following equation:
| mg/m3 = | (µg/mL analyte) (2-mL
desorption volume) (1 mg)
(air volume in m3) (1000 µg) (solvent adsorption effect) |
Note: No solvent adsorption effect correction will need to be applied if standards have been prepared in solutions containing XAD-7 resin.
To convert to ppm at 760 mm and 25°C:
| 24.46 | = | the molar volume at 760 mm and 25°C. |
| MW | = | 266.35 for pentachlorophenol |
3.8. Safety precautions
- 3.8.1. Minimize exposure to pentachlorophenol by performing
standard preparations in a well ventilated hood.
3.8.2. Avoid all skin contact with pentachlorophenol.
3.8.3. Restrict the use of solvents to hoods which provide adequate ventilation.
3.8.4. Wear safety glasses in laboratory areas at all times.
4. Backup Data
- 4.1. Detection limit for analytical procedure
The detection limit for the analytical procedure is 4.1 ng for PCP. This is based on a 25-µL injection of a 0.163-ng/µL standard, and represents approximately five times the baseline noise. (Figure 4.1.)
4.2. Detection limit of the overall procedure and reliable quantitation limit
The detection limit of both the overall procedure and the reliable quantitation limit is 0.33 µg per sample (0.007 mg/m3) for PCP.
Six XAD-7 sampling tubes were spiked with 8 µL of 40.4 µg/mL PCP in methanol, then capped and stored overnight in a laboratory drawer. Assuming complete recovery, this amount of analyte is equivalent to the detection limit of the analytical procedure. The following day the samples were desorbed in 2 mL of methanol and analyzed. The percent recoveries (corrected for the solvent adsorption effect) are reported below.
Detection Limit Data
|
| |||
| % recovery | statistics | ||
|
| |||
| 104 104 104 98 110 104 |
SD 1.96 SD |
= = = |
104 3.8 7.4 |
|
| |||
4.3. Precision
The pooled coefficient of variation over a range of 0.5 to 2 times the target concentration for pentachlorophenol was obtained from multiple 25-µL injections of three standard solutions. The results are listed in Table 4.3.
Sensitivity and Precision Data
|
| |||
| × target conc. µg/mL |
0.5× 5.99 |
1× 12.0 |
2× 24.0 |
|
| |||
| area
counts SD CV |
137934 138276 137543 136555 137254 137470 600 0.0044 |
283403 287608 278956 278511 276153 280856 4085 0.015 |
560373 561265 554505 548815 550071 555574 5122 0.0092 |
|
| |||
4.4. Sensitivity
The slope of the calibration curve over the range of 0.5 to 2 times the target concentration for the analytes represents the sensitivity of the method. The sensitivity determined in this manner is 23,184 area units per µg/mL for PCP (Figure 4.4.).
4.5. Breakthrough
Breakthrough studies for PCP were performed using both aerosol generation and vapor generation systems. The aerosol generation system used in this study consists of a syringe drive pump which was used to meter a 0.313 mg/mL standard of PCP in isopropanol into a model 3050 Bergland-Liu Vibrating Orifice Monodisperse Aerosol Generator (Thermo-System Inc., St. Paul, Minn.) at 0.16 mL/min. The frequency of the orifice was set at 7.5 KHz with a Precision Dynascan model 3010 function generator (Chicago, IL.) to produce a 2.2 µm monodisperse aerosol of PCP. The aerosol was then diluted with 2 m3/h. of dry laboratory air as it passed vertically up through the electrostatic neutralizer. After exiting the neutralizer at a right angle, the aerosol then passed through approximately 3 ft of 1.5-in. flexible plastic tubing before entering the sample chamber. The sampling chamber consists of a 9-in. by 20-in. cylinder of clear acrylic plastic in which a diffuser plate is mounted near the top. Samples were collected via any of seven sampling ports mounted in the bottom of the chamber. Flow rates were controlled by means of critical orifices attached to a vacuum pump.
Attached to one of the sampling ports in the chamber was a section of tubing used to sample the total mass of the aerosol in the chamber with a Thermo System Model 3203 Particle Mass Monitor. An air flow of approximately 20 L/min was maintained in the sampling chamber by means of a calibrated pressure-vacuum pump connected via 3/4-in. PVC tubing to three outlets in the bottom of the sampling chamber. Excess air flow from the aerosol generator and all sampled air was first drawn through a HEPA filter before it was vented to the atmosphere.
Ideally this aerosol generation system, when used in conjunction with the particle mass monitor to measure the actual concentration of the aerosol generated, should permit the evaluation of a test method independent of other sampling methods. In practice, however, some discrepancies were obtained between the amount of PCP present in the generated atmosphere as measured by the analysis of various sampling tubes or bubblers and with the levels measured by the particle mass monitor.
Several preliminary evaluations of mass monitor results versus the results of various sampling methods for PCP were in good agreement. Subsequent studies, however, indicated that although the total PCP concentration had increased in the system, the particle mass monitor was underestimating the total PCP concentration. This difference in results could possibly be attributed to an increase in the amount of vapor present in the generated atmosphere which would not be detected by the particle mass monitor.
Although the particle mass monitor could not be used as an independent measure of the total PCP present in the system, the good agreement obtained using various sampling methods to measure the total PCP concentration establishes confidence that the actual PCP concentration is known with some degree of certainty.
The average concentration of the atmosphere generated in the chamber was determined to be 0.82 mg/m3 for the breakthrough study. This was determined by analysis of a series of XAD-7 tubes and one silica gel packed sampling tube which sampled adjacent to the XAD-7 tube used for the breakthrough study. This concentration was considerably less than the calculated concentration of 1.5 mg/m3. The observed deposition of the aerosol in the neutralizer may have accounted for the difference.
In this study, no breakthrough was observed for XAD-7 after sampling 275 L of the dry laboratory air at 1 L/min. It might also be noted that a similar volume of silica gel also did not exhibit breakthrough under these conditions. Adequate capacities for PCP vapors under high humidity conditions have also been observed by silica gel. The selection of XAD-7 as the sampling medium for PCP is based largely upon the desire to develop a single sampler for a wide variety of phenolic compounds. Since silica gel has been shown to be much less effective than XAD-7 in collecting phenol and cresol, it is anticipated that silica gel will lack the versatility offered by XAD-7.
A second attempt at evaluating breakthrough for PCP was performed using a crude vapor generation system. This system consisted of a syringe drive pump which delivered 0.01 mL/min of 4 mg/mL PCP in methanol into one end of a glass tee which was wrapped with heat tape and packed with silanized glass wool. A rheostat set at 65% of full scale was used to heat the sampling tee. Air, with a relative humidity of 80% at 23°C, was drawn through the tee at 1 L/min and onto an XAD-7 sampling tube mounted at the other end of the tee. No breakthrough was observed during a 6.3 h sampling period. The total amount of PCP collected on the sampling media was 3912 µg. This corresponds to an average concentration of 10 mg/m3 over the entire sampling period. Of this total only 105 µg was found on the glass fiber disc which preceded the resin bed. The actual amount of PCP deposited on the sample tube is considerably less than expected. However, considerable condensation of PCP ahead of the sampling media was observed.
This vapor study performed under humid conditions, and the aerosol study performed with dry laboratory air indicate the recommended sampling device is very effective for sampling PCP vapors or aerosols.
4.6. Desorption efficiency (solvent adsorption effect)
The average desorption efficiency from spiked samples over the range of 0.5 to 2 times the target concentration is 118%. This high desorption efficiency is probably due to a solvent adsorption effect as discussed in Section 2.5. Six XAD-7 sampling tubes were each spiked with either 1.5, 3, or 6 µL of a 7492 µg/mL PCP standard in methanol. All of the 18 samples were prepared by spiking PCP standard directly on the glass fiber disc contained within the XAD-7 sampling tube. The samples were then capped and stored over night under ambient conditions and analyzed the following day. The results are presented in Table 4.6. (Similar high desorption efficiencies are obtained for samples prepared by spiking PCP standard directly onto the XAD-7 resin.)
Desorption Efficiency
|
| |||
| × target conc. | 0.5× | 1× | 2× |
|
| |||
| desorption, efficiency, % |
121 121 126 121 121 113 120 |
118 120 123 122 114 119 119 |
117 119 117 113 116 109 115 |
|
| |||
4.7. Storage test
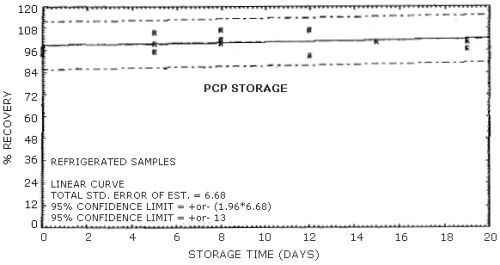
No stability problems were observed for PCP upon storage at either ambient or refrigerated conditions. Storage samples were generated using approximately the same aerosol concentration as was used in the breakthrough study. Mass monitor determinations of the total aerosol concentration generated for storage samples were lower than the results observed from the analysis of sampling tubes. This may be due to the presence of a volatile fraction of PCP in the atmosphere which was not detected by the particle mass monitor. Since there exists no reliable means of independently determining the total PCP concentration generated in this study, the percent recovery of each sample was determined relative to the overall average PCP concentration. The overall average PCP concentration for the ambient and the refrigerated stored samples were 0.77 and 0.79 mg/m3 respectively. All of the storage samples were generated in groups of six by sampling the aerosol atmosphere for approximately 48 min at 1 L/min. Seven sets of samples were generated over a one-day period. One sample of each set was analyzed immediately to ensure that no major fluctuations in the PCP concentration had occurred during the generation process. Of the remaining 35 samples, two were randomly discarded and three were analyzed immediately for the zero day storage. The remaining 30 samples were then randomly split into equal ambient and refrigerated groups for storage. All of the stored samples were capped and stored in the dark either at ambient conditions on a laboratory shelf or in a refrigerator at -5°C prior to analysis. Results corrected for the solvent desorption effect are presented in Table 4.7. and Figures 4.7.1. and 4.7.2.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 5 8 12 15 19 |
94.1 99.7 99.6 93.5 101 97.1 |
97.1 95.2 107 107 101 101 |
97.2 106 102 107 lost 101 |
96.9 99.0 94.3 92.1 94.2 96 |
100 101 102 98.5 111 95 |
100 104 104 104 102 102 | |
|
| |||||||
4.8. Reproducibility
XAD-7 packed glass tubes containing a glass fiber filter disc were spiked either in the resin bed or on the surface of the glass fiber filter with either 1.5, 3.0, or 5.0 µL of a 8094 µg/mL standard of PCP in methanol. The samples were capped with plastic caps and then stored in the dark at room temperature until submitted for analysis. The samples were analyzed after 22 days by a chemist unassociated with the method. The recoveries are shown in Tables 4.8.1. and 4.8.2.
Resin Spiked Samples
|
| |
| µg spiked | % recovery |
|
| |
| 12.1 24.3 40.5 |
98 105 100 |
|
| |
Filter Spiked Samples
|
| |
| µg spiked | % recovery |
|
| |
| 12.1 24.3 40.5 |
89 85 65 |
|
| |

5. References
- 5.1. "NIOSH Manual of Analytical Methods", 2nd ed.;
USDHEW/PHS/CDC/ NIOSH, USDHEW (NIOSH) Publication No. 78 - 175, Vol.
4, Method S297, 1978.
5.2. Cummins, Kevin, "Phenol and Cresols," Method No. 36, Organic Methods Evaluation, OSHA Laboratory, Salt Lake City, Utah 1982 (unpublished).
5.3. Beaulieu, Harry J.; Eidino, A.V.; Arlington, Kim L.B.; Bachan, Roy M. American Ind. Hygiene Assoc. J. (1980), 41, (10), 758-65.
5.4. Ivanov, Zlata; Magee, R.J. Microchemical Journal (1980), 25, 543-47.
5.5. Ugland, Karin; Ludanes, Elsa; Greibrokk, Tyge J. Chromatogr. (1981), 213, 83-90.
5.6. Ervin, H.E.; McGinnis, G.D. J. Chromatogr. (1980), 190, 203-207.
5.7. Daniels, C.R.; Swan, E.P. J. Chrom. Sci. (1979), 17, 628-30.
5.8. Mundy, D.E.; Machin, A.F. J. Chromatogr. (1981), 216, 229-38.
5.9. Edgerton, Thomas R.; Moseman, Robert F. J. Chrom. Sci. (1980), 18, 25-29.
5.10. Mackinson, Frank W.; Stricoff, R. Scott; Partridge, Lawrence J., editors, "Occupational Health Guideline for Pentachlorophenol", USDHHS/PHS/CDC/NIOSH, (NIOSH) Publication No. 81-123, Vol. II, 1981.
5.11. Weinbach, E.C. Proc. Nat. Acad. Sci. (1956), 43, (6), 393-97.
5.12. "Documentation of the Threshold Limit Values", 4th ed., American Conference of Government Industrial Hygienists, Cincinnati, OH., (1980), p. 323.
5.13. Choudhary, Gangadhar, "Chemical Hazards in the Workplace" ACS Symposium Series 149, American Chemical Society, Washington, D.C., (1981), 319-342.
5.14. International Agency for Research on Cancer, "IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans", IARC, Lyon, Switzerland, (1979), Vol. 20, 303-325.
5.15. E.R. Freiter, "Chlorophenols", "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd ed., John Wiley and Sons, N.Y., (1980), Vol 5, 864-872.
5.16. "Pentachlorophenol and Sodium Pentachlorophenate". Hygienic Guide Series, American Industrial Hygiene Association, Vol. II, Akron, Ohio, (1970).