| Method no.: | 35 |
| Matrix: | Air |
| Target concentration: | 10 ppm (OSHA PEL) |
| Procedure: | Samples are collected on Chromosorb 106 tubes, desorbed with carbon disulfide and analyzed by gas chromatography with a flame ionization detector (FID). |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min |
| Reliable quantitation limit: | 0.08 ppm (0.4 mg/m3) |
| Standard error of estimate at the target concentration: (Figure 4.6.2.) |
6.4% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1982 | Chemist: Mary Eide |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
NIOSH method S292 recommends collection of naphthalene on charcoal tubes and desorption with carbon disulfide (Ref. 5.1.). Naphthalene desorption efficiency in this method (S292) is dependent upon the mass of naphthalene on the charcoal tube. NIOSH requires a 75% or better desorption efficiency for its methods. A loading of 10 mg of naphthalene on the charcoal tube gives a 76.2% desorption efficiency. To obtain a loading of 10 mg of naphthalene from an atmosphere at the PEL of 10 ppm, 200 L of air must be sampled. This is the air volume recommended by NIOSH. With lower loading the desorption efficiency decreases, for example at 0.5 mg, equivalent to the PEL of 10 ppm with a 10-L air volume, the desorption efficiency is 61%.
Other solvents were considered for desorbing charcoal tubes, but no solvent better than carbon disulfide was found. Other collection tubes tried were XAD-4, Coated Resin (SKC, cat. no. 226-23), and Chromosorb 106. Chromosorb 106 had a 100% desorption efficiency over the range of 0.004 mg to 1.02 mg loading, when desorbed with carbon disulfide, and samples were stable over a 17-day storage time.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis for OSHA policy).
Naphthalene is readily absorbed through the skin and can cause skin irritation, corneal ulcerations, and cataracts (Refs. 5.2. and 5.3.). Naphthalene inhalation and ingestion can cause headaches, nausea, extensive sweating, and confusion. Symptoms of severe poisoning are vomiting, then coma, and hematuria (Ref. 5.4.). Sensitive individuals can have a "hemolytic crisis" (rupture of red blood cells) when exposed to naphthalene (Ref. 5.5.). Naphthalene has been shown to be a teratogen in rats (Ref. 5.6.).
1.1.3. Potential workplace exposure
The major uses of naphthalene in 1978 were: phthalic anhydride production (141,000 metric tons), insecticide production (35,000 metric tons), 2-naphthol production (16,000 metric tons), synthetic tanning agents (12,000 metric tons), moth repellents (4,000 metric tons), surfactant production (2,000 metric tons), and miscellaneous (2,000 metric tons) (Ref. 5.7.). The total consumption in 1979 was 212,000 metric tons, while the production in 1979 was 226,000 metric tons. The major sources of naphthalene are from coal tars and petroleum refineries (Ref. 5.7.).
1.1.4. Physical properties (Ref. 5.8.).
| molecular weight: | 128.2 |
| boiling point: | 218°C |
| melting point: | 80.2°C |
| color: | white crystals |
| vapor pressure: | 0.05 mm Hg at 20°C |
| flash point: | 79°C (174°F) (closed cup) |
| odor: | moth balls |
| specific gravity: | 1.14 |
| lower explosive limit: | 0.9% |
| odor threshold: | 0.3 ppm |
| structural formula: | Figure 1.1.4. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 4 ng per injection. This is the amount of analyte that gives a peak area similar to the peak area of trace contaminants in the desorbing solution. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 4 µg per sample (0.08 ppm or 0.4 mg/m3, assuming the recommended air volume). This is the amount of analyte spiked on the sampling tube which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 4 µg per sample (0.08 ppm or 0.4 mg/m3, assuming the recommended air volume). This is the smallest amount of analyte which can be quantitated within the criteria of 75% recovery and 95% confidence limits of ±25%. (Section 4.3.)
1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 684 area units per µg/mL. This is determined by the slope of the calibration curve. (Section 4.4.) The sensitivity may vary with the particular instrument used in the analysis. The instrument used in this evaluation was a Hewlett-Packard 5840A.
1.2.5. Recovery
The recovery of analyte from the collection medium during storage must be 75% or greater. The recovery of naphthalene from samples used in a 17-day storage test remained above 94% when the samples were stored at both 23°C and -5°C. (Section 4.6.)
1.2.6. Precision (analytical method)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0058. (Section 4.4.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 17-day storage test is ±12.5%. (Figure 4.6.2.) This includes an additional ±5% for sampling error.
1.3. Advantages
- 1.3.1. The sampling procedure is convenient.
1.3.2. The analytical method is reproducible and sensitive.
1.3.3. Re-analysis of samples is possible.
1.3.4. Samples are stable, even at room temperature.
1.3.5. It may be possible to analyze other compounds at the same time.
1.3.6. Interferences may be avoided by proper selection of column and GC parameters.
1.3.7. The desorption efficiency remained high over a range of 0.004 to 1.02 mg per tube.
1.4. Disadvantages
- 1.4.1. The precision, in part, is limited by the reproducibility
of the pressure drop across the tubes. This applies, in general, to
all sampling procedures using sampling tubes.
1.4.2. There are trace amounts of naphthalene in the Chromosorb 106 packing. This was confirmed by GC/MS. A blank from the same lot as the tubes used in sampling must be sent to the laboratory and analyzed with the samples.
1.4.3. The carbon disulfide contained trace contaminants that may interfere with the naphthalene analysis. Instrument parameters must be chosen to avoid this.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. Personal sampling pump whose flow can be determined
within ±5% at the recommended flow.
2.1.2. Chromosorb 106 tubes containing a 100-mg adsorbing section with a 50-mg backup section separated by a 2-mm portion of urethane foam, with silanized glass wool before the adsorbing section and a 3-mm plug of urethane foam at the back of the backup section. The ends are flame sealed and the glass tube containing the adsorbent is 70 mm × 6-mm o.d. × 4-mm i.d. SKC tubes or equivalent.
2.2. Reagents
None required
2.3. Sampling technique
- 2.3.1. The ends of the Chromosorb 106 tubes are broken
immediately before sampling.
2.3.2. Connect the Chromosorb 106 tube to the sampling pump with flexible tubing.
2.3.3. Tubes should be placed in a vertical position to minimize channeling, with the smaller section towards the pump.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the Chromosorb 106 tubes.
2.3.5. Seal the Chromosorb 106 tubes with plastic caps immediately after sampling. Seal each sample lengthwise with OSHA Form 21.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break ends, seal, transport) except no air is drawn through it.
2.3.7. Ship the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. Bulks submitted for analysis must be shipped in a separate container from the samples.
2.4. Sampler capacity
- 2.4.1. Breakthrough was determined two ways. First, a standard
naphthalene concentration was prepared in a gas bag filled with
humid air (80% relative humidity). When samples were drawn from the
gas bag atmosphere, no breakthrough was observed with a loading of
3.72 mg on the sampling tube. The total sampling time was 283 min at
a flow rate of 0.192 L/min. This concentration is equivalent to an
air concentration of 13 ppm. (Section 4.7.1.).
2.4.2. Breakthrough was also studied with a tube packed with silanized glass wool in series with the sampling and backup tubes. A solution of naphthalene was injected onto the glass wool and 33 L of humid air were drawn through. No breakthrough was observed with up to 998 µg of naphthalene on the sampling tube. No breakthrough was observed at three times the recommended air volume in either breakthrough study (Section 4.7.2.).
2.5. Desorption efficiency
The desorption efficiency was essentially 100% for 0.5 to 2 times the PEL for a 10-L air sample (Section 4.5. and 4.2.).
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences
Suspected interferences should be listed on sample data sheets.
2.8. Safety precautions
- 2.8.1. Sampling equipment should be placed on an employee in a
manner that does not interfere with work performance or safety.
2.8.2. Safety glasses should be worn when breaking the ends of the adsorbent tubes.
2.8.3. Follow all safety practices that apply to the workplace being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. GC column capable of separating naphthalene and an internal standard from any interferences. The column used for validation studies was 10 ft × 1/8 in., stainless steel, packed with 3% SP-2100 on 100/120 Supelcoport.
3.1.3. An electronic integrator or some other suitable method of measuring peak areas.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. A 1-µL syringe or other convenient size for sample injection.
3.1.6. Pipets for dispensing desorbing reagent. The Glenco 1-mL dispenser was used in this method.
3.1.7. Volumetric flasks, 25-mL and other convenient sizes for preparing standards.
3.1.8. A balance capable of weighing microgram quantities.
3.1.9. Pipets of convenient sizes for standard preparation.
3.2. Reagents
- 3.2.1. Naphthalene, reagent grade.
3.2.2. Carbon disulfide, chromatographic grade.
3.2.3. An internal standard, such as n-hexylbenzene, reagent grade.
3.2.4. Desorbing reagent - 1 µL internal standard/mL CS2.
3.2.5. Purified GC grade nitrogen or helium, hydrogen, and air.
3.3. Standard preparation
- 3.3.1. Standards are prepared by diluting a known quantity of
naphthalene in desorbing reagent. Fresh standards should be prepared
daily.
3.3.2. A concentration of 524 µg of naphthalene per mL desorbing reagent is equivalent to 10 ppm for a 10-L air sample desorbed with 1 mL of desorbing reagent.
3.3.3. At least two separate stock standards should be prepared.
3.4. Sample preparation
- 3.4.1. Sample tubes are opened and the adsorbent from each
section of each tube is placed in separate 2-mL vials. Foam and
glass wool plugs are discarded.
3.4.2. Each section is desorbed with 1 mL of desorbing reagent.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with occasional shaking.
3.5. Analysis
- 3.5.1. GC conditions
| flow rates (mL/min) | temperature (°C) | ||
| nitrogen: | 28 | injector: | 200 |
| hydrogen: | 22 | detector: | 250 |
| air: | 240 | column: | 120 |
| injection size: | 1 µL | ||
| elution time: | 4.62 min | ||
| chromatogram: | Figure 4.8. | ||
3.5.2. Peak areas are measured by an integrator or other means.
3.5.3. An internal standard procedure is used. The integrator is calibrated to report results in parts per million based on a 10-L air sample and a desorption efficiency of 100% (Section 3.7.).
3.6. Interferences
- 3.6.1. Any compound having a retention time similar to that of
naphthalene or the internal standard is an interference. Possible
interferences should be listed on the sample data sheet. GC
parameters should be adjusted if necessary so these interferences
will pose no problems.
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the PEL should be confirmed by GC/MS or other suitable means.
3.6.3. There are trace amounts of naphthalene present in the Chromosorb 106 tubes. A blank of the same lot as the samples must be analyzed at the same time, and a blank correction must be made.
3.6.4. Phthalic anhydride, 1-naphthol, and 2-naphthol do not interfere with naphthalene when the recommended column and conditions of this method are used.
3.7. Calculations
- 3.7.1. To calculate the equivalent ppm air concentration of
analytical standards, assuming a 10-L air sample, use the following
equation:
| where | A | = | µg/mL of naphthalene in standard |
| 128.2 | = | molecular weight of naphthalene | |
| 1 mL | = | desorption volume | |
| 24.46 | = | molar volume (L/mole) at 25°C and 760 mm Hg |
3.7.2. Since the integrator was calibrated to report results in ppm based on a 10-L air sample, the following calculation is used:
| where | A | = | ppm calculated by integrator |
| B | = | air volume (L) | |
| C | = | blank amount (ppm) |
3.7.3. This calculation is done for each section of the sampling tube and the results added together.
3.8. Safety precautions
- 3.8.1. All handling of solvents should be done in a hood.
3.8.2. Avoid skin contact with all solvents.
3.8.3. Wear safety glasses at all times.
4. Backup Data
- 4.1. Detection limit data
The detection limit of the analytical procedure was determined by injecting 1 µL of a 4.0 µg/mL standard prepared in carbon disulfide containing 1 µL/mL n-hexylbenzene internal standard. The detection limit of the analytical procedure is 4 ng per injection. Chromatograms of such a determination are shown in Figure 4.1.
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure was determined from the following desorption data. This data is presented graphically in Figures 4.2.1. and 4.2.2.
Detection Limit Data
|
| ||||||
| µg/sample | 1020.8 | 500.2 | 268.6 | 131.6 | 26.3 | 4.4 |
|
| ||||||
| µg recovered |
1041.5 1037.8 1044.4 1040.4 1038.5 1032.3 |
513.9 514.6 507.9 513.1 514.5 511.8 |
277.8 273.8 270.6 267.7 270.7 269.3 |
131.8 131.1 131.6 133.2 130.7 133.3 |
28.0 26.6 25.9 23.8 |
4.6 4.7 4.5 4.3 |
|
| ||||||
4.3. Reliable quantitation limit
The recovery of naphthalene from three samples spiked with an amount equivalent to the detection limit of the overall procedure, was 103.6%, 102.03% and 99.25%. The average of these values is 101.7% and the standard deviation is 2.20%. Therefore, the 95% confidence limits (±1.96 SD) is ±4.32%.
4.4. Sensitivity and precision data (analytical method)
Four analytical standards whose concentrations are indicated below were each analyzed six times. The data listed in Table 4.4 were used to determine the calibration curve shown in Figure 4.4. The precision of the analytical method was determined with the 0.5×, 0.9×, and 2× data.
Sensitivity and Precision
|
| ||||
| × target conc. µg/mL |
0.5× 286.8 |
0.9× 451.4 |
2× 1063 |
3.28× 1718.4 |
|
| ||||
| area counts SD CV |
209900 210950 211200 209950 210400 210300 210450 1179.0 0.00560 |
306900 304200 309050 305550 308300 307850 306975 1820.1 0.00593 |
727800 740800 734100 737900 734200 735200 735000 4367.6 0.00594 |
1188800 1185000 1180500 1176500 1181500 1184000 |
|
| ||||
4.5. Desorption efficiency
Desorption efficiencies were determined by injecting a solution of naphthalene in o-xylene onto Chromosorb 106 tubes and analyzing them a day later. The desorption efficiencies were determined with sample loadings equivalent to 0.5, 1, and 2 times the target concentration based on the recommended air volume of 10 L. Six samples were prepared and analyzed at each concentration.
Desorption Efficiency
|
| |||
| × target conc. | 0.5× | 1× | 2× |
|
| |||
| desorption efficiency, % |
100.3 103.4 101.9 100.7 99.6 100.8 101.2 |
102.7 102.9 101.5 102.6 102.9 102.3 102.5 |
102.0 101.7 102.3 101.9 101.7 101.1 101.8 |
|
| |||
4.6. Storage
Storage samples were prepared by vapor spiking Chromosorb 106 tubes with naphthalene. Tubes containing silanized glass wool were liquid spiked with 5.0 µL of 100.12 mg/mL naphthalene in o-xylene. These tubes were placed in series ahead of Chromosorb 106 tubes and humid air was drawn through them. The naphthalene was leached from the glass wool and adsorbed on the Chromosorb 106. Six samples were analyzed the day after generation. They had been stored in a refrigerator at -5°C. Fifteen samples were stored at -5°C and 15 samples were stored at room temperature (23°C). Three samples from each set were analyzed at intervals of a few days over a 17-day period. Results are shown graphically in Figures 4.6.1. and 4.6.2.
Storage Tests
|
| |||||||
| storage time (days) |
%
recovery (refrigerated) |
%
recovery (ambient) | |||||
|
| |||||||
| 1 1 6 9 13 15 17 |
91.8 98.8 99.2 96.0 93.9 96.0 100.0 |
98.3 100.3 100.2 97.0 96.9 101.6 98.8 |
100.2 99.4 99.1 96.2 96.5 100.9 97.7 |
99.6 97.2 94.7 96.6 97.9 |
99.5 97.0 85.3 89.7 96.5 |
94.7 90.7 94.7 97.6 96.4 | |
|
| |||||||
4.7. Sampler capacity
- 4.7.1. Breakthrough study using a Teflon gas bag
A Teflon gas bag was filled with 60 L of humid air at 80% relative humidity. A solution containing 24 mg of naphthalene in xylene was injected into the gas bag. The gas bag was allowed to equilibrate for 25 h. Naphthalene crystals were observed in the gas bag after this period of time. Samples were taken from the gas bag using a Chromosorb 106 tube containing only the 100-mg section of adsorbent connected in series with a normal Chromosorb 106 tube. The flow was from the gas bag, through the tube with the main section only, then through the second Chromosorb 106 tube. This second Chromosorb 106 tube was changed at timed intervals and analyzed for the presence of naphthalene. The air was drawn at 0.192 L/min.
Breakthrough Study Using a Gas Bag
|
| |||
| tube no. |
total time (min) |
total air drawn (L) |
µg found |
|
| |||
| 1 2 3 4 main tube |
110 210 260 283 283 |
21.08 40.24 49.82 54.23 54.23 |
0 0 0 0 3720 |
|
| |||
4.7.2. A second breakthrough study was performed using a similar sampling train to that used for storage sample generation. A tube packed with silanized glass wool was placed in series with a Chromosorb 106 tube with the backup portion removed. These tubes were followed by another Chromosorb 106 tube which was changed at timed intervals. The silanized glass wool packed tube was injected with 20 µL of a 49.9 mg/mL naphthalene in o-xylene solution. The total loading was 998 of µg naphthalene. The air drawn was at 23.5°C and relative humidity of 72.34%. The flow rate was 0.193 L/min.
Breakthrough Study Using Spiked Glass Wool
|
| |||
| tube no. |
total time (min) |
total air drawn (L) |
µg found |
|
| |||
| 1 2 3 4 5 glass wool tube main tube |
42 62 96 126 171 171 171 |
8.11 11.97 18.53 24.32 33.00 33.00 33.00 |
0 0 0 0 0 0 976 |
|
| |||
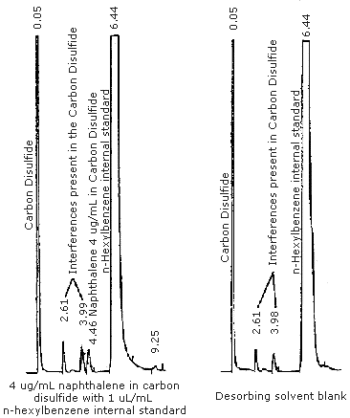
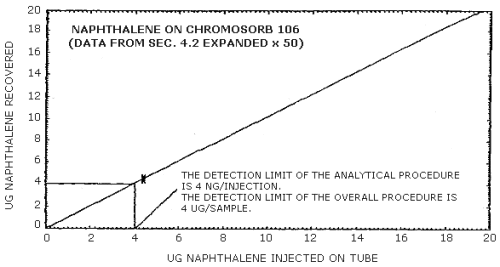
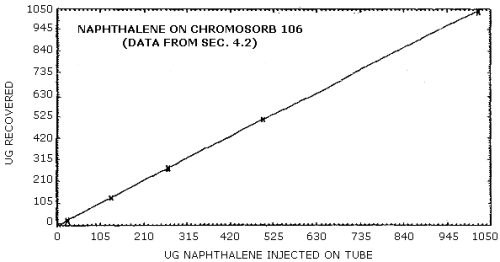
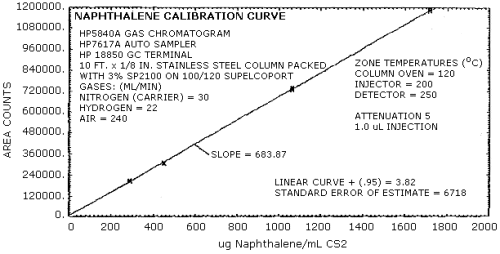
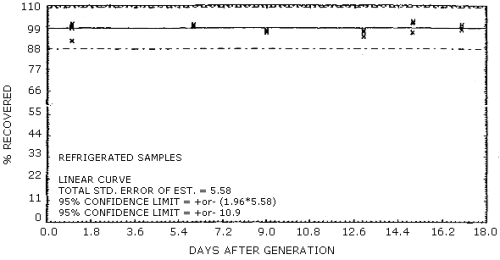
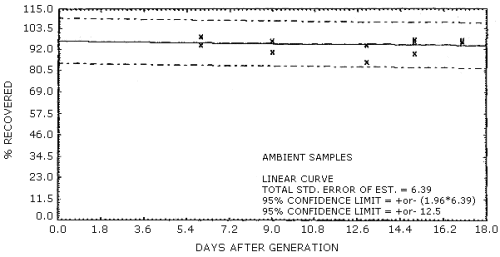
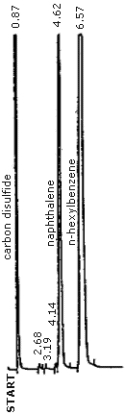
Figure 4.8. Chromatogram of a 369 µg/mL naphthalene standard in
CS2 with 1 µl/mL
5. References
- 5.1. "NIOSH Manual of Analytical Methods", U.S. Department of
Health, Education and Welfare, Public Health Service, Center for
Disease Control, National Institute for Occupational Safety and
Health, Second Edition, Vol. 3, Method S292.
5.2. Meyer, R.T. N. Engl. J. Med. 1955, 252, 622-628.
5.3. Adams, D.R. BR. J. Ophthanol. 1930, 14, 545-576.
5.4. Patty, F.A. "Industrial Hygiene and Toxicology", John Wiley and Sons, New York, Second Edition, Vol. 2, p. 1238.
5.5. Athreya, B.K.; Swain, A.K.; and Dickstein, B. Indian J.
Child. Health 1961, 10,
5.6. Harris, S.J.; Bond, G.P.; and Riemeir, R.W. Toxicology Appl. Pharmacol. 1979, 48-1 part 2, A35.
5.7. "Encyclopedia of Chemical Technology." John Wiley and Sons, New York, Vol. 15, p. 710, 714.
5.8. "Occupational Health Guidelines for Chemical Hazards", U.S. Department of Health and Human Services, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, U.S. Department of Labor, Occupational Safety and Health Administration, Vol. 2, Naphthalene 1-5.