| Method no.: | 31 |
| Matrix: | Air and bulk process samples |
| Target concentration: | 4.2 µg/m3 |
| Procedure: | Air samples are collected using Gelman Type A glass
fiber filters in open-face cassettes without backup pads. The
samples are extracted with |
| Recommended air volume and sampling rate: |
480 L and 2 L/min |
| Reliable quantitation limit: | 0.42 µg/m3 |
| Standard error of estimate at the target concentration: (Figure 4.7.2.) |
6.2% |
| Special requirements: | The filter must be protected from light during and after sampling because light will decompose NDELA. |
| Status of method: | A sampling and analytical method that has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: July 1981 | Chemist: Warren Hendricks |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
N-nitrosodiethanolamine (NDELA) is found in many complex matrices such as cutting and grinding fluids used for metal working (Ref. 5.1.) and cosmetics (Ref. 5.2.). Analytical procedures for NDELA are complicated by the matrix and many require a cleanup technique prior to quantitation of the analyte. A typical cleanup procedure recommends that ammonium sulfamate be added to the sample to prevent the artifactual formation of N-nitrosamines. Ethyl acetate is added to the sample, the mixture is filtered and then chromatographed on an open silica gel column. Ethyl acetate is used to wash off unwanted components and then acetone is added to elute NDELA. The acetone fraction is evaporated under vacuum at 50°C and the residue is diluted with an appropriate solvent (Ref. 5.2.).
Many analytical methods for NDELA require derivatization. Derivatives of NDELA have been prepared by acylation, trifluoracylation, trimethylsilylation, and methylation. The derivatives have been analyzed by gas chromatography (GC) using flame ionization and mass spectrometric detectors. Underivatized NDELA has been successfully chromatographed but the detection limit was found to be about 2000 times higher than for the derivatized analyte (Ref. 5.3.). A derivative of NDELA has been prepared for analysis by GC with electron capture detection (Ref. 5.2.).
NDELA has been separated by thin-layer chromatography and then visualized with a spray which formed a colored complex with the analyte after exposure to ultraviolet (UV) light (Ref. 5.4.). NDELA has also been determined by differential pulse polarography (Ref. 5.5.).
NDELA has been analyzed by GC and high performance liquid
chromatography (HPLC) with Thermal Energy Analyzer (TEA) detection.
These procedures utilize cleanup techniques and recommend that
ammonium sulfamate or sodium ascorbate and
NDELA is considered to be a non-volatile nitrosamine and if the airborne compound is present, it is probably contained in an aerosol. Since the collection efficiency for aerosols on solid sorbents has not been established, the alternative air sampling devices were filters and bubblers.
Philip Issenberg, of the Eppley Institute for Research on Cancer,
suggested during a personal conversation in 1978, that airborne
NDELA could probably be collected on a glass fiber filter using
open-face cassettes. Based on this recommendation and the fact that
bubblers are inconvenient, it was decided to evaluate glass fiber
filters as an air sampling medium for NDELA. It was also decided to
adopt the recommended
The sampling method does not preclude all possibility of the artifactual formation of the analyte on the sampling device. The significance of the problem is unknown because no NDELA has been found in air samples taken inside workplaces where materials known to contain NDELA and its precursors are in use (Section 4.11.).
In fiscal year 1980, the Carcinogen/Pesticide Branch of the OSHA Analytical Laboratory completed more than 400 determinations for NDELA. This was the ninth most requested compound for analysis by that branch. Most of the determinations were done on bulk samples.
The purpose of this work is to evaluate an air sampling and analytical method for NDELA and to provide a confirmatory procedure for the analyte found in bulk samples.
The analytical section of this procedure recommends that bulk samples be screened for NDELA using HPLC with UV detection. Those bulk samples which give positive HPLC/UV results are confirmed using either GC/TEA or HPLC/TEA. Air samples are analyzed by GC/TEA and the presence of NDELA is confirmed by HPLC/TEA.
The HPLC/UV bulk sample screening procedure is recommended because its use reduces laboratory turnaround time for these samples. The GC/TEA method for air samples was selected because it is convenient and the highly selective TEA detector is more sensitive than the UV detector.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis for OSHA policy).
NDELA has been shown to be carcinogenic to rats and hamsters. For a detailed discussion of the toxic effects see Section 4.9.
The International Agency for Research on Cancer (IARC) states that there is sufficient evidence of a carcinogenic effect of NDELA in two animal species. Given the potentially widespread exposure to the agent, the IARC recommends that efforts should be made to collect epidemiological data. Further, NDELA should be regarded for practical purposes as a human carcinogen (Ref. 5.15.).
1.1.3. Potential exposure
NDELA has been determined to be present in processed tobacco, pesticides, cosmetics, cutting fluids and grinding fluids. Cutting and grinding fluids probably represent the sources of most occupational exposure to NDELA. Skin absorption of NDELA is probably a significant means of exposure of the analyte to man. For a more detailed discussion of these topics see Section 4.10.
1.1.4. Physical properties (Ref. 5.15.)
| physical appearance: | yellow, viscous oil. |
| boiling point: | 114°C at 1.5 mm Hg, decomposes at about 200°C at 14 mm Hg |
| refractive index: | 1.4540 |
| UV absorption data: | l = 234 nm, e = 470.7 |
| (in water) | l = 345 nm, e = 5.3 |
| solubility: | miscible with water in all proportions, soluble in polar organic solvents, insoluble in non-polar organic solvents. |
| volatility: | not steam-volatile. |
| molecular weight: | 134.1 |
| structure: | 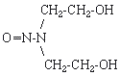 |
| CAS no. (Ref. 5.10) |
1116-54-7 |
| synonyms: (Ref. 5.10) |
bis(beta-hydroxyethyl)nitrosamine;
diethanolnitrosamine; |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 200 pg per injection. This is the amount of analyte which will give a peak whose height is about 5 times the height of the baseline noise (Section 4.1.).
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 200 ng per sample (0.42 µg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure (Section 4.2.).
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 200 ng per sample (0.42 µg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25% (Section 4.2.).
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
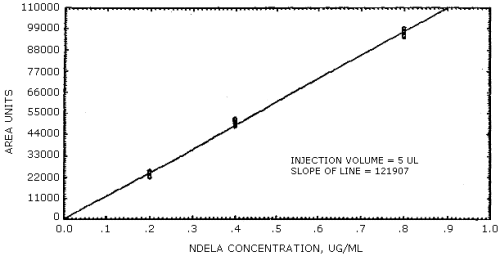
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 121,907 area units per µg/mL. This is determined by the slope of the calibration curve (Section 4.4.). The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of analyte from the collection medium must be 75% or greater. The average recovery from spiked samples at the target concentration is 99.8% (Section 4.6.).
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration was 0.031 (Section 4.3.).
1.2.7. Precision (overall procedure)
The overall procedure must provide results at the target
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level for the
1.3. Advantages
- 1.3.1. The analytical method provides a procedure to confirm the
presence of NDELA.
1.3.2. The sampling and analytical procedures are precise, reliable, safe and convenient.
1.3.3. The samples are stable when stored at ambient temperature for at least 16 days.
1.4. Disadvantages
- 1.4.1. Smaller laboratories may not be able to support the cost
of the recommended analytical instrumentation.
1.4.2. The sampling procedure may not be artifact free under certain conditions.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An air sampling pump, the flow of which can be determined
to within ±5% at the recommended air flow rate with the air sampler
in line.
2.1.2. Glass fiber filters, 37-mm, Gelman type A or equivalent.
2.1.3. Filter holder, (cassette, 37-mm, polystyrene, 3-section.)
2.1.4. Shrinkable cellulose bands for cassettes.
2.1.5. Equipment to measure the air flow rate through the sampling device.
2.1.6. Scintillation vials, 20-mL with Teflon-lined caps.
2.1.7. A suitable means to protect individual samples from light such as aluminum foil, electrical tape or masking tape.
2.2. Reagents
None required
2.3. Technique
- 2.3.1. Prior to sampling, assemble the cassette for open-face
sampling. This is accomplished by placing the glass-fiber filter in
the cassette base and using the center cassette section to hold the
filter in place. Do not use backup pads. Cover the junction between
the cassette base and center section with a shrinkable band to
prevent sampled air from by-passing the filter. Attach the third
(inlet) section of the cassette to prevent contamination of the
filter. Remove this section just before sampling.
2.3.2. Attach the sampling device in the breathing zone of the employee to be monitored. Position the open face of the cassette downward.
2.3.3. The filter must be protected as much as possible from light during and after sampling.
2.3.4. After sampling for the appropriate time, remove the sampler and replace the inlet section of the three-section cassette. Insert the small plastic plugs into the inlet and outlet orifices of the assembled cassette device. Wrap the air sample with official OSHA seals.
2.3.5. Submit at least one blank with each set of samples. The blank should be subjected to the same handling as the sample except that no air is drawn through it.
2.3.6. It is strongly recommended that a sample of each material suspected to contain NDELA be submitted for analysis. Submit the bulk samples in an opaque scintillation vial sealed with a Teflon-lined cap. Wrap the vial with official OSHA seals. Do not ship bulk samples with air samples.
2.3.7. List potential interferences on the sample data sheet.
2.3.8. Place the samples in a freezer if they are to be stored before shipping to the laboratory.
2.4. Retention efficiency
- 2.4.1. No significant loss of NDELA, spiked on glass fiber
filters at the target concentration, was observed after drawing the
recommended air volume at the recommended rate through the filter
(480 L at 2 L/min). The average recovery for 6 spiked samples was
97.1%. (Section 4.5.)
2.4.2. When twice the target level NDELA was spiked on filters and twice the recommended air volume (960 L) was drawn through the samples at 2 L/min, the average recovery was 92.6%. (Section 4.5.)
2.5. Extraction efficiency
The average recovery for NDELA spiked on glass fiber filters at the
target level was 99.8%. The filters were placed in scintillation vials
and extracted with
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 480 L.
2.6.2. The recommended sampling rate is 2 L/min.
2.7. Interferences (sampling)
The recommended air sampling procedure for NDELA is not artifact free under certain conditions. For a more complete discussion of artifact problems associated with air sampling for NDELA see Section 4.11.
2.8. Safety precautions (sampling)
- 2.8.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.8.2. Follow all safety practices that apply to the work area to be monitored.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph.
3.1.2. An HPLC pump.
3.1.3. An HPLC sample injector.
3.1.4. An HPLC ultraviolet (UV) detector. Either a variable or fixed wavelength detector is adequate.
3.1.5. A Thermal Energy Analyzer (TEA), Thermo Electron Corporation, Waltham, Mass. A TEA Model 502 analyzer with a retrofitted Explosives Analysis Package was used for this evaluation.
3.1.6. A GC column capable of resolving NDELA from the solvent
and potential interferences. The column used for this work was 3 ft
× 1/4-in. o.d. (2-mm i.d.) glass,
3.1.7. HPLC analytical columns capable of resolving NDELA from the solvent and potential interferences. The column used for the reversed phase HPLC/UV work was a DuPont Zorbax ODS, 4.6-mm i.d. × 25 cm. The column used for the normal phase HPLC/UV and HPLC/TEA work was a DuPont Zorbax CN, 4.6-mm i.d. × 25 cm.
3.1.8. The necessary hardware to interface the TEA, GC, HPLC and UV apparatus.
3.1.9. An electronic integrator or other suitable means to measure detector response and record chromatograms.
3.1.10. Vials, 2-mL with Teflon-lined caps.
3.1.11. Vials, scintillation, 20-mL with Polyseal caps.
3.1.12. Volumetric pipets, 5-mL and other convenient sizes.
3.1.13. Volumetric flasks, 5-mL and other convenient sizes.
3.1.14. Syringes, 10-µL and other convenient sizes.
3.1.15. Pipets, disposable Pasteur type.
3.1.16. Dewar flasks, convenient size for liquid nitrogen.
3.1.17. Analytical microbalance, with sufficient precision and accuracy to weigh samples and standards.
3.1.18. A mechanical shaker, Eberback 6000 or equivalent.
3.1.19. A 1-mL scoop-type measure for dispensing Dowex 1-X8 resin.
3.2. Reagents
- 3.2.1. NDELA, analytical standard of known assay.
3.2.2. Water, acetone, isooctane,
3.2.3. Nitrogen, liquid.
3.2.4. Helium, GC grade.
3.2.5. Oxygen, medical grade.
3.2.6. Dowex 1-X8 anion exchange resin, 20-50 mesh.
3.3. Standard preparation
- 3.3.1. Keep the exposure of standards to light at a minimum to
avoid decomposition of NDELA.
3.3.2. Preparation of standards for use in the reversed phase HPLC/UV bulk screening method:
Dilute a known quantity of NDELA to the working range with water. The working range should include a standard at about 0.7 µg/mL NDELA. Store the standards in well sealed, dark containers under refrigeration.
3.3.3. Preparation of standards for use in the normal phase HPLC/UV bulk screening method:
Dilute a known quantity of NDELA to the working range with
3.3.4. Preparation of standards for use in the HPLC/TEA and GC/TEA methods:
- 3.3.4.1. Dilute a known quantity of NDELA to the working range
with
3.3.4.2. Place 5 mL of each standard to be analyzed in separate 20 mL scintillation vials. Add 1 mL of Dowex 1-X8 anion exchange resin (about 0.60 g) to each vial and then shake the vials on a mechanical shaker for 1 h. Some operations associated with the use of the anion exchange resin are critical, therefore, the recommended method should be followed closely. See Section 4.12. for a more complete discussion.
3.3.4.3. Immediately after shaking, remove about 1.5 mL of the solution from each scintillation vial and place it in a separate 2-mL vial for analysis. Make sure that no anion exchange resin is transferred to the small vials. The standard is now ready for analysis.
3.4. Sample preparation
- 3.4.1. Keep the exposure of samples to light at a minimum
because light will decompose NDELA.
3.4.2. Insure that the official OSHA seal is intact and complete.
3.4.3. Check the laboratory sample number against the field identification number to be sure that the sample has been properly identified.
3.4.4. Remove the filter from the cassette and place it in a scintillation vial.
3.4.5. Add 1 mL of Dowex 1-X8 anion exchange resin to each sample vial.
3.4.6. Add 5 mL of
3.4.7. Immediately after shaking, remove about 1.5 mL of the solution from each sample vial and place it in a separate 2-mL vial for analysis. Make sure that no anion exchange resin is transferred to the small vials. The sample is now ready for analysis.
3.4.8. Preparation of bulk samples for the reversed phase HPLC/UV screening method:
- 3.4.8.1. Accurately weigh about 0.25 g of bulk sample into a
5-mL volumetric flask, dilute to the mark with water and mix the
contents of the flask by shaking. Some samples may not be
completely water soluble but any NDELA present will be extracted
into the aqueous phase.
3.4.8.2. Transfer the entire contents of the flask to a 20-mL scintillation vial and add 5 mL of chloroform.
3.4.8.3. Shake the vial using a mechanical shaker for 1 h. Transfer about 1.5 mL of the aqueous phase to a 2-mL vial for analysis.
3.4.9. Preparation of bulk sample for the normal phase HPLC/UV screening method:
- 3.4.9.1. Accurately weigh about 0.25 g of bulk sample into a
5-mL volumetric flask, dilute to the mark with
3.4.9.2. Transfer about 1.5 mL of the contents of the flask to a 2-mL vial for analysis.
3.4.10. Preparation of bulk sample for the HPLC/TEA or GC/TEA methods:
- 3.4.10.1. Accurately weigh about 0.25 g of bulk sample into a
5-mL volumetric flask, dilute to the mark with
3.4.10.2. Transfer the contents of the flask to a 20-mL scintillation vial and add 1 mL of Dowex 1-X8 anion exchange resin and shake the vial for 1 h using a mechanical shaker.
3.4.10.3. Immediately after shaking, remove about 1.5 mL from each sample vial and place it in a separate 2-mL vial. Make sure that no anion exchange resin is transferred to the small vial. The sample is now ready for analysis.
3.5. Analysis
- 3.5.1. Instrument conditions for the reversed phase HPLC/UV bulk
screening method:
| analytical column: | DuPont Zorbax ODS, 4.6-mm i.d. × 25 cm |
| mobile phase: | 100% water |
| flow rate: | 1 mL/min |
| UV detector: | variable wavelength - 234 nm fixed wavelength - 254 nm |
| injection volume: | 25 µL |
| chromatogram: | Section 4.8.1 |
3.5.2. Instrument conditions for the normal phase HPLC/UV bulk screening method:
| analytical column: | DuPont Zorbax CN, 4.6-mm i.d. × 25 cm |
| mobile phase: | 70% isooctane 20% dichloromethane 10% methanol |
| flow rate: | 1 mL/min |
| UV detector: | variable wavelength - 234 nm Fixed wavelength - 254 nm |
| injection volume: | 25 µL |
| chromatogram: | Section 4.8.2. |
3.5.3. Instrument conditions for the HPLC/TEA and GC/TEA method:
| HPLC conditions | |
| analytical column: | DuPont Zorbax CN, 4.6-mm i.d. × 25 cm |
| mobile phase: | 60% isooctane 40% acetone |
| flow rate: | 1 mL/min |
| HPLC/TEA interface temp.: | 150°C |
| injection volume: | 10 µL |
| chromatogram: | Section 4.8.3. |
| GC conditions | |
| analytical column: | 3 ft × 1/4-in. o.d. (2-mm i.d.) glass, on-column injection, 10% SP-1000 on 80/100 Supelcoport. |
| injection temp.: | 245°C |
| column temp.: | 245°C |
| GC/TEA interface temp.: | 245°C |
| helium (carrier gas) flow rate: |
30 mL/min |
| injection volume: | 5 µL |
| chromatogram: | Section 4.8.4. |
| TEA conditions | |
| GC pyrolyzer temp.: | 500°C |
| HPLC pyrolyzer temp.: | 550°C |
| oxygen flow rate: | 5 mL/min |
| pressure: | 0.5 mm Hg |
| attenuation: | 4 |
| cold trap temp.: | HPLC: -80°C (n-propanol, water and liquid
nitrogen). GC: -130°C (n-propanol and liquid nitrogen) |
| HPLC/TEA helium carrier flow rate: |
Adjust helium flow rate until chamber pressure is 0.5 mm Hg in the HPLC mode. |
3.5.4. Detector response is measured with an electronic integrator or other suitable means.
3.5.5. An external standard procedure is used to prepare a calibration curve using at least 3 standard solutions of different concentrations. The calibration curve is prepared daily. The integrator is calibrated to report results in µg/mL.
3.5.6. Good analytical practice requires that samples be compared to standards of similar concentrations.
3.6. Interferences (analytical)
- 3.6.1. GC and HPLC parameters may be changed to circumvent
interferences. Potential interferences are listed on the sample data
sheets.
3.6.2. Retention time on a single HPLC or GC column is not proof of chemical identity. For methods to confirm the presence of NDELA and a brief description of the TEA detector see Section 4.13.
3.7. Calculations
- 3.7.1. The integrator value in µg/mL is used for reference only.
More reliable results are obtained by use of a calibration curve.
The detector response, for each standard, is compared to its
equivalent concentration in µg/mL and the best straight line through
the data points is determined by linear regression.
3.7.2. The concentration, in µg/mL, for a particular sample determination is obtained by comparing its detector response to the calibration curve.
3.7.3. Bulk samples: % weight = (A)(100)/W
| where | A | = | µg/mL of NDELA from Section 3.7.2. |
| W | = | sample concentration in µg/mL |
3.7.4. Air samples: µg/m3 = (A)(B)(1000)/(E)(V)
| where | A | = | µg/mL of NDELA from Section 3.7.2. |
| B | = | extraction volume in milliliters | |
| E | = | extraction efficiency for air samples | |
| V | = | air volume in liters |
3.8. Safety precautions (analytical)
- 3.8.1. NDELA is a documented animal carcinogen and utmost care
must be exercised when working with this compound.
3.8.2. Avoid skin contact with liquid nitrogen and the solvents.
3.8.3. Use suitable means to avoid exposure to solvent vapors.
3.8.4. Wear safety glasses in all laboratory areas.
3.8.5. Check to be sure that the TEA exhaust is connected to a fume hood.
4. Backup Data
- 4.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is the amount of NDELA which will produce a peak whose height is about 5 times the height of the base line noise. The injection size recommended in the analytical procedure (5 µL) was used in the determination of the detection limit for the analytical procedure.
Figure 4.1. is a chromatogram obtained from a 5-µL injection of a solution containing 0.04 µg/mL NDELA. The mass is 0.2 ng per injection.
4.2. Detection limit of the overall procedure and reliable quantitation limit
The detection limit of the overall procedure is the amount of NDELA spiked on filters which will allow recovery of an amount equivalent to the detection limit of the analytical procedure. The reliable quantitation limit is the smallest amount of NDELA spiked on filters which can be quantitated within the requirements of at least 75% recovery and 95% confidence limits of ±25%. The injection size recommended in the analytical procedure (5 µL) was used in the determination of the detection limit of the overall procedure and of the reliable quantitation limit.
The detection limit of the overall procedure and the reliable quantitation limit were the same because the extraction efficiency for NDELA is near 100% and the 95% confidence interval was ±13.4% at this level. This amount was 200 ng/sample or 0.42 µg/m3 based on the recommended air volume.
The following data represent the recovery of 200 ng (20 µL × 10 µg/mL) NDELA spiked on glass fiber filters.
Detection Limit of the Overall Procedure
and the Reliable Quantitation Limit
|
| ||||
| sample | % recovery | statistics | ||
|
| ||||
| 1 2 3 4 5 |
100.0 109.5 101.2 97.5 90.5 |
SD 1.96 SD |
= = = |
99.8 6.84 ±13.4 |
|
| ||||
4.3. Precision data
The following data were obtained from multiple injections of analytical standards.
Precision Data
|
| |||
| µg/mL | 0.2 | 0.4 | 0.8 |
|
| |||
| area counts SD CV |
24475 22834 22632 25089 23045 22290 23394.2 1120.4 0.048 |
49047 48583 51778 50558 49998 49239 49867.2 1171.5 0.023 |
95108 97657 95561 95957 98001 99454 96956.3 1684.2 0.017 |
|
| |||
4.4. Sensitivity data
The sensitivity of the analytical procedure was determined by the slope of the calibration curve.
Figure 4.4. is the calibration curve which is a graphical representation of the data in Table 4.3.
4.5. Retention efficiency
Six glass fiber filters were each spiked with 2 µg of NDELA (1× target concentration) and then 480 L of air were drawn through the filters at 2 L/min. The relative humidity of the air was approximately 80% and the temperature was 22°C. The results are presented in Table 4.5.1.
Two glass fiber filters were each spiked with 4 µg of NDELA (2× target concentration) and then 960 L of air were drawn through the filters at 2 L/min. The relative humidity of the air was approximately 80% and the temperature was 23°C. The results are presented in Table 4.5.2.
Retention Efficiency (1×)
|
| |
| % recovery | |
|
| |
| 93.8 101.1 100.8 93.6 92.9 99.5 |
|
|
| |
Retention Efficiency (2×)
|
| |
| % recovery | |
|
| |
| 93.5 91.8 |
|
|
| |
4.6. Extraction efficiency
Six glass fiber filters were each spiked with 2 µg of NDELA. The
filters were placed in scintillation vials, 1 mL of Dowex 1-X8 anion
exchange resin was added to each vial and then 5 mL of
Extraction Efficiency
|
| |
| % recovery | |
|
| |
| 102.5 102.8 99.1 98.9 100.4 96.9 |
|
|
| |
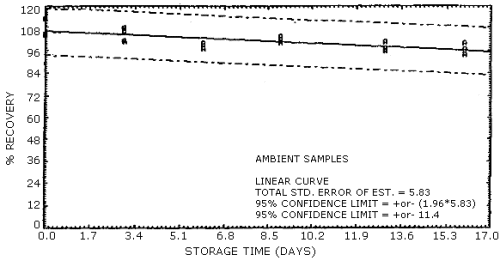
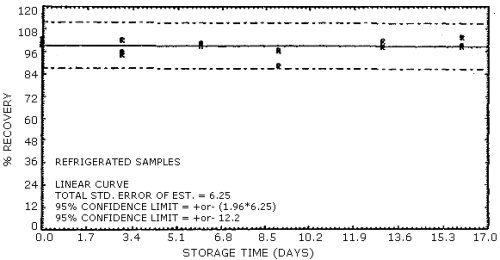
4.7. Storage data
The data in Table 4.7. represents the effects of storage at ambient
(21 to 26°C) and reduced
Storage Stability
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 9 13 15 |
102.8 102.5 100.6 97.1 98.4 99.2 |
102.8 96.0 100.2 96.7 101.6 103.8 |
100.2 94.7 99.4 88.4 99.5 98.7 |
96.2 92.3 91.2 91.6 87.7 91.4 |
95.6 96.9 88.9 94.7 88.8 88.4 |
104.0 99.0 91.4 92.9 92.0 85.8 | |
|
| |||||||
4.8. Chromatograms
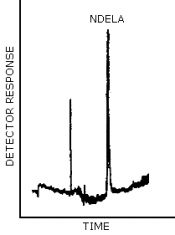
- 4.8.1. Reversed Phase HPLC/UV Chromatogram
The chromatogram in Figure 4.8.1. was obtained from the injection of 25 µL × 0.7 µg/mL NDELA.
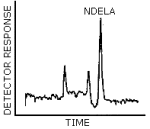
4.8.2. Normal Phase HPLC/UV Chromatogram
The chromatogram in Figure 4.8.2. was obtained from the injection of 25 µL × 0.7 µg/mL NDELA.
4.8.3. HPLC/TEA Chromatogram
The chromatogram in Figure 4.8.3. was obtained from the injection of 5 µL × 0.4 µg/mL NDELA.
4.8.4. GC/TEA Chromatogram
The chromatogram in Figure 4.8.4. was obtained from the injection of 5 µL × 0.4 µg/mL NDELA.
4.9. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
The LD50 for NDELA administered orally to the rat was 7500 mg/kg and when given by subcutaneous injection to the hamster, 11 g/kg. The lowest published toxic dose administered orally to the rat was 150 g/kg, given over 41 weeks in discrete doses. The toxic effects were carcinogenic. The lowest published toxic dose for the hamster was 1540 mg/kg given over 3 weeks in discrete subcutaneous injections. The toxic effects were neoplastic in nature. In another experiment performed with hamsters, the toxic dose was 15 g/kg. NDELA was administered by subcutaneous injection over 5 weeks in separate doses and the toxic effects were carcinogenic (Ref. 5.10.).
Druckrey, et al., observed no lethal effects in acute experiments
when 7.5 g/kg of NDELA was administered to rats. When four rats were
given increasing oral doses of from 250 mg/kg to 3 g/kg (mean dose: 1
g/kg) per day, all rats developed liver cancer between the 285th and
325th day. One animal also had metastases in the lungs and kidneys.
Another group of 16 rats were given half the increasing oral doses
(mean value: 600 mg/kg) up to a total dose of 150 g/kg over 240 days
and then the treatment was discontinued. All animals died of liver
cancer between day 242 and day 300. Seven animals died within four
days of each other. Four rats also had kidney tumors. Because of the
high tumor yield and relatively short induction period in which the
tumors appeared in rapid succession, NDELA was termed a certain
carcinogen. The required total dose to result in cancer is 200 times
greater for NDELA than for
NDELA in saline was administered by subcutaneous injection to two groups of 15 male and 15 female Syrian golden hamsters. The LD50 was determined to be 11.3 g/kg. The first group was given 2260 mg/kg (1/5 of the LD50) in 7 applications for a total of 15.8 g/kg. The second group received 565 mg/kg (1/20 of the LD50) in 27 applications for a total dose of 15.3 g/kg. Extensive local necrosis was observed at the injection site for both groups of animals. All surviving animals were sacrificed after 78 weeks. In the first group, 20 of 28 effective animals had tumors of the nasal cavity, trachea, liver, adrenal gland cortex or thyroid gland. Of the second group, 19 of 27 effective animals had tumors located in the nasal cavity, trachea or at the injection site. The main target organs for both groups were the nasal cavity and trachea. NDELA was determined to be a more effective carcinogen for Syrian golden hamsters than for rats (Ref. 5.12.).
NDELA was administered in drinking water to rats at concentrations of from 3900 to 31,250 parts per million (ppm) for about six months. All of the animals developed liver cancer. At the higher dose levels many of the liver carcinomas metastasized into the lung and peritoneal cavity. Since liver tumors were induced following exposure to the 3900 ppm NDELA solution in only 34 weeks it was considered likely that even lower concentrations would be effective. The total dose, for the 3900 ppm solution was estimated to be 30 g/kg for males and 50 g/kg for females. This dose is considerably lower than the 150 to 300 g/kg study conducted by Druckrey et al. Therefore, NDELA is probably a more potent liver carcinogen than previously suspected. NDELA is inactive or very weakly active in the Ames Salmonella Mutagenesis Test (Ref. 5.13.).
The fact that carcinogenesis by NDELA requires relatively high
doses, when compared to
4.10. Potential exposure
NDELA has been determined to be present in processed tobacco. The source of the agent is believed to be the nitrosation of diethanolamine, which is employed with a herbicide for tobacco crops. There appears to be a correlation between tobacco chewing and cancer of the oral cavity and esophagus (Ref. 5.12.).
NDELA has been found to be a contaminant of the triazine pesticide atrazine (Ref. 5.15.).
NDELA was found in cosmetics, hand and body lotions and hair shampoos. The amounts varied from 1 ng/g to 48,000 ng/g. The highest amount was found in a facial cosmetic. Human exposure was estimated to be as high as 50 to 100 µg of NDELA per day. Some of the lotions are recommended for use on infants at each diaper change. The source of NDELA in these products was not specified (Ref. 5.7.).
NDELA has been found to be present in several brands of synthetic cutting fluids at concentrations up to 3% weight. The source of NDELA is thought to be a consequence of the formulation of cutting fluids. Synthetic cutting fluids contain up to 45% triethanolamine and 18% sodium nitrate (Ref. 5.18.). It has been shown that solutions containing triethanolamine and sodium nitrate can form NDELA upon storage even at basic pH (Ref. 5.1.).
There are four major types of cutting fluids:
- Cutting oils or straight oils - contain mineral oil, fat and
other additives. These oils are not water soluble.
Soluble cutting oils - contain mineral oil, fat, emulsifiers, additives and water. These oils may or may not contain amines and nitrates.
Semi-synthetic cutting oils - contain mineral oil, water, fat, soluble base, emulsifiers and additives. These oils usually contain amines and nitrates.
Synthetic cutting fluids - contain a soluble base, additives and water. These fluids usually contain amines and nitrate.
Synthetic cutting fluids, semi-synthetic cutting oils and soluble cutting oils may contain NDELA or other nitrosamines as a contaminant of the amines or as reaction products of amines with nitrite. Straight oils may contain polynuclear aromatic compounds but probably not nitrosamines. NIOSH estimates that 780,000 people are occupationally exposed in the manufacture and use of cutting fluids (Ref. 5.19.).
Percutaneous absorption of NDELA is probably a significant means of
exposure of the agent to man. NDELA penetrated slowly through excised
human skin when applied in hydrophilic solvents but the rate was
significantly increased when NDELA was applied in a lipoidal vehicle.
The permeation constants for NDELA in the following solvents applied
to human skin were: water = 5.5 × 10-6 cm/h,
propylene glycol = 3.2 × 10-6 cm/h, and
isopropyl
In an experiment designed to demonstrate that NDELA can be absorbed by human skin, a commercial facial cosmetic contaminated with NDELA was applied to the skin of a human subject. The total NDELA applied in the cosmetic was 980 µg. The cosmetic remained in contact with the subject's skin for 7.75 h and then was removed by washing. The subject's urine, collected for about 22 h after removal of the cosmetic, contained 17.3 µg NDELA (Ref. 5.17.).
4.11. Interferences to the sampling procedure
The recommended air sampling procedure for NDELA is not artifact free under certain conditions. This means that NDELA can be formed on the air sampling device from the chemical reaction of di- and triethanolamine with a suitable nitrosating species such as nitrogen oxides. Therefore, if the amines are collected and retained by the filter and also if a nitrosating agent is encountered, results may be somewhat high.
Cutting fluids may represent a source of considerable occupational exposure to NDELA. It must be recognized that the fluids in which NDELA has been found are usually formulated with precursors of the nitrosamine - triethanolamine and sodium nitrate. Since neither NDELA nor its formulated precursors are volatile, if NDELA is present in air it will probably be a component of an aerosol. Other components of the aerosol may include NDELA precursors. If NDELA precursors are collected and retained, artifactual NDELA may be produced.
The recommended sampling procedure may give somewhat high results because of possible nitrosation artifact formation. Alternative air sampling techniques suffer similar liabilities. Bubblers are inconvenient for use in the field and laboratory turn-around time would be increased by their use. Also, a portion of the aerosol could be collected in the bubbler inlet and form nitrosation artifacts. The collection efficiency of aerosols on solid sorbents such as ThermoSorb/N, the commercial N-nitrosamine air sampling device, has not been established. In addition to unknown collection efficiency, a portion of the aerosol could be collected at the ThermoSorb/N inlet or on the stainless steel sorbent retaining screen and form nitrosation artifacts. Therefore, in light of the obvious problems, it seems logical to select the most convenient and least expensive sampling technique and this is the glass fiber filter.
David Fine, of New England Institute for Life Sciences, reported that no NDELA was detected when air samples were taken in large machine shops using cutting fluids containing NDELA (Ref. 5.8.). Similar negative results have been obtained by OSHA industrial hygienists. Given these results and the fact that neither NDELA not its precursors are volatile, it seems probable that dermal absorption of NDELA is a more likely means of exposure than inhalation. If airborne NDELA is not encountered then the limitations of the air sampling method are not significant. The air sampling method should not underestimate NDELA exposure.
4.12. Addition of Dowex 1-X8 anion exchange resin to samples and standards
Dowex 1-X8 anion exchange resin is added to samples that are to be subjected to GC/TEA or HPLC/TEA analysis. Dowex 1-X8 is a strongly basic anion exchange resin in the chloride form. The advertised dry basis total exchange capacity is 3.6 meq/g. The resin is added to samples to remove nitrate ions. The treatment is necessary because the TEA detector will respond to nitrite and the large peak may mask any NDELA response. Another advantage is that if nitrite is removed from solution then potential analytical nitrosation artifacts are eliminated.
In addition to effectively removing ionic nitrite, the recommended Dowex 1-X8 anion exchange resin treatment will remove some NDELA from solution. The loss of NDELA appears to be more dependent on the amount of resin added and the length of time the resin is in contact with the NDELA solution than on the concentration of NDELA in solution. The results of an experiment in which increasing amounts of resin were added to 5-mL aliquots of NDELA standard of different concentrations are shown in Table 4.12.1.
The Effects of Increasing Amounts of Resin
on Different Concentrations of NDELA Solutions
|
| |||
| anion exchange | NDELA concentration, µg/mL | ||
| resin added, mL | 0.2 | 0.4 | 0.8 |
|
| |||
| percent recovery | |||
| 0 1 2 3 4 |
100 76 52 41 34 |
100 78 58 41 35 |
100 77 53 41 35 |
|
| |||
The results of an experiment designed to show the effects of NDELA solution/resin contact time are shown in Table 4.12.2. One milliliter of resin was added to 5 mL of NDELA standard solution (0.8 µg/mL), the mixture was shaken for 1 h and then allowed to stand undisturbed. The mixture was stored in a light proof container.
The Effect of Standing
Time on NDELA/Resin Solutions
|
| |
| time after resin treatment, h |
NDELA recovery, % |
|
| |
| 0 6 24 96 168 |
72 61 57 61 57 |
|
| |
The recoveries were determined by comparing the NDELA/resin solutions to a NDELA standard without resin.
The data in Table 4.12.1. indicates that the same amounts of resin must be added to standards and samples. Therefore, if it is necessary to add 2 mL anion exchange resin to a sample then it is also necessary to add that amount to the analytical standards. The loss of NDELA from solution appears constant over a narrow range but good analytical practice requires that samples be compared to standards of similar concentration.
The data presented in Table 4.12.2. indicates that it is prudent to separate the liquid from the resin immediately after shaking so that no further loss of NDELA will occur.
4.13. Confirmation of NDELA results
- 4.13.1. Retention time on a single HPLC or GC column is not
proof of chemical identity. Therefore, because mass spectrometric
confirmation of NDELA has been unsuccessful, it is necessary to use
the recommended analytical methods in combination with
4.13.2. The Thermal Energy Analyzer (TEA) is a highly selective
detector for



5. References
- 5.1. Zingmark, P.A.; Rappe, C. Ambio 1977, 6, 137-238.
5.2. Rollmann, B.; Lombart, P.; Rondelet, J.; Mercier, M. J. Chrom. 1981, 206, 158-163.
5.3. Ohshima, H.; Masami, M.; Tochiharu, K. J. Chrom. 1979, 169, 279-286.
5.4. Williams, D.T.; Benoit, F.; Mazika, K. Bull. Environ.
Contam. Toxicol. 1978, 20,
5.5. Smyth, M.R.; Osteryoung, J.G.; Rowley, P.G.; Weiniger, S.J. Fresenius Z. Anal. Chem. 1979, 298, 17-22.
5.6. Thermo Electron Corporation, A.I.D.S. CL-79.
5.7. Fan, T.Y.; Goff, U.; Song, L.; Fine, F.H. Fd. Cosmet. Toxicol. 1977, 15, 423-430.
5.8. Fine, D.H. Oncology 1980, 37, 199-202.
5.9. Beaulieu, H.J.; Fidino, A.V.; Arlington, K.L.; Buchan, R.M. Am. Ind. Hyg. Assoc. J. 1980, 41, 758-765.
5.10. "Registry of Toxic Effects of Chemical Substances", 1978 Edition, (Lewis, R.J. and Tatken, R.L., Eds.) U.S. Dept. of H.E.W., PHS, CDC, NIOSH, U.S. Gov't. Printing Office, Washington, D.C. (1978).
5.11. Druckery, D.; Preussmann, R.; Ivankovic, S.; Schmahl, D.
Z. Krebsforsch 1967, 69,
5.12. Hilfrich, J.; Schmeltz, I.; Hoffmann D. Cancer Letters 1977, 4, 55-60.
5.13. Lijinsky, W.; Reuben, M.D.; Manning, W.B. Nature 1980, 288, 589-590.
5.14. Preussmann, R.; Wurtele, G.; Eisenbrand, G.; Spiegelholder, B. Cancer Letters 1978, 4, 207-209.
5.15. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Some N-nitroso Compounds, Vol. 17, (International Agency for Research on Cancer, Lyon 1978).
5.16. Bronaugh, R.L.; Congdon, E.R.; Scheuplein, R.J. J. Invest.
Dermatol. 1981, 76,
5.17. Spiegelhalder, B.; Kann, J. Toxicol. Letters 1979, 4, 217-222.
5.18. Fan, T.Y.; Morrison, J.; Rounbehler, D.P.; Ross, R.; Fine, D.H.; Miles, W.; Sen, N.P. Science 1977, 196, 70-71.
5.19. NIOSH Current Intelligence Bulletin; Nitrosamines in Cutting Fluids, No. 15, U.S. Dept. H.E.W., PHS, CDC, NIOSH, DHEW (NIOSH) Publication No. 78-127 (1976).
5.20. Instruction Manual - Thermal Energy Analyzer, Model 502/LC, Thermo Electron Corp., Waltham, Mass. 02145, 3-I(1977).