| Method no.: | 28 |
| Matrix: | Air |
| Target concentration: | 2 ppm (5.9 mg/m3) (Section 1.1.2.) |
| Procedure: | Samples are collected by drawing a known volume of air through two XAD-8 sampling tubes connected in series. Samples are desorbed with methanol/water (1:1) and analyzed by high performance liquid chromatography (HPLC) using an ultraviolet (UV) detector. |
| Recommended air volume and sampling rate: |
24 L at 0.1 L/min |
| Reliable quantitation limit: | 0.014 ppm (0.042 mg/m3) |
| Standard error of estimate at the target concentration: |
7.13% |
| (Section 4.8.) | |
| Status of method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1981 | Chemist: Kevin Cummins |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
A number of analytical methods are reported in the literature for the analysis of acrylic acid. Although a polarographic method has been published, most of these methods involve either gas, liquid, or paper chromatographic techniques (Ref. 5.1.). A direct method of analysis using reverse phase high performance liquid chromatography was developed and used in this study. This method is sensitive, selective, and easy to apply, and it also permits the simultaneous analysis of a number of other acrylate monomers and acrylic acid precursors.
A previous attempt by Brown to use octadecasilane (ODS) based HPLC columns for the analysis of acrylic acid was unsuccessful (Ref. 5.2.). It has been recognized in this laboratory for some time that polar molecules of low molecular weight can often be retained and chromatographed in the reverse phase mode using Zorbax ODS packed columns and a high percent of water in the mobile phase. This method, when coupled with an ion suppression technique, proved successful for the retention and separation of acrylic acid. A retention time of approximately 6 min is obtained with a Dupont Zorbax ODS 8-Fm silica packed column and a water/acetonitrile (96:4) mobile phase containing 0.1% by volume of phosphoric acid. The phosphoric acid serves to suppress the ionization of acrylic acid resulting in the retention of the undissociated form of the molecule. Under these conditions acrylic acid is separated from the potential interferences: methacrylic acid, acrylamide, acrolein, acrylonitrile, and acetic acid. Propanoic acid, a saturated precursor of acrylic acid, can be resolved from acrylic acid in a 13 min analysis at 1 mL/min flow rate using a 0.1% aqueous phosphoric acid mobile base. Acrylic acid, because of its unsaturated nature, is approximately 100 times more sensitive at 210 nm on a weight basis than propanoic acid. This method permits the detection of acrylic acid in the presence of very high levels of propanoic acid.
No published data was found regarding a collection method for acrylic acid from air. In a personal communication, it was reported that silica gel tubes coated with either hydroquinone or p-methoxyhydroquinone were being evaluated as a means of sampling acrylic acid in air (Ref. 5.4.). Both of these compounds are commonly used in the acrylicacrylate industry to prevent polymerization of a variety of monomeric substances. No decomposition of acrylic acid was observed in evaluations performed at this laboratory using either hydroquinone treated or untreated silica gel tubes. It should be noted, however, that the standard used in this evaluation contained low, unspecified levels of p-methoxyhydroquinone inhibitor.
Further evaluations indicated that some problems with the retention of acrylic acid on SKC silica gel tubes could arise if air sampling is being performed for an extended time in humid atmospheres. No loss of acrylic acid from the front section of a silica gel tube occurred if 80% relative humidity air was sampled for 1 h at a 0.1 L/min flow rate. With longer sampling times, a considerable migration from the front section of the sampling tube was observed. When 46.7 µg of acrylic acid in methanol was spiked into an atmosphere ahead of two silica gel tubes mounted in series, and humid air was drawn through the system for 4 h at a 0.1 L/min flow rate, only 15% of the total analyte was retained on the front section of the first silica gel tube. (Section 4.9.)
Differences in retention efficiency between two different lots of SKC silica gel tubes are also apparent from this data. The recently purchased lot 119 silica gel tubes are less effective in retaining acrylic acid in a humid atmosphere than the older SKC silica gel tubes which do not have a lot number designation. (Section 4.9.) In addition to silica gel, several other solid sorbent materials were determined to be inadequate for sampling acrylic acid. Low disruption efficiencies were obtained for both charcoal and Porapak T sorbents using various ratios of methanol and water to desorb the spiked tubes. XAD-2 and XAD-4, both non-polar, styrene-divinyl benzene copolymers, gave 100% desorption efficiencies using methanol. However, neither of these two sorbents were totally effective in retaining acrylic acid when humid air was sampled. Six XAD-2 tubes retained an average of only 60% of 120-µg acrylic acid spikes when 80% relative humidity air was drawn through each tube for 3 h at a 0.1 L/min flow rate. Although more effective in retaining acrylic acid than XAD-2, the higher surface area XAD-4 sorbent still lost an average of 20% of a 327-µg acrylic acid spike when 80% relative humidity air was drawn through duplicate sample tubes at a 0.1 L/min flow rate for 7.5 h. Further studies on the collection of acrylic acid from air indicated that the solid sorbent, XAD-8, an acrylic ester polymer, was quite effective in collecting and retaining acrylic acid. Amounts equivalent to twice the target concentration for a 4-h air sample (327 µg) could be spiked into an atmosphere ahead of the sampling tube and effectively collected and recovered after 80% relative humidity air is drawn through the tube for 4 h at 0.1 L/min. No breakthrough onto a second tube mounted in series was observed for acrylic acid collected from a spiked atmosphere, even though 80% relative humidity air was drawn through the system for 8 h. Similar results were observed when relatively dry laboratory air was sampled. (Section 4.5.)
Although no problems were encountered with the use of XAD-8 in sampling for acrylic acid, it should be noted that there exists a similar polymeric acrylic ester, XAD-7, which because of its higher surface area may be a more effective sampling media for low molecular weight, polar substances. (Ref. 5.5.) However, based on the evaluation procedures performed to date, an XAD-8 sorbent packed tube is currently recommended as the sampling media for acrylic acid in air.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy).
Acrylic acid is an acute local irritant. Exposure to its vapors can produce an irritating effect to the skin, eyes, nasal and bronchial passages. (Ref. 5.3.) An exposure of 300 ppm for 6 h per day for 20 days resulted in nasal irritation, lethargy, and weight loss in three male and three female rats. A one time 5-h exposure at saturated conditions (6000 ppm) produced nose and eye irritation, respiratory impairment, and death in one of four exposed rats. (Ref. 5.6.)
A large variability in the LD50 value is reported for both rabbits and mice. LD50 values for a single skin application ranging from 295 mg/kg to 950 mg/kg are reported in rabbits. Oral LD50 values in rats vary from 193 mg/kg to 3200 mg/kg. (Ref. 5.1.)
In a fetal rat toxicity study conducted by Singh, et al, a dose related increase in the incidences of skeletal abnormalities, reduced birth weights, and resorptions was observed with exposure of pregnant rats to acrylic acid. (Ref. 5.7.) The authors noted however, that the effects observed by acrylic acid and several methacrylate esters were not as pronounced as was observed for phthalate ester treated rats in previous studies.
The International Agency for Research in Cancer (IARC) reports that there is no data available regarding the carcinogenic potential of acrylic acid, and recommends study in this area. (Ref. 5.1.)
The selected target concentration of 2 ppm is based on the results of a recent industry sponsored subchronic inhalation study of mice and rats. A slight local degeneration of the olfactory mucosa was observed in a portion of the mice exposed to 5 ppm acrylic acid for 90 days. The 2 ppm level is a suggested TWA exposure limit of the Health and Safety Division of Rohm and Haas. (Ref. 5.8.)
1.1.3. Exposure
Exposure to acrylic acid vapors is primarily confined to production processes because most acrylic acid is used as a precursor in the production of a variety of different acrylates. The alkyl esters of acrylic acid are used to produce a number of products including acrylic fibers, emulsion and solution polymers, and surface coatings. Some of the free acid is used to produce polyacrylic acid, which has industrial uses as a thickener, flocculant, and binder. In 1976, three U.S. companies reported a production of 116.5 million kg of acrylic acid. (Refs. 5.1. and 5.3.)
1.1.4. Physical properties (Ref. 5.9. unless otherwise indicated)
| molecular weight: | 72.06 |
| solubility: | miscible in alcohol and ether. Soluble in acetone and benzene. |
| boiling point: | 141°C at 760 mm Hg |
| flash point: | 155°F (Ref. 5.10.) Cleveland open cup. |
| specific gravity: | 1.05 (20/4°C) |
| color: | Clear, colorless (Ref. 5.10.) |
| odor: | Pungent, irritating, odor resembling acetic acid. (Ref. 5.10.) |
| synonyms: | Acroleic acid, propenoic acid, ethylene carboxylic acid, propene acid, vinyl formic acid (Ref. 5.11.) |
| formula: | H2C=CHCOOH |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
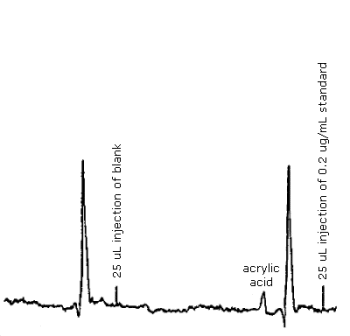
The detection limit of the analytical procedure is 5 ng per injection. This is the amount of analyte which will give a peak whose height is about 5 times the amplitude of the baseline noise. (Section 4.1., Figure 4.2.).
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1 µg per sample (0.014 ppm or 0.042 mg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 1 µg per sample (0.014 ppm or 0.042 mg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25%. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
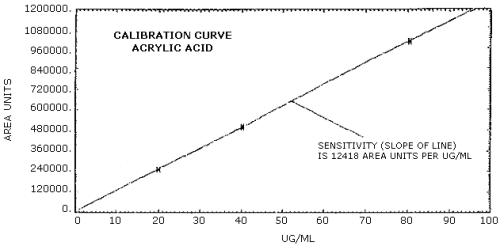
The sensitivity of the analytical procedure over a concentration range representing 0.7 to 2.9 times the target concentration based on the recommended air volume is 12,415 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.4.) The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Recovery
The average recovery from spiked samples over the range of 0.6 to 2.3 times the target concentration is 102%. (Section 4.7.) The recovery of analyte from the collection medium must be 75% or greater.
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from eight replicate determinations of analytical standards at 0.7, 1.4, and 2.9 times the target concentration is 0.0085. (Section 4.6.)
1.2.7. Precision (overall procedure)
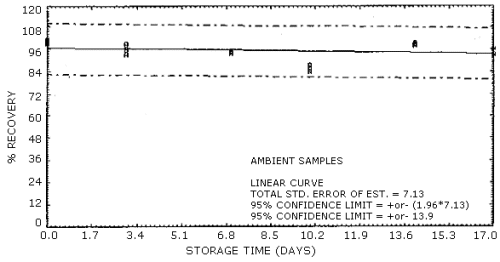
The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level. The average precision at the 95% confidence level for the ambient storage tests is ±14%. (Section 4.8.) This includes an additional ±5% for sampling error.
1.3. Advantages
- 1.3.1. The sensitivity of the analytical method permits sampling
times as short as 15 min.
1.3.2. HPLC analysis of acrylic acid is rapid, direct, and sensitive.
1.3.3. Reanalysis of samples is possible.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump which can be calibrated to
within 5% of the recommended 0.1 L/min flow rate while the sampling
tubes are in line.
2.1.2. Glass tubes of 4to 5-cm length with a 4-mm i.d. and a 6-mm o.d. are packed with approximately 100 mg of XAD-8 solid sorbent of 16-50 mesh size. Small silanized glass wool plugs on each end of the tube are used to retain the sorbent. These packed XAD-8 tubes are currently available from the laboratory upon request.
2.1.3. The XAD-8 solid sorbent is an acrylic ester polymer manufactured by Rohm and Haas. The sorbent was Soxhlet extracted for three days with methanol to remove any trace contaminants, and then dried by rotary evaporation before being used.
2.2. Sampling technique
- 2.2.1. Connect two small sampling tubes in series using a small,
red plastic SKC cap with the end removed to join the two tubes
together. Label each tube in order to distinguish the front and back
tubes. Use a portion of Tygon tubing to attach the sampling tubes to
the pump and then attach the sampling device to the worker's
clothing in such a manner that the device will monitor the worker's
breathing zone. Turn on the personal pump and begin to record the
total sampling time. Discontinue sampling at the appropriate time.
2.2.2. After sampling for the appropriate time, disconnect the sampling tubes from the pump and seal them with plastic end caps. Wrap each tube lengthwise with an official OSHA seal (Form 21).
2.2.3. Avoid exposure of the sampling tubes to heat or light whenever possible.
2.2.4. Include all necessary paperwork with the samples for shipment to the laboratory. Insure that air volume corrections have been recorded and all possible interferences or other pertinent information is included.
2.2.5. Submit any bulk samples in sealed containers under a separate cover.
2.3. Sampler capacity
No breakthrough of acrylic acid onto a second sampling tube has been observed in studies performed in this laboratory using both humid and dry atmospheres. An amount of acrylic acid equivalent to twice the target concentration for a 4-h air sample was spiked onto glass wool contained in a section of glass tubing that was mounted ahead of two sampling tubes. In different experiments, both 80% relative humidity air and relatively dry laboratory air were drawn through the tubes for up to 8 h at a 0.1 L/min flow rate. Recoveries from the front tube averaged 99.4% for three pairs of samples in which 24, 36 or 48 L of 80% relative humidity air were drawn. Similar recoveries were observed when dry air was sampled. (Section 4.5.) Acrylic acid was not detected on the backup tube in any of the studies performed.
2.4. Recommended air volume and sampling rate
A 24-L air sample obtained from a 4-h sampling period at 0.1 L/min flow rate is recommended for acrylic acid. If necessary, the sensitivity of the analytical method will permit a sampling period as short as 15 min at a 0.1 L/min flow rate.
2.5. Interferences
- 2.5.1. Low results due to polymerization of acrylic acid on the
sampling tube may result from the presence of polymer initiators
collected from the atmosphere.
2.5.2. The presence of other substances in the work atmosphere may interfere with the collection efficiency of acrylic acid.
2.6. Safety precautions
- 2.6.1. Attach the sampling equipment to the worker in such a
manner that it will not interfere with work performance or safety.
2.6.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. HPLC equipped with sample injector, variable wavelength
detector, chart recorder and all necessary hardware.
3.1.2. A 25-cm × 4.6-mm i.d. stainless steel column, slurry packed with Zorbax 8-Fm, ODS bound, spherical silica particles.
3.1.3. An electronic integrator, or other suitable method of measuring detector response. A Hewlett-Packard 3354 data system was used in this study.
3.1.4. Microliter syringe, or automatic sampling device for making sample injections. A Waters WISP auto sampler was used for this evaluation.
3.1.5. Volumetric glassware for sample and standard preparations.
3.1.6. Ten-milliliter glass syringe fitted with plastic tubing for use in desorbing sample tubes in situ.
3.2. Reagents
- 3.2.1. HPLC grade methanol and acetonitrile.
3.2.2. HPLC grade water. Our laboratory employs a commercially available water filtration system for the preparation of HPLC grade water.
3.2.3. Acrylic acid, K & K Labs., ICN Pharmaceuticals, Lot 12906-A, containing trace amounts of p-methoxyhydroquinone as an inhibitor. Standards should be refrigerated, but not frozen, during storage in order to avoid the danger of local overheating and possible polymerization during thawing (Ref. 5.10.).
3.3. Standard preparation
- 3.3.1. Stock standards of acrylic acid are weighed in volumetric
flasks and diluted to volume with methanol/water (1:1) to give a
solution of approximately 1 mg/mL concentration.
3.3.2. Appropriate dilutions of the stock standard in methanol/water (1:1) are made to give working standards in the 0.1 to 100 µg/mL range. All standards should be stored under refrigeration in tightly sealed dark bottles. Standards are stable for at least four months with proper storage.
3.4. Sample preparation
A dynamic desorption method was used to extract the sorbent material since considerable difficulty was encountered in quantitatively transferring the XAD-8 beads to a small vial for solvent desorption. This is accomplished by first attaching the sampling tube to the end of a 10-mL vertically-mounted glass syringe using a small section of plastic tubing. With the plunger removed, methanol/water (1:1) desorption solution is added to the top of the syringe and the eluting solvent is collected in a 5-mL volumetric flask. Gentle pressure can be applied to the system with the plunger in place, although flow rates in excess of 1 mL/min should be avoided. A second, 5-mL rinse of the sampling tube is collected, to insure a thorough desorption. The two 5-mL rinse solutions are then ready for direct analysis by HPLC.
3.5. Analysis
- 3.5.1. HPLC conditions:
| column: | 25-cm × 4.6-mm i.d. stainless steel column packed with Zorbax 8-Fm ODS-bound, spherical, silica particles |
| mobile phase: | 96:4 (v/v) water/acetonitrile containing 0.1% by volume phosphoric acid. |
| flow rate: | 1 mL/min |
| UV detector: | 210 nm |
| injection volume: | 25 µL |
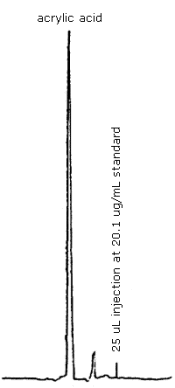
| retention time | 6.0 min. |
3.5.2. Chromatogram: Figure 4.0.
3.5.3. At the expense of some loss in selectivity, a greater than twofold increase in sensitivity can be obtained by monitoring acrylic acid at 195 nm. This requires a comparatively new deuterium lamp, since the lamp output at this wavelength deteriorates rapidly.
3.5.4. Detector response is measured by electronic integration or other suitable means.
3.5.5. An external standard procedure is used for quantitation. A calibration curve of at least three different concentrations is used. Although the acrylic acid response is very linear over a broad range, it is good laboratory practice to bracket the sample values with standards.
3.6. Interferences
A number of potential interferences were evaluated. These compounds were selected both for their structural similarity and for their potential in being present in the work atmosphere. The following compounds, with their retention times listed, were not analytical interferences: acetaldehyde, not retained; acrylamide, 4.1 min; acetic acid, 4.3 min; acrolein, 6.9 min; acrylonitrile, 7.0 min; methacrylic acid, 15 min.
Propanoic acid, the saturated form of acrylic acid, is only partially resolved from acrylic acid under the routine analytical conditions. An essentially complete resolution of these two compounds can be attained in 13 min at a 1 mL/min flow rate by using water containing 0.1% phosphoric acid as the mobile phase.
No analytical problems were encountered when an atmosphere spiked with a mixture of acrylic acid and the above compounds, less acetic and propanoic acid, was drawn through an XAD-8 tube. The mixture, containing approximately equal concentrations of the analytes in methanol, was spiked onto a piece of glass wool contained within a section of glass tubing that was mounted ahead of an XAD-8 tube. Air at 80% relative humidity was drawn through the system for 1 h at 0.1 L/min. No acetaldehyde, acrolein or acrylonitrile were retained on the XAD-8 sorbent after the 1 h sampling. Methacrylic acid and acrylamide were retained, but presented no problems for the analysis of acrylic acid.
3.7. Calculations
- 3.7.1. A linear least-squares fit of standard concentrations
versus response is determined. The response values of the sample are
used to determine the concentration from the equation.
3.7.2. The air concentration for a sample in mg/m3 is determined from the following formula:
| mg/m3 = | (µg/mL acrylic acid in sample)
(5 mL desorption solution) (1 mg)
(air volume in cubic meters) (1000 µg) |
The result converted to ppm at 25°C and 760 mm Hg:
24.46 is the molar volume at 25°C and 760 mm Hg
72.06 is the
formula weight for acrylic acid
3.8. Safety precautions
- 3.8.1. Exposure to acrylic acid should be minimized by
performing standard preparations in a well ventilated hood.
3.8.2. Avoid skin contact with acrylic acid and all solvents.
3.8.3. Restrict the use of solvents to well ventilated areas.
3.8.4. Wear safety glasses in laboratory areas.
4. Backup Data
- 4.1. Detection limit for analytical procedure
The detection limit of the analytical procedure is 5 ng per injection (0.2 ng/µL × 25 µL). This is the amount of analyte which will give a peak whose height is about 5 times the amplitude of the baseline noise. (Figure 4.1.)
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1 µg per sample (0.014 ppm or 0.042 mg/m3):
| (0.2 µg/mL) (5 mL desorbing
solution) (1 mg) (1000 L)
(24 L of air sampled in 4 h) (1000 µg) (1 m3) |
= 0.042 mg/m3 |
0.014 ppm = (0.042 mg/m3)(24.46)/72.06
4.3. Reliable quantitation limit
The reliable quantitation limit is the same as the detection limit of the overall procedure since the recovery at this level is greater than 75% and the 95% confidence limit is within ±25%. Three XAD-8 sampling tubes were spiked with 1 µg of acrylic acid (10 µL of 100.8 µg/mL acrylic acid standard), capped and allowed to sit overnight in a laboratory drawer. The following day the samples were desorbed and analyzed by HPLC. The results are reported below in Table 4.3.
|
| ||||
| µg/sample | 1 | statistics | ||
|
| ||||
| µg | 0.99 | = | 0.97 | |
| recovered | 0.93 | SD | = | 0.0346 |
| 0.99 | CV | = | 0.0357 | |
| 1.96(CV) | = | 0.0707 | ||
|
| ||||
4.4. Sensitivity
The calibration curve for acrylic acid is shown in Figure 4.4. The slope of line, 12,415 area units per µg/mL, is a measure of the sensitivity of the analytical method.
4.5. Retention efficiency
Retention efficiency of acrylic acid in either a relatively dry laboratory atmosphere or 80% relative humidity was examined. Fourteen or twenty-eight microliters of a 23.36 µg/µL standard of acrylic acid in methanol was spiked onto a piece of glass wool contained within a section of glass tubing that was mounted ahead of two XAD-8 sampling tubes connected in series, resulting in sample loadings of 327 µg or 654 µg respectively. Either 80% relative humidity air or dry laboratory air was drawn through each of six samples at a 0.1 L/min flow rate for different periods of time. Both front and back sampling tubes were desorbed and analyzed for acrylic acid. In no case was acrylic acid detected on a backup sampling tube.
Retention Efficiency
|
| |||||
| dry air | humid air | ||||
|
|
| ||||
| 327 µg/sample | 654 µg/sample | 327 µg/sample | |||
| air vol.(L) | % recov. | air vol.(L) | % recov. | air vol.(L) | % recov. |
|
| |||||
| 27 | 103 | 27 | 96 | 24 | 93.2 |
| 30 | 100.5 | 30 | 97.1 | 24 | 101.4 |
| 42 | 89 | 42 | 97 | 36 | 96.5 |
| 36 | 100.3 | ||||
| 48 | 102.5 | ||||
| 48 | 102.7 | ||||
|
| |||||
4.6. Precision of the analytical method
Acrylic acid standards at 0.7, 1.4, and 2.9 times the target concentration were each injected eight times into the liquid chromatograph using a Waters WISP autosampler. The area response was determined by integration with a Hewlett-Packard 3354 data system and converted to concentration units based on the target concentration standard. The pooled coefficient of variation for these results is reported in Table 4.6.
|
| |||
| µg/mL | 20.15 | 40.30 | 80.61 |
| × target conc. | 0.7× | 1.4× | 2.9× |
|
| |||
| µg/mL found | 20.36 20.06 19.94 19.85 20.24 20.02 20.15 20.15 |
40.81 40.21 39.49 40.48 39.54 39.98 39.73 39.92 |
80.02 80.08 80.00 79.59 79.42 79.25 79.72 79.54 |
| 20.10 | 40.02 | 79.69 | |
| SD | 0.164 | 0.460 | 0.309 |
| CV | 0.0082 | 0.0115 | 0.0039 |
|
| |||
4.7. Recovery
The average percent recovery of acrylic acid spiked onto XAD-8 tubes over a 0.6 to 2.3 times the target concentration was 102%. A total of 18 XAD-8 sampling tubes were spiked with variable amounts of acrylic acid equivalent to 0.6, 1.2, and 2.3 times the target concentration of acrylic acid based on a 24-h air sample. Six samples each were spiked with 3.5, 7.0 and 14 microliters respectively of 23.36 µg/µL acrylic acid in methanol. The sample tubes were capped and stored overnight in a laboratory drawer. The next day, each sample was desorbed with 5 mL of methanol/water (1:1) solution and analyzed.
Desorption Efficiency
|
| |||
| × target conc. | 0.6× | 1.2× | 2.3× |
| µg/sample | 81.8 | 163.5 | 327 |
|
| |||
| desorption | 107 | 105 | 105 |
| efficiency, | 99.5 | 96.7 | 103 |
| % | 103 | 101 | 82* |
| 102 | 95.4 | 103 | |
| 103 | 100 | 104 | |
| 104 | 101 | 87* | |
| 103 | 98.9 | 104 | |
|
| |||
| * Excluded from the results
becauseof an excessively rapid desorption | |||
4.8. Storage
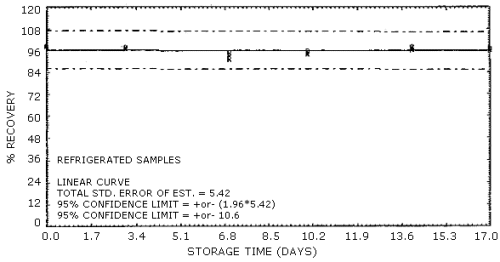
The percent recovery of acrylic acid from XAD-8 tubes after storage at both ambient and refrigerated conditions is reported in Table 4.8. Thirty-six samples were prepared for storage by spiking 7 µL of a 23.36 µg/µL standard of acrylic acid in methanol onto a piece of glass wool contained within a section of glass tubing that was mounted ahead of an XAD-8 sampling tube. Approximately 80% relative humidity air was drawn through each sample for 1 h at a 0.1 L/min flow rate. Six of the samples were desorbed immediately with 5 mL of methanol/water (1:1) solution and analyzed the same day. Of the remaining 30 samples, half were stored at room temperature in a laboratory drawer and the other half in a refrigerator at -5°C. At the indicated time intervals, three samples each were removed from storage, desorbed and analyzed for acrylic acid. The results of the ambient and the refrigerated storage are presented graphically in Figures 4.8.1. and 4.8.2.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 98.8 | 98.8 | lost | 99.4 | 101 | 100 | |
| 3 | 97.2 | 98 | lost | 93.4 | 96.2 | 99 | |
| 7 | 91.5 | 95.1 | 93.3 | 93.8 | 94.3 | 95.1 | |
| 10 | 94.2 | 94.8 | 96 | 87.5* | 83.8 | 85 | |
| 14 | 96.6 | 97 | 98.1 | 98.6 | 99.4 | 98.2 | |
| 17 | 96.4 | 97 | 97.6 | 93.5 | 96.7 | 96.6 | |
|
| |||||||
4.9. Retention efficiency on silica gel
Two microliters of a 23.36 µg/µL standard of acrylic acid were spiked onto a piece of glass wool contained within a section of glass tubing that was mounted ahead of two standard size silica gel tubes connected in series. Air at 80% relative humidity was drawn through each system at 0.1 L/min for various time intervals. Front and back sections of each silica gel tube were placed in separate 4-mL WISP vials and desorbed with 2 mL of methanol/water (1:1) solution and analyzed by HPLC. SKC silica gel tubes from two lots (an unlabled lot and lot 119) were tested.
Retention Efficiency on Silica Gel Tubes
|
| |||||||
| lot no. | unlabled | 119 | unlabled | 119 | unlabled | 119 | unlabled |
|
| |||||||
| air volume, L: | 18 | 18 | 21 | 24 | 28.5 | 0 | 0 |
| total amount | |||||||
| recovered, µg: | 45.1 | 45.6 | 48.9 | 49.3 | 49.6 | 48.3 | 48.0 |
| amount recovered from first tube | |||||||
| front section | |||||||
| front, µg: | 42.10 | 27.2 | 44.3 | 7.25 | 22.5 | 48.3 | 48.0 |
| %: | 93 | 60 | 91 | 15 | 45 | 100 | 100 |
| back, µg: | 3.03 | 16.0 | 4.6 | 28.8 | 26.3 | N.D. | N.D. |
| %: | 7 | 35 | 9 | 58 | 53 | 0 | 0 |
| amount recovered from second tube | |||||||
| front, µg: | N.D. | 2.30 | N.D. | 13.3 | 0.79 | ||
| %: | 0 | 5 | 0 | 27 | 2 | ||
| back, µg | N.D. | N.D. | N.D. | N.D. | N.D. | ||
|
| |||||||
5. References
- 5.1. "IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Humans", International Agency for Research in Cancer,
Vol. 19, 1979, pp 47-71, Lyon, France, WHO Publications Center,
Albany, N.Y.
5.2. Brown, L. Analyst, (1979), 104, 1165-1170.
5.3. Nemec, Joseph W.; Bauer, William Jr. in "Kirk-Othmer Encyclopedia of Chemical Technology", 3rd ed., Interscience, N.Y., 1978; Vol. 1, pp. 330-354.
5.4. Personal communication with W. J. Vincent, Union Carbide, South Charleston, W.V., Aug. 1980.
5.5. Sydor, Robert; Pietrzyk, Donald J., Analytical Chemistry, (1978) 50, (13), 1842-1847.
5.6. Gage, J.C., Brit. J. Industr. Med., (1970) 27, 1-18.
5.7. Singh, A.R.; Lawrence, W.H.; Autian, J., J. Dent. Res., (1978), 51, (6), 1632-1638.
5.8. Communication with American Conf. of Government Industrial Hygienists, from I. Rosenthal, Director Corporate Health and Safety, Rohm and Haas Co., Bristol, PA., Feb. 1980.
5.9. "CRC Handbook of Chemistry and Physics", 60th ed.; Weast, Robert C., Ed.; CRC Press, Inc., Boca Raton, FL., 1980.
5.10. Miller, M.L., in "Encyclopedia of Polymer Science and Technology, Plastics, Rubbers, Fibers", Bikales, N.M., ed.; Vol. 1, Interscience, N.Y., 1964, pp. 197-226.
5.11. "Register of Toxic Effects of Chemical Substances", 1975 ed.; Department of Health Education and Welfare, National Institute for Occupational Safety and Health: Cincinnati, OH, 1975.2