| Method no.: | 25 |
| Matrix: | Air |
| Target concentration: | 1 mg/m3 (OSHA PEL) |
| Procedure: | Collection and derivatization on a sampling train
consisting of two adsorption tubes connected in series. The first
tube contains |
| Recommended air volume and sampling rate: |
20 L at 0.1 L/min |
| Reliable quantitation limit: | 0.005 mg/m3 |
| Standard error of estimate at the target concentration: (Section 4.7.) |
7.6% |
| Special requirements: | |
| Status of method: | An air sampling and analytical method that has been evaluated according to the criteria established by the Organic Methods Evaluation Branch. |
| Date: February 1981 | Chemists: Keith Motley Tom Plummer |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
The procedure for determining maleic anhydride air concentrations which employs isopropanol impingers encounters a serious interference with the presence of maleic acid. The monoisopropyl ester of maleic acid was previously considered to be formed solely from the reaction of maleic anhydride with isopropanol and hence was the compound considered to exclusively represent the concentration of maleic anhydride. It can be shown, however, that maleic acid also reacts with isopropanol to form this ester in significant quantities. Further, the maleic acid itself may be either of ambient origin or formed from the hydrolysis of maleic anhydride utilizing trace amounts of water in the isopropanol impingers. In view of the myriad of equilibria and unknown factors involved, the use of isopropanol impingers for the quantitative determination of maleic anhydride is found to be lacking in both specificity and accuracy. (Section 4.8.)
During the past several months, attempts have been made at this
lab to selectively derivatize maleic anhydride in the presence of
maleic acid. Among the derivatizing agents tried were triphenyl
phosphine, various Diels-Alder reagents such as cyclopentadiene and
1,3-diphenyl isobenzofuran, imidazoles and thiazoles. All of the
above fell short of the desired goal due either to stability
problems or the inability to chromatograph and analyze the resultant
derivative.
The collection and analysis of maleic anhydride using
1.1.2. Toxicology (This section is for information only and should not be taken as the basis for OSHA policy).
Maleic anhydride is a severe irritant to the eyes, skin and respiratory tract which can, upon exposure, produce intense burning sensations in the eyes and throat with coughing and vomiting. Among workers repeatedly exposed to 5-10 mg/m3, toxic effects included ulceration of nasal mucous membranes, chronic bronchitis, and in some cases, asthma. Other potential effects of exposure are dermatitis, pulmonary edema, respiratory sensitization, skin sensitization, photophobia and double vision. (Ref. 5.2.)
1.1.3. Uses where exposure may occur
Most maleic anhydride now produced in the United States is obtained from the catalytic oxidation of either benzene or butene. An exception is the isolation of maleic anhydride as a by-product in the production of phthalic anhydride. Approximately 50% of the maleic anhydride produced is used in the manufacture of polyester resins. The remaining maleic anhydride is used in the preparation of fumaric acid, agricultural pesticides, alkyd resins and miscellaneous chemical products. No literature data indicating the number of workers potentially exposed to maleic anhydride were found. (Ref. 5.3.)
1.1.4. Physical properties
Maleic Anhydride (Ref. 5.6.)
| molecular weight: | 98.06 |
| melting point: | 53°C |
| boiling point: | 202°C |
| vapor pressure: | 1 mm Hg at 44.0°C |
| structure: | Figure 1.1.4. |
| synonyms: | 2,5-furandione, toxilic anhydride, cis butenedioic anhydride. |
Maleic Anhydride -
| molecular weight: | 221.21 |
| melting point: | 184°C |
| boiling point: | unknown |
| appearance: | yellow-green powder or crystals |
| structure: | Figure 1.1.4. |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 2.4 ng of maleic anhydride per injection. This is the amount of maleic anhydride necessary to produce a derivative peak whose height is approximately five times the baseline noise height. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 97 ng of maleic
anhydride per sample or 0.005 mg/m3 based
on the recommended air volume of 20 L. This is the amount of maleic
anhydride spiked on the sampling device as the maleic anhydride -
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 97 ng of maleic anhydride per sample or 0.005 mg/m3 based on the recommended air volume. This is the smallest amount of maleic anhydride which can be quantitated within the requirements of 75% recovery and 95% confidence limits of less than ±25%. (Section 4.2.)
1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the PEL based on the recommended air volume is 2019 area units/ng of maleic anhydride detected as the derivative. The sensitivity is determined by the slope of the calibration curve. (Section 4.4.)
1.2.5. Recovery
The recovery of analyte from the sorbent tubes must be greater than 75%. The average recovery over the range of 0.5 to 2 times the PEL is 96%. (Section 4.6.)
1.2.6. Precision of the analytical method
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the PEL is 0.0095. (Section 4.3.)
1.2.7. Precision of the overall procedure
The overall procedure must provide results that are ±25% or better at the 95% confidence level. The precision at the 95% confidence level for the 15-day storage test is ±15.9%. This figure includes an additional ±5% for sampling error. (Section 4.7.)
1.3. Advantages
- 1.3.1. The major advantage is the quantitative recovery of
maleic anhydride as the derivative without interferences from maleic
acid or degradation of the highly unstable and reactive anhydride.
1.3.2. The sampling apparatus is compact, easy to use, and has no liquid spill potential.
1.3.3. The analytical procedure is straight-forward and reproducible with detection limits well below the PEL value.
1.4. Disadvantages
- 1.4.1. The derivatization tube contains
1.4.2. The capacity of the treated sorbent tube is limited but should give quantitative recovery up to 4 times the PEL at the recommended air volume.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1.
2.1.2. Untreated
2.1.3. An air sampling pump with a flow rate which can be calibrated to within ±5% of the recommended 0.1-L/min flow rate while the sampler is in line.
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. The air sampling train is composed of one treated
2.3.2. Connect the sampling train to the sampling pump with a piece of flexible tubing. Cover each tube with masking tape or other material to prevent exposure to sunlight.
2.3.3. The air sampler should be placed in a vertical position to minimize channeling.
2.3.4. Sampled air should pass directly into the sampling train without use of extraneous tubing.
2.3.5. Immediately after sampling, separate the air sampling train into its component tubes, identify each tube as treated or untreated tube and seal each tube with plastic end caps. Also, wrap each sample end to end with official OSHA seals.
2.3.6. With each batch of samples, submit at least one blank
2.3.7. The presence of phthalic or trimellitic anhydride in the sampling area must be reported to the lab.
2.3.8. Transport the samples and paperwork to the lab for analysis.
2.3.9. Sampling tubes are stable for at least 30 days if shielded from light and stored in a freezer.
2.4. Breakthrough (Breakthrough for the purposes of this study will be defined as the presence of the maleic anhydride derivative on the "B" section of the treated sorbent tube).
- 2.4.1. Vapor generation system
A vapor generation system for maleic anhydride was constructed by
filling an empty silanized sorbent tube with glass wool and placing
it in front of the treated
2.4.2. Laboratory experiments consisted of the following: 1) drawing 60 L of humid air through the treated sorbent tube to simulate a humid sampling atmosphere, 2) transferring the tube to the dry air vapor generator and introducing a known quantity of maleic anhydride, and 3) subsequently passing more humid air through the sampling system. It is this volume of humid air which is presented in the third column of Table 4.5.2.
2.4.3. Breakthrough of the maleic anhydride from the "A" section to the "B" section of the tube is primarily a function of the flow rate at which the derivative will all form on the "A" section and secondarily a function of the air volume at which migration of the derivative will take place. At 0.1 L/min, all of the derivative will be formed on the "A" section and up to 150 L of humid air could be pulled without appreciable migration. A 20-L air volume at 0.1 L/min falls well within the parameters of providing a sensitive detection limit , quantitative recovery of maleic anhydride and a practical sampling period of 200 min. This study used an amount of maleic anhydride approximately equal to 2 times the PEL based on the recommended air volume. (Section 4.5.)
2.5. Desorption efficiency
The average desorption efficiency from
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 20 L.
2.6.2. The recommended sampling rate is 0.1 L/min.
2.7. Interferences (sampling)
Generic anhydrides present in the sampled atmosphere will react
with the
2.8. Safety precautions (sampling)
- 2.8.1. Observe due care when working with the sharp ends of the
air sampler.
2.8.2. Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance.
2.8.3. Assure that the untreated
2.8.4. Follow all safety practices that apply to the work area being sampled.
3. Analytical Method
- 3.1. Apparatus
- 3.1.1. A High Performance Liquid Chromatograph interfaced to a
UV absorbance detector.
3.1.2. A reverse phase C18 liquid chromatographic column.
3.1.3. An electronic integrator or other suitable method to measure peak magnitude.
3.1.4. An analytical balance.
3.1.5. 2-mL vials with Teflon-lined caps.
3.1.6. Syringes, 50-µL for sample injections.
3.1.7. Pipets of convenient sizes for diluting standards and a 1-mL pipet or dispenser for the desorbing solvent.
3.1.8. Volumetric flasks of convenient sizes for diluting standards.
3.1.9. Shaking device for desorption of samples.
3.2. Reagents
- 3.2.1.
3.2.2. Maleic anhydride, reagent grade.
3.2.3. Methyl alcohol, chromatographic grade.
3.2.4. Water, LC grade.
3.2.5. Phosphoric acid.
3.2.6. Chloroform, chromatographic grade.
3.3. Sample preparation
- 3.3.1. The status of the OSHA seal on each sample is noted and
recorded as intact, broken, or none.
3.3.2. The field and laboratory numbers are checked against those on the sample identification sheets.
3.3.3. The "A" and "B" sections of the treated sorbent tube should be transferred to separate 2-mL vials. The front glass wool plug should be included with the "A" section and great care should be exercised with the sorbent beads closest to the front of the tube as this is where the maleic anhydride derivative concentration will be greatest.
3.3.4. To each vial, add 1.0 mL of methanol and seal immediately with Teflon-lined caps.
3.3.5. The vials should be shaken vigorously for 60 min.
3.4. Standard preparation
- 3.4.1. Neat standard preparation is accomplished by dissolving
stoichiometric quantities of
3.4.2. Weigh out the derivative into a volumetric such that the concentration of the stock solution is no more than 1 mg/mL after addition of methanol. Sonication, shaking and/or warming this standard may facilitate the derivative's dissolution in the methanol. Dilute to the working range with methanol. A derivative solution of 45.1 µg/mL is equivalent to a maleic anhydride air concentration of 1.0 mg/m3 for a 20-L air sample desorbed with 1 mL of methanol. This amount is uncorrected for the desorption efficiency.
3.4.3. Standards are stored in dark bottles under refrigeration.
3.5. Analysis
- 3.5.1.
| LC conditions - | Waters M-6000A HPLC Pump |
| column: | Nucleosil C18 (25 cm × 4.6 mm) |
| eluent: | 50% MeOH, 50% H2O, 0.1% phosphoric acid |
| flow: | 1.5 mL/min |
| pressure: | 2600 psi |
| injection volume: | 25 µL |
| elution time: | 6.5 min |
3.5.2.
| Detector conditions - | Waters 440 Dual Wavelength Absorbance Detector |
| wavelength: | 313 nm primary, 254 nm secondary |
| attenuation: | vary as per need. |
3.5.3. Chromatogram (Figure 4.3.)
3.5.4. Peak magnitude is measured by electronic integration or other suitable means.
3.5.5. An external standard procedure is used to prepare a calibration curve from the analysis of at least six different standards diluted from two separate weighings.
3.5.6. Bracket the samples with analytical standards.
3.6. Interferences (analytical)
- 3.6.1. Any collected compound that has the same LC retention
time as the derivative and exhibits a UV absorbance at 313 nm is an
interference.
3.6.2. LC parameters may be changed to circumvent most interferences.
3.6.3. Retention time alone is not proof of a chemical identity. Samples should be confirmed by GC/MS or other suitable means when required.
3.7. Calculations
- 3.7.1. The integrator value in area units for each standard is
plotted against its concentration in µg/mL and a calibration curve
using the best straight line through the points obtained.
3.7.2. The concentration values in µg/mL for both "A" and "B"
sections of the treated
3.7.3. The air concentration of maleic anhydride (MA) for a sample is calculated by the following equation:
mg/m3 = (A)(B)(C)/D
| where | A | = | µg/mL derivative from Section 3.7.2. |
| B | = | desorption volume in mL | |
| C | = | molecular weight ratio
maleic anhydride/derivative = 0.443 | |
| D | = | sample air volume in liters |
3.8. Safety precautions (analytical)
- 3.8.1. Maleic anhydride is an extremely dangerous irritant with
a high vapor pressure that readily sublimes at room temperature.
Avoid all skin contact and use only in a well ventilated area.
3.8.2.
3.8.3. Confine the use of solvents to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
4. Backup Data
- 4.1. Detection limit
A 0.22 mg/mL derivative standard is prepared by injecting 4.0 µL of
a 55.10 µg/mL derivative standard into 1.0 mL of MeOH.
Detection Limit Data
|
| |||
| peak height (cm) | statistics | ||
|
| |||
| 0.54 | |||
| 0.54 | = | 0.586 | |
| 0.62 | SD | = | 0.049 |
| 0.58 | CV | = | 0.084 |
| 0.65 | |||
|
| |||
4.2. Reliable quantitation limit
Exactly 4.0 µL of a 55.10 µg/mL standard of the derivative was
injected onto five
Reliable Quantitation Limit Data
|
| |||||
| peak height | % recovered | ||||
| (cm) | (0.586 cm = 100%) | statistics | |||
|
| |||||
| 0.50 | 85.3 | ||||
| 0.49 | 83.6 | = | 93.1 | ||
| 0.58 | 98.9 | SD | = | 7.93 | |
| 0.58 | 98.9 | ±1.96 | SD | = | ±15.5 |
| 0.58 | 98.9 | ||||
|
| |||||
4.3. Precision
The data in Table 4.3. represent replicate 25-µL injections of
three standard solutions. The concentrations of the standards were
9.53 19.05 and 41.50 µg/mL maleic anhydride as the
Precision of the Analytical Method
|
| |||
| × target concentration | 0.5× | 1× | 2× |
| µg MA | 9.53 | 19.05 | 41.50 |
| µg adduct formed | 21.48 | 42.96 | 93.59 |
|
| |||
| area counts | 501687 | 1030920 | 2045560 |
| 507853 | 1036560 | 2062770 | |
| 507141 | 1017060 | 2064960 | |
| 503126 | 1054290 | 2072360 | |
| 504952 | 1034707 | 2064556 | |
| SD | 3035 | 15410 | 12075 |
| CV | 0.0060 | 0.0149 | 0.0058 |
|
| |||
4.4. Sensitivity
A calibration curve is shown in Figure 4.4. using data from Table 4.3. The slope of the curve indicates the sensitivity of the method over the range of 0.5 to 2 times the PEL based on the recommended air volume.
4.5. Breakthrough
- 4.5.1. The data in Table 4.5.1. represent the vapor generation
unit's efficiency at producing maleic anhydride vapors at
quantitative levels. Ten microliters of a 1905 µg/mL maleic
anhydride standard was injected onto the glass wool tube and then
dry air was pulled through the system.
Vapor Generation System Efficiency
|
| ||
| air volume (L) | % recovered | |
| dry air | as derivative | |
| tube no. | at 0.2 L/min | from treated tube |
|
| ||
| 1 | 2 | 98 |
| 2 | 3 | 100 |
| 3 | 5 | 96 |
| 4 | 4 | 100 |
| 5 | 4 | 96 |
| 6 | 4 | 100 |
|
| ||
4.5.2. The data in Table 4.5.2. represent areas obtained from "A" and "B" sections of treated tubes using different flow rates for the vapor generation. The percent B figure is indicative of breakthrough being flow related.
Variation of Sampler Flow Rates During Vapor Generation
|
| |||
| vapor generation | |||
| 3-L vol. dry air | humid air | % found | |
| number | flow rate (L/min) | volume (L) | on "B" section |
|
| |||
| 1 | 0.2 | 60 | 2.6 |
| 2 | 0.2 | 60 | 2.3 |
| 3 | 0.1 | 60 | 0.08 |
| 4 | 0.1 | 60 | 0.06 |
| 5 | 0.1 | 150 | 0.15 |
|
| |||
4.6. Desorption
Samples representing maleic anhydride concentrations of 0.49, 0.98
and 1.95 mg/m3, based on a 20-L air volume
and a 1-mL desorption volume, were prepared by injecting 4, 8, and 16
µL of a MeOH solution of derivative, whose concentration was 5510
µg/mL, onto
Desorption Efficiency Data
|
| |||
| mg/m3 | 0.49 | 0.98 | 1.95 |
|
| |||
| desorption | 89.78 | 100.00 | 99.62 |
| efficiency, | 92.63 | 100.13 | 100.10 |
| % | 94.57 | 99.46 | 99.10 |
| 92.94 | 97.83 | 97.48 | |
| 87.61 | 98.02 | 92.71 | |
| 92.14 | 99.09 | 97.43 | |
|
| |||
4.7. Storage
Storage samples were prepared by injecting 8 µL of a 55.10 mg/mL
standard of the derivative onto 36 treated
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 94.0 | 94.0 | 98.0 | 99.0 | 95.0 | 96.0 | |
| 3 | 94.0 | 93.0 | 96.0 | 95.0 | 92.0 | 86.0 | |
| 7 | 78.0 | 83.0 | 95.0 | 100.0 | 88.0 | 94.0 | |
| 10 | 99.0 | 99.0 | 99.0 | 99.0 | 93.0 | 91.0 | |
| 14 | 92.0 | 99.0 | 93.0 | 98.0 | 87.0 | 94.0 | |
| 15 | 92.0 | 98.0 | 94.0 | 101.0 | 102.0 | 89.0 | |
|
| |||||||
4.8. The data in Table 4.8. represent the esterification of maleic acid in isopropanol under various conditions. A 0.5-mL aliquot of a 222 ng/µL solution of maleic acid in isopropanol was added to each of 8 vials containing 4.5 mL of isopropanol with the pH adjusted as follows:
1) 3 vials adjusted to pH 1.8 with
H2SO4
2) 3
vials left neutral
3) 2 vials adjusted to pH 13.0 with KOH
All vials were subjected to a temperature of 41°C over a 36-h period and analyzed for the monoisopropyl ester of maleic acid by HPLC. Results are shown in Table 4.8. Note that the ester was previously believed to have been formed only from maleic anhydride in isopropanol. A significant doubt could be raised as to the validity of maleic anhydride air concentration results determined in this manner.
Esterification of Maleic Acid in IPA Under Various Conditions
|
| ||
| % maleic acid | ||
| esterified | ||
| vial no. | pH condition | (vs. 22.2 ng/µL std) |
|
| ||
| 1 | acid | 12.2 |
| 2 | acid | 12.4 |
| 3 | acid | 13.3 |
| 4 | neutral | 2.7 |
| 5 | neutral | 3.0 |
| 6 | neutral | 3.3 |
| 7 | basic | 1.6 |
| 8 | basic | 1.6 |
|
| ||
4.9. Preparation of
- 4.9.1. Reagents
Methylene chloride, chromatographic grade.
4.9.2. Preparation
The tubes are prepared by breaking both ends of untreated
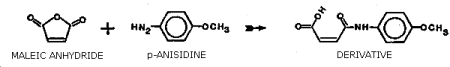
Figure 1.1.1. Derivatization reaction between maleic anhydride and
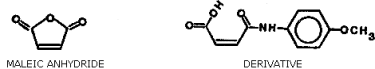
Figure 1.1.4. Structure of maleic anhydride and of the
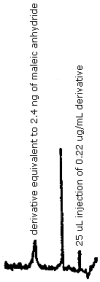
Figure 4.1. Detection limit of the analytical procedure.
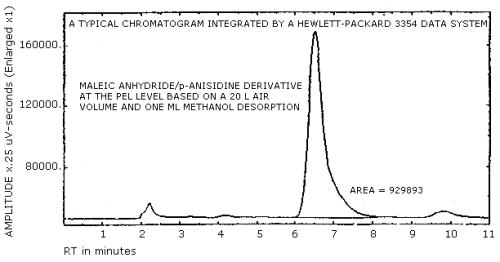
Figure 4.3. A typical chromatogram for the
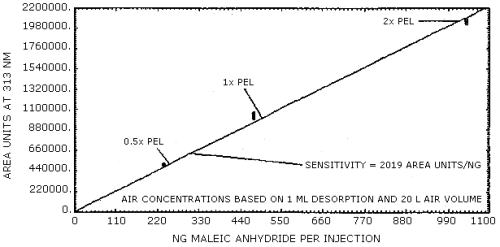
Figure 4.4. Calibration curve.
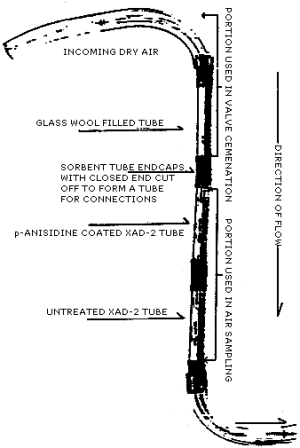
Figure 4.5. Vapor generation and sampling system for maleic
anhydride.
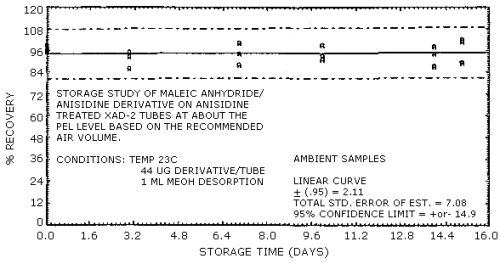
Figure 4.7.1 Ambient temperature storage test.
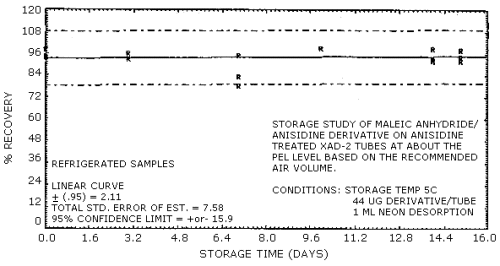
Figure 4.7.2. Refrigerated temperature storage test.
5. References
- 5.1. March, J.: "Advanced Organic Chemistry: Reactions, Mechanisms
and Structure", p. 336. McGraw-Hill, New York (1968).
5.2. Proctor, N., Hughes, J.: "Chemical Hazards of the Workplace", p. 318, J.B. Lippincott Company, Philadelphia (1978).
5.3. SRI International: "1980 Directory of Chemical Procedures United States of America", pp. 680-681, SRI International, Menlo Park, CA (1980).
5.4. U.S. Department of HEW, 1977. "NIOSH Manual of Analytical Methods", 2nd Edition, Vol. 5 Method No. S163, Anisidine (o,p isomers).
5.5. Sax, N.I.: "Dangerous Properties of Industrial Materials", p. 883 Van Nostrand Reinhold Company, New York (1975).
5.6. "The Merck Index", Ninth Edition, p. 5540, Merck & Company, Inc. Rahway, N.J.