4,4'-METHYLENEBIS(O-CHLOROANILINE) [MOCA]
| Method no.: | 24 |
| Matrix: | Air |
| Target concentration: | 0.2 mg/m3 (0.02 ppm) 0.02 ppm is the TLV of the American Conference of Governmental Industrial Hygienists. A skin notation is attached to the standard. The NIOSH recommended standard is 3 µg/m3 based on a |
| Procedure: | Collection in a bubbler containing 15 mL of 0.1 N HCl and analysis by HPLC using UV detection. |
| Recommended air volume and sampling rate: |
100 L at 1 L/min |
| Reliable quantitation limit: | 3.6 µg/m3 |
| Standard error of estimate at the target concentration: (Figure 4.5.1.) |
6.06% |
| Special requirements: | After air sampling is completed, the inlet tube of the bubbler should be thoroughly rinsed with the fresh collecting solution. The rinse solution should then be combined with the remaining collecting solution for later analysis. (Section 2.3.) |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: February 1981 | Chemist: Kevin Cummins |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
1.1. Background
1.1.1. History
A variety of sampling and analytical methods have been employed
in the past for monitoring exposure to MOCA. OSHA field samples have
been collected using
Since 1977 the analysis of MOCA in the OSHA Laboratory has been performed using HPLC. Prior to that time, analysis was performed by the gas chromatographic determination of the fluoroacetyl derivatives using electron capture detection. Analysis of the fluoroacetyl derivatives using FID detection has also been reported in the literature. (Ref. 5.1.)
MOCA is a solid at room temperature and has a relatively low
vapor pressure (3.7 ×
Rappaport and Morales of the Los Alamos Laboratory, University of California, evaluated this sampling method using an aerosol generation system. (Ref. 5.2.) They concluded that the glass fiber filter quantitatively trapped the MOCA aerosol, and no MOCA was detected on the silica gel backup portion of the sampler. MOCA aerosol from 3.6 to 54.6 µg/m3 were generated at relative humidities ranging from 5 to 90%. The ability of the sampler to collect MOCA vapor was not reported in this study. The rationale for recommending silica gel for sampling MOCA vapor was based on the work of Wood and Anderson also from the Los Alamos Laboratory. (Ref. 5.4.) Although MOCA was not evaluated in this study, they found silica gel to be an effective sorbent for the collection of a variety of volatile aromatic amines.
Yasuda, of Los Alamos, reported that Gas Chrom S was an effective solid sorbent for trapping MOCA vapor. (Ref. 5.1.) The vapor in this study was generated by adding a known amount of MOCA to a diffusion chamber contained within a temperature controlled oven. A MOCA concentration of 0.06 µg/L was generated at an oven temperature of 120°C. Gas Chrom S tubes attached directly to the oven outlet resulted in collection efficiencies of 100% for sampling times of 0.5 to 8 h at a 1 L/min sampling rate.
1.1.2. Scope of this study
The evaluation of a MOCA sampling method was pursued in order to
develop a reliable collection method for OSHA use. Air sampling over
a flask of molten MOCA was performed using a variety of collection
methods to test relative collection efficiencies. The design of the
sampling apparatus employed did not permit independent determination
of the concentration of the MOCA generated, nor could information
about the physical state of the MOCA generated be determined. The
solid sorbents
When relatively dry laboratory air was drawn through the
apparatus,
The initial results of this study indicated that a glass fiber
filter followed in series with a silica gel tube might be an
effective sampling method. However, it was later determined that low
recoveries were obtained when MOCA was spiked onto glass fiber
filters and air was drawn through the system. Similar low recoveries
were also observed with air drawn through MOCA spiked silica gel,
Gas Chrom R or
In breakthrough studies of MOCA, it was observed that the degree
of decomposition or oxidation was not simply a function of the air
volume sampled through the collection media. The recovery from
spiked glass fiber filters, with equal volumes of air sampled,
varied from approximately 46 to 99% with either dry laboratory air
or 70 to 80% relative humidity air. Similar results were observed
for silica gel, Gas Chrom R and
In an attempt to determine if the loss of MOCA was due to
oxidation, nitrogen was passed through a sodium
Storage studies were conducted for glass fiber filters, cellulose backup pads, silica gel tubes, and 0.1 N HCl aqueous bubbler solutions. The results indicate that no further degradation occurs for filters after the initial loss. Further degradation is observed however for backup pads and silica gel tubes stored both at 4°C and ambient conditions. No degradation is observed with storage for the 0.1 N HCl bubbler solution.
Because of the problems encountered with glass fiber filters and solid sorbents, the 0.1 N HCl bubbler was selected as the sampling device for MOCA. This sampling procedure passes all of the criteria established by the Methods Evaluation Branch.
In order to further evaluate the efficiency of the bubbler as a sampling method, comparative sampling over molten MOCA was conducted.
Two 0.1 N HCl bubblers connected in series were attached to one
side of a sampling arm, and a glass fiber
It is recognized that some convenience in sampling is sacrificed
with the use of a bubbler. It is also recognized that there are
inherent limitations in this evaluation process. For this reason it
is believed that side by side field sampling will provide valuable
information regarding the relative collection efficiency of the
bubbler versus the
1.1.3. Toxic effects (This section is for information only and should not be taken as the basis for OSHA policy.)
Like other aromatic amines, MOCA can produce a
Of greater concern is the evidence that MOCA is a carcinogen as
indicated by five separate animal studies using rats, mice, and
dogs. (Ref. 5.7.) In a
In another study, continuous daily oral doses of 8 to 15 mg/kg of MOCA administered to female beagles for up to nine years produced evidence of urinary bladder cancer in three of five dogs. No cases were observed in six controls. (Ref. 5.7.)
A positive Ames test for MOCA has also been demonstrated using Salmonella Typhimurium indicating that MOCA is mutagenic in an in vitro system. (Ref. 5.7.)
Evaluation of human exposure to MOCA is limited to one study
conducted by DuPont on 209 employees who were exposed to MOCA over a
Some pathological disorders in urinary tract cells were observed
among the 209 current or former employees as determined by the
Papanicolaon (Pap) technique. Of 178 former employees two deaths due
to cancer were reported. According to the report, the overall
average cancer death rate for a
1.1.4. Exposure
MOCA is used as a curing agent in the production of polyurethane elastomers. Hard tires, rollers, seals, crash pad foam and vibration dampeners are products produced from urethane elastomers. It is estimated that 3.3 million kg of MOCA were produced in 1972. (Ref. 5.2.)
NIOSH reports that in the early 1970s approximately 55,000 U.S. workers were potentially exposed to MOCA. (Ref. 5.7.)
1.1.5. Physical properties (Ref. 5.7.)
| CAS no.: | 101-14-4 |
| synonyms: | Bis Amine, Curalin M., Curene 442
|
| physical properties: | Yellow to tan pellets, nearly odorless. |
| melting point: | 99-107°C |
| solubility: | Slightly soluble in water. Soluble in alcohol, ketones, esters and many organic solvents. |
| vapor pressure: | 3.7 × 10-6 mm Hg at 20°C (Ref. 5.2.) |
| specific gravity: | 1.44 at 24°C |
| chemical formula: | C13H12N2C12 |
| molecular weight: | 267.16 |
1.2. Limit defining parameters
1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.48 ng of MOCA per injection. This is the amount of analyte which will give a peak whose height is approximately five times the amplitude of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.36 µg per sample (3.6 µg/m3). This is the amount of analyte spiked in the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.1.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.36 µg per sample (3.6 µg/m3). This is the smallest amount of analyte which can be quantitated within the required 95% confidence limits of ±25%. (Section 4.1.)
The reliable quantitation limit and detection limits reported in this method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration is 53,542 area units per µg MOCA/mL. The sensitivity is determined from the slope of the calibration curve. Variations in sensitivity may be observed with different instruments. (Section 4.2.)
1.2.5. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.0282. (Section 4.4.)
1.2.6. Precision (overall procedure)
The overall procedure must provide results at the target
concentration that are ±25% or better at the 95% confidence level.
The precision at the 95% confidence level is 14.4% for the
1.3. Advantages
1.3.1. The analytical procedure is rapid, sensitive, and reproducible.
1.3.2. Direct injection of sample is used for the analysis since no derivatization steps are required.
1.3.3. Reanalysis of samples is possible.
1.4. Disadvantages
1.4.1. Bubbler collection solutions are cumbersome to use. Loss of sample can easily occur.
1.4.2. Detection limits for bubbler solutions are greater than filters or sorbent tubes since the sample is more dilute.
1.4.3. The sampling procedure has not been field tested.
2. Sampling Procedure
2.1. Apparatus
2.1.1. An air sampling pump with a flow rate which can be calibrated to within ±5% of the recommended 1 L/min flow rate while the sampler is in line.
2.1.2. Clean, dry
2.1.3. Clean, dry
2.1.4. Glass Pasteur type pipettes equipped with small rubber bulbs for rinsing the bubbler, etc., and transferring the collection solution.
2.2. Reagents
0.1 N Hydrochloric acid collecting solution.
2.3. Sampling technique
2.3.1. Place 15 mL of the 0.1 N HCl aqueous solution into a clean, dry bubbler. Connect the bubbler to the sampling pump using flexible tubing, and maintain the device in an upright position throughout the sampling period. Fifteen milliliters of solution is an adequate volume for at least 5 h of sampling at 25°C.
2.3.2. After having completed sampling, transfer the entire contents of the bubbler to the scintillation vial for shipping to the laboratory. Rinse the inlet tube of the bubbler and the bubbler with several small volumes of fresh 0.1 N HCl solution and add these rinses to the shipping vial.
2.3.3. Insure that the vial is leakproof, and sealed with the properly labeled OSHA seal.
2.3.4. Avoid unnecessary exposure of the sample to direct light and/or heat.
2.3.5. Include all necessary paper work with the samples for shipping to the laboratory. Insure that all possible interferences or other pertinent information is included.
2.3.6. Submit any bulk samples in sealed containers under separate cover.
2.4. Breakthrough
2.4.1. Retention efficiency
Three bubblers containing 15 mL of 0.1 N HCl solution were spiked
with 5.87 µg of MOCA in methanol.
2.4.2. Collection efficiency
In order to evaluate the collection efficiency of MOCA, air was sampled over a flask of molten MOCA using an apparatus diagrammed in Figure 4.6.
Air concentrations of MOCA generated ranged from 0.062 to 0.43 mg/m3 as determined by the analysis of the bubbler system.
With the exception of the
The results of this experiment must be viewed with caution. The
actual amount, as well as the physical state of the MOCA generated,
is not known. In two of the four comparative samplings, the sections
of tubing connecting the glass Y tube to the sampling device were
rinsed with methanol and analyzed for MOCA. For the
These results indicate that both sides of the sampling device are apparently being exposed to a similar MOCA atmosphere. It is possible that the difference in recovery for the filter assembly is due to oxidation or decomposition. (Section 4.8.)
2.5. Recommended air volume and sampling rate
2.5.1. The minimum recommended air volume is 100 L.
2.5.2. The recommended sampling rate is 1 L/min.
2.6. Interferences
There are no known interferences involved in the sampling procedure.
2.7. Safety precautions
2.7.1. Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance or safety.
2.7.2. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
3.1. Apparatus
3.1.1. High performance liquid chromatograph equipped with pump, sample injector, UV detector, chart recorder and necessary hardware.
3.1.2. HPLC analytical column capable of separating aromatic
amines. A
3.1.3. An electronic integrator, or other suitable method to measure detector response.
3.1.4. Microliter syringes or automatic sampling device for making sample injections.
3.1.5. Volumetric glassware for sample and standard preparations.
3.1.6. A pH meter or pH indicating paper for adjusting pH of collecting solution to neutral conditions.
3.2. Reagents
3.2.1 HPLC grade methanol.
3.2.2. HPLC grade water. Our laboratory uses a commercially available water filtration system for the preparation of HPLC grade water.
3.2.3. Sodium hydroxide, reagent grade.
3.2.4. Purified MOCA standard. (Section 3.3.1.)
3.3. Standard preparation
3.3.1. HPLC analysis of technical grade MOCA standards at 254 nm
indicates the presence of contaminants. A cyclohexane extraction
method for purification of MOCA gave low recoveries and was found to
be time consuming. (Ref. 5.2.) In lieu of this method a
3.3.2. Stock standards of MOCA are prepared by weighing a portion of the purified MOCA standard into HPLC grade methanol. These solutions stored in dark bottles in a refrigerator are stable for an indefinite period of time. Working range standards (0.02 to 10 µg/mL) are prepared by making dilutions of the stock solution into HPLC grade water. These dilute MOCA standards in water are also quite stable, although decomposition of dilute standards in methanol has been observed. (Section 3.6.6.)
3.4. Sample preparation
3.4.1. Neutralize the 0.1 N HCl collecting solution with several drops of saturated NaOH. Check the pH with pH paper or a pH meter.
3.4.2. Measure and record the total volume of the collecting solution with a graduated cylinder.
3.5. Analysis
3.5.1. HPLC conditions
| column: | Zorbax ODS |
| mobile phase: | methanol/water 80:20 (v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 254 nm or 280 nm |
| injection volume: | 20 µL |
| retention time: | approximately 6 min |
3.5.2. Use of a dual wavelength detector permits simultaneous detection at an alternate 280 nm wavelength. Since UV response varies with wavelength, this information can be useful for confirmatory purposes as well as for the recognition of interferences. A UV scan of the purified MOCA standard in methanol is shown in Figure 4.7.
3.5.3. A representative chromatogram is shown in Figure 4.3.
3.5.4. Detector response is measured by electronic integration.
3.5.5. An external standard procedure is used for quantitation. A calibration curve of at least three different MOCA concentrations is used. Although the MOCA response is linear over a broad concentration range, it is good laboratory practice to bracket the sample values with standards.
3.6. Interferences
3.6.1. There are no known interferences to MOCA which cannot be resolved by changes in mobile phase conditions.
3.6.2.
3.6.3. Benzidine, a- and
ß-naphthylamine, and
3.6.4.
3.6.5. A matrix effect for MOCA in a mixture of aromatic amines has been observed. In an amine standard mixture MOCA elutes slightly earlier than if it is analyzed separately.
In order to identify MOCA in a complex sample, it may be necessary to spike a portion of the field sample with MOCA and reanalyze.
3.6.6. In the course of this study, it was observed that some
standards and spiked samples stored in methanol had decomposed with
time. Since this decomposition has not been observed for samples or
standards in water, the problem appears to apply only to the
analysis of samples dissolved in methanol. The nature of this
decomposition in methanol is not understood. One of the
decomposition products may
3.7. Calculations
3.7.1. A linear
3.7.2. The air concentration for a sample in µg/m3 is determined from the following formula:
| µg/m3 = | (µg/mL MOCA in sample) (total
mL of collecting solution)
air volume in cubic meters |
3.8. Safety precautions
3.8.1. Sample and standard preparations should be performed in a fume hood. Avoid exposure to both standards and samples.
3.8.2. Avoid all possible skin contact with MOCA.
3.8.3. Confine the use of solvents to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
3.8.5 MOCA should be handled with extreme care since it is an animal carcinogen.
4. Backup Data
4.1. Detection limits
4.1.1. The analytical detection limit for MOCA is 0.48 ng per injection (20 µL × 0.024 ng/µL). This amount of analyte gave a peak whose height was approximately five times the amplitude of the baseline noise. (Figure 4.1.)
4.1.2. The overall detection limit of the procedure is 0.36 µg per sample (0.024 µg/mL × 15 mL).
4.1.3. The reliable quantitation limit is the same as the detection limit of the overall procedure since the precision at the detection limit is better than ±25% at the 95% confidence level. This was determined with replicate 20-µL injections of a 0.024 µg/mL MOCA in water standard.
Table 4.1.3.
Reliable Quantitation Limit Data
|
| |||
| peak height (mm) | statistics | ||
|
| |||
| 4.2 | |||
| 4.5 | = | 4.47 | |
| 4.8 | SD | = | 0.207 |
| 4.3 | CV | = | 4.63% |
| 4.5 | ±1.96(CV) | = | ±9.1% |
| 4.5 | |||
|
| |||
4.2. Sensitivity
The calibration curve for MOCA is shown in Figure 4.2. The slope of the regression line is a measurement of the sensitivity of the analytical method.
4.3. Chromatogram
A typical chromatogram for MOCA is presented in Figure 4.3.
4.4. Precision of the analytical method
MOCA standards at 0.5, 1 and 2 times the target concentration were each injected five times using a Waters WISP automatic sampler. The area response for each injection was measured by electronic integration and the results used to construct a calibration curve. The calculated best fit values in µg/mL of each injection are used to determine a pooled coefficient of variation.
Table 4.4.
Precision of the Analytical Method
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/mL | 0.609 | 1.218 | 2.43 |
|
| |||
| µg/mL found | 0.615 | 1.183 | 2.416 |
| 0.621 | 1.231 | 2.436 | |
| 0.618 | 1.215 | 2.306 | |
| 0.610 | 1.198 | 2.481 | |
| 0.580 | 1.261 | 2.510 | |
| 0.609 | 1.218 | 2.430 | |
| SD | 0.0166 | 0.0302 | 0.0784 |
| CV | 0.0273 | 0.0248 | 0.0320 |
|
| |||
4.5. Storage
The percent recovery of MOCA after storage in aqueous 0.1 N HCl
collecting solution is reported in Table 4.5.
Over the same time period a similar storage study was conducted
with MOCA spiked on glass fiber filters, cellulose support pads, and
the front silica gel sections of the SKC sorbent tubes. All of these
sampling media were spiked with MOCA and stored in
Recoveries of MOCA on the filter are constant after the initial loss upon spiking. A further loss of MOCA is observed for cellulose support pads and silica gel stored at both ambient and refrigerated conditions. No losses are observed on day zero for controls of spiked methanol solutions on each of the sampling media.
Table 4.5.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 102.0 | 102.0 | 103.0 | 97.1 | 94.0 | 102.0 | |
| 4 | 101.0 | 104.0 | 102.0 | 105.0 | 97.1 | 104.0 | |
| 7 | 102.0 | 106.0 | 107.0 | 107.0 | 107.0 | 110.0 | |
| 11 | 97.1 | 102.0 | 103.0 | 102.0 | 104.0 | 102.0 | |
| 18 | 103.0 | 103.0 | 103.0 | 100.0 | 97.1 | 101.0 | |
| 21 | 93.3 | 103.0 | 112.0 | 93.0 | 97.1 | 95.2 | |
|
| |||||||
4.6. Bubbler retention efficiency
4.6.1. Four bubblers containing 15 mL of aqueous 0.1 N HCl were spiked with 8.75 µg of MOCA (5 µL × 1.75 µg/mL) in methanol. Three of the bubblers were placed on the humid air generator and 92 L of air at 72% relative humidity was drawn through each bubbler at 1 L/min. No air was drawn through the fourth bubbler (control). The percent recovery of MOCA from the four bubblers was 103.0, 100.0, 100.0, and 99.0 (control).
4.6.2. A similar study was done with a 117.5-µg spike of MOCA
into 15 mL of 0.1 N HCl collecting solution. One hundred and
4.7. Glass fiber filter retention efficiency
4.7.1. The average recovery for 12 glass fiber filters spiked with either 4.77 µg or 5.96 µg MOCA on different days was 80%. The recoveries ranged from a low of 45% to a high of 99%. Air volumes used ranged from 60 to 68 L. Both relatively dry laboratory air and 80% relative humidity air was used at a flow rate of 1 L/min. No correlation was observed between the percent recovery and the volume or the relative humidity of the air sampled. (Table 4.7.)
4.7.2. To examine sample loss from a glass fiber filter, a cassette containing a spiked glass fiber filter was backed by a bubbler containing 15 mL of 0.1 N HCl. Three filters were spiked with 117.5 µg of MOCA and 147 L of humid air was sampled. No MOCA was detected in any of the three bubbler backup systems although the average loss from the filters was 18%. MOCA was also not detected in cassette rinses, or on connecting pieces to the bubbler.
Recoveries for spiked
Table 4.7.
Retention Efficiency
of Spiked
Glass Fiber Filters at Ambient Conditions
|
| ||||
| amount | air | |||
| spiked | recovered | percent | volume | relative |
| (µg) | (µg) | recovery | (L) | humidity |
|
| ||||
| 5.96 | 4.28 | 71.8 | 68 | 5% |
| 4.37 | 73.3 | 68 | ||
| 5.35 | 89.8 | 68 | ||
| 5.60 | 93.9 | 0 | ||
| 5.96 | 4.99 | 83.7 | 60 | 75% |
| 5.67 | 95.1 | 60 | ||
| 4.87 | 82.0 | 60 | ||
| 6.8 | 114.0 | 0 | ||
| 5.96 | 2.74 | 45.9 | 60 | 75% |
| 5.50 | 92.3 | 60 | ||
| 5.88 | 98.6 | 60 | ||
| 6.60 | 111.0 | 0 | ||
| 4.77 | 3.21 | 67.3 | 60 | 80% |
| 4.13 | 86.6 | 60 | ||
| 3.46 | 72.5 | 60 | ||
| 4.34 | 90.9 | 0 | ||
|
| ||||
4.8. Collection efficiency
A
The collection efficiency of two
Table 4.8.
Comparative Sampling Over Molten MOCA
|
| ||||||||
| amount found in filter- | amount found in | |||||||
| silica gel assembly (µg) | bubbler assembly (µg) | |||||||
|
|
| |||||||
| air | glass | |||||||
| volume | fiber | support | silica | first | second | |||
| (L) | filter | pad | cassette | gel tube | total | bubbler | bubbler | total |
|
| ||||||||
| 142 | 18.02 | 0.85 | 13.14 | 0.882 | 32.89 | 57.65 | 3.33 | 60.98 |
| 143 | 15.88 | 3.11 | 4.17 | 0.59 | 23.75 | 39.43 | 3.47 | 42.90 |
| 205 | 9.51 | 2.13 | 4.70 | 1.30 | 17.64 | 18.75 | 0.60 | 19.35 |
| 206 | 3.07 | 0.38 | 2.20 | ND | 5.65 | 11.70 | 0.78 | 12.72 |
|
| ||||||||
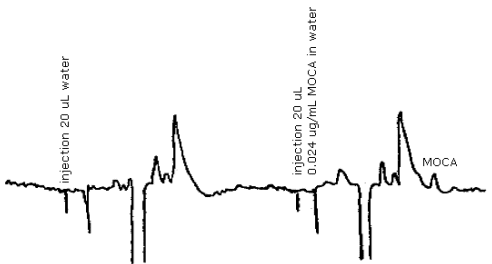
Figure 4.1. Detection limit for MOCA. 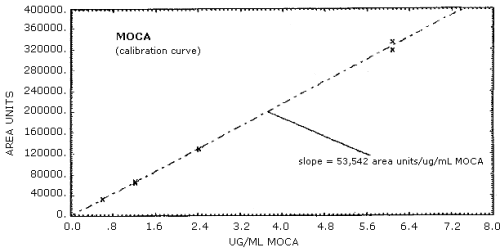
Figure 4.2. Calibration curve for MOCA.

Figure 4.3. Chromatogram of MOCA standard.
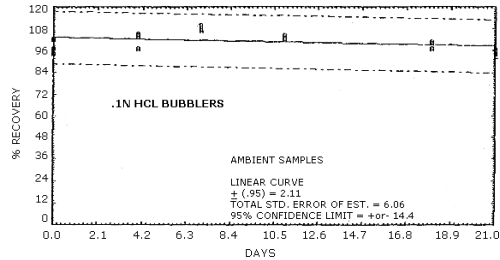
Figure 4.5.1. Ambient storage of MOCA in acid.
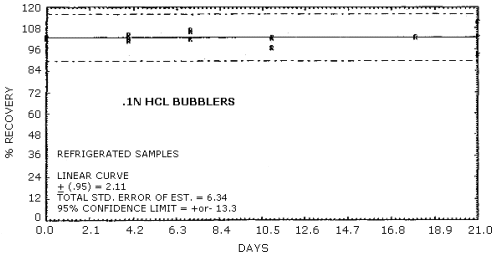
Figure 4.5.2. Refrigerated storage of MOCA in acid.
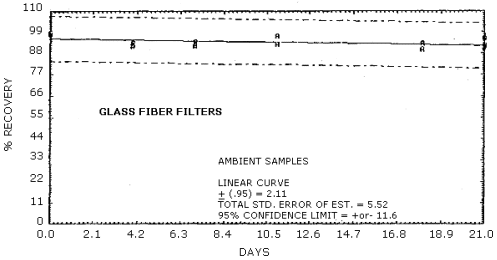
Figure 4.5.3. Ambient storage of MOCA on glass fiber
filters. 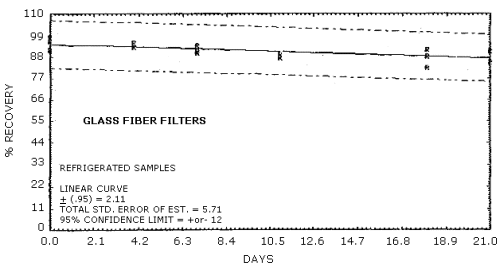
Figure 4.5.4. Refrigerated storage of MOCA on glass fiber
filters. 
Figure 4.5.5. Ambient storage of MOCA on
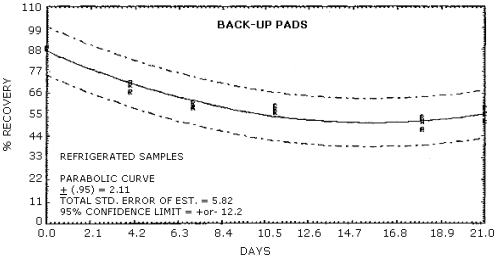
Figure 4.5.6. Refrigerated storage of MOCA on

Figure 4.5.7. Ambient storage of MOCA on silica gel.
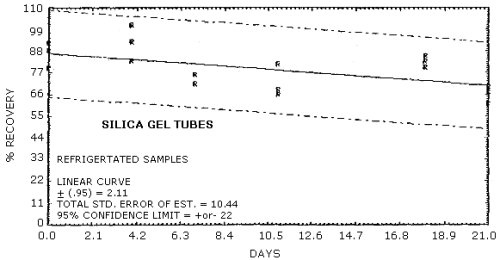
Figure 4.5.8. Refrigerated storage of MOCA on silica gel.
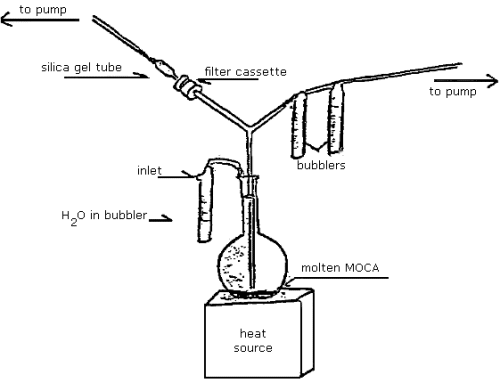
Figure 4.6. Sampling apparatus for MOCA.
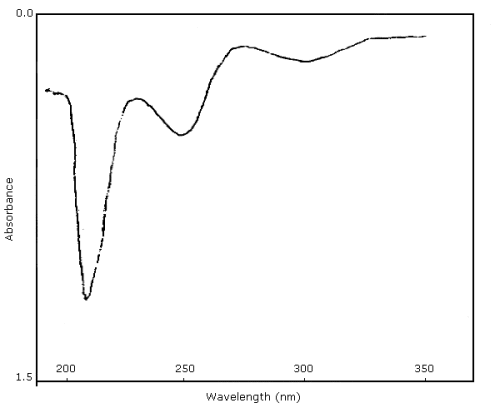
Figure 4.7. UV spectrum of MOCA in methanol.
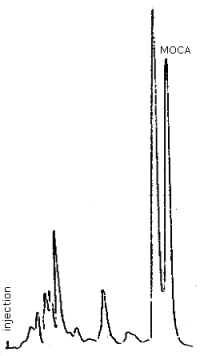
Figure 4.8. Chromatogram of MOCA in the presence of decomposition products.
5. References
5.1. Yasuda, Stanley, K., J. of Chromatography, 1975, 104,
5.2. Rappaport, S.M.; Morales, R. Anal. Chemistry 1979
51(1),
5.3. "NIOSH Manual of Analytical Methods," 2nd Edition, Volume 1,
April, 1977, NIOSH, Cincinnati, Ohio,
5.4. Wood, G.O.; Anderson, R.G., Am. Ind. Hygiene. Assoc.
Journal 1975, 36,
5.5. Elskamp, Carl J. "Benzidine Status Report" August 1979, OSHA Laboratory, Salt Lake City, Utah.
5.6. Linch, A.L.; O'Conner, G.B.; Barnes, J.R.; Killian, A.S., Jr.; and Neeld, W.E., Jr. Am. Ind. Hygiene Assoc. Journal, 1971, 32, 802-819.
5.7. "Special Hazard Review with Control Recommendation,