DIPHENYLAMINE
| Method no.: | 22 |
| Matrix: | Air |
| Target concentration: | 10 mg/m3 (ACGIH TLV) |
| Procedure: | Collection using a bubbler containing isopropanol. Analysis is by high performance liquid chromatography with ultraviolet detection. |
| Recommended air volume and sampling rate: |
250 L at 1.0 L/min |
| Reliable quantitation limit: | 1.0 µg/m3 |
| Standard error of estimate at the target concentration: |
5.5% |
| (Section 4.7.) | |
| Status of the method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: August 1980 | Chemist: Warren Hendricks |
Organic Method Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
1.1. Background
1.1.1. History
Diphenylamine (DFA) has been determined using thin layer (Refs. 5.1. and 5.2.), liquid (Ref. 5.3.), and gas (Ref. 5.4.) chromatographic techniques. Spectrophotometric analytical procedures are also available for the compound. (Ref. 5.5.)
A literature search resulted in no citations regarding air sampling methods for DFA. Since the vapor pressure of DFA is 1 mm Hg at 108°C, both aerosol and vaporous DFA may be encountered in the field. A general sampling procedure should provide a means to collect both phases. It was determined by sampling over an open container of DFA, that glass fiber and PTFE filters would not retain DFA vapors. Florisil tubes did collect DFA vapors, but since the collection efficiency of aerosols on adsorption tubes has not been well established, it was decided not to pursue this approach. Therefore, the use of a bubbler containing a collection medium was evaluated for the sampling procedure. Isopropanol was selected as the collection medium because DFA is very soluble in that solvent.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis for OSHA policy.)
The LD50 for DFA was 300 mg/kg when administered orally to the guinea pig. The lowest published oral lethal dose for the rat and for the hamster was 3000 and 500 mg/kg respectively. The lowest published oral toxic dose administered to rats 17 to 22 days pregnant was 7500 mg/kg and the toxic effects were teratogenic in nature. The lowest published oral lethal dose for humans was 500 mg/kg. (Ref. 5.6.)
The probable human oral lethal dose was estimated to be 0.5 to 5 g/kg. DFA, when given by mouth (in an oil solution) to laboratory animals, causes loss of appetite, diarrhea, emaciation, hypothermia, and general physical weakness, probably from protracted inflammation of the membrane lining of the stomach and the intestines. Death may not result until 2 to 3 weeks after a single lethal dose. (Ref. 5.7.)
Male and female albino rats fed 0.5 to 1.0% dietary concentrations of DFA for 2 years showed growth arrests which, in part, were due to decreased food intake. Moderate degrees of anemia were also observed, but return to normal hemoglobin levels followed feeding of the control diet. Leukocyte numbers and percentages remained within normal ranges in anemic rats. DFA did not increase proteinuria or cause glycosuria. An apparent decrease in litter sizes, number of pups weaned, and decreased weights of pups at weaning, observed in gestating and lactating rats fed DFA was probably due to reduced food intake. The 2-year ingestion of DFA caused lesions only in the urinary tract, namely, cystic dilatation of renal tubules with inflammation between the tissues. The glomeruli were never altered. Lower tubules were sometimes filled with a fluid. The incidence of tumors was due to the age of the rats at autopsy, not because of treatment with DFA. (Ref. 5.8.)
DFA was reported to be less toxic and less readily absorbed through the skin and respiratory tract than aniline, but the acute and chronic systemic toxicity potentials were considered to be high for both compounds. Industrial poisoning with DFA has been encountered and reported symptoms included bladder and skin problems, abnormal heartbeat, and high blood pressure. It was also reported that animals exposed to DFA dust developed definite changes in their liver, spleen and kidneys. Based on industrial experience, a TLV of 10 mg/m3 was suggested for DFA dust because that value was known to be sufficiently low to prevent systemic poisoning. (Ref. 5.9.)
While there is no experimental or epidemiological evidence that DFA is a carcinogen, commercial DFA usually contains 4-aminodiphenyl (a very potent carcinogen) as an impurity. The expanding use of DFA requires the continuing investigation of this compound to be maintained. (Ref. 5.10.)
1.1.3. Operations where exposure occurs
Diphenylamine is widely used as a rubber antioxidant and accelerator, an insecticide (directly and by fusion with sulfur), solid fuel rocket propellant, stabilizer for explosives, preparation of azo dyes (Acid Yellow 36, Acid Yellow 63, and Acid Orange 5), pharmaceuticals, veterinary medicine, storage preservation of apples, and as a reagent in analytical chemistry. (Refs. 5.11 and 5.12.)
1.1.4. Number of workers that face exposure - Unknown
1.1.5. Physical properties (Refs. 5.12. - 5.14.)
| CAS no. | 122-39-4 |
| synonyms: | aniline, N-phenyl; anilinobenzene; Big Dipper; NCI 10355; DFA; DPA; NO SCALD; N-phenylaniline; Scaldip |
| molecular structure: | Figure 1.1.5. |
| molecular weight: | 169.24 |
| physical appearance: | white to grayish crystals |
| odor: | floral odor |
| vapor pressure: | 1 mm Hg at 108.3°C |
| solubility: | Soluble in carbon disulfide, benzene, alcohol, and ether. Insoluble in water. |
| melting point: | 52.9°C |
| boiling point: | 302°C, 179°C at 22 mm Hg |
| flash point: | 307°F (closed cup) |
| autoignition temp.: | 1173°F (combustible) |
| density 22/20: | 1.160 |
| vapor density: | 5.82 |
| absorp. spectroscopy: | l max: 208 nm (log e = 4.33) |
| (in alcohol) | l max: 286 mm (log e = 4.29) |
1.2. Limit defining parameters
1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.42 ng per injection. This is the amount of analyte which will give a peak whose height is about five times the height of the baseline noise. (Section 4.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 0.26 µg per sample (1.0 µg/m3). This is the amount of analyte spiked into the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.26 µg per sample (1.0 µg/m3). This is the smallest amount of analyte which can be quantitated within 95% confidence limits of ±25%. (Section 4.3.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
1.2.4. Sensitivity
The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 178.9 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.4.) The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.5, 1, and 2 times the target concentration is 0.033. (Section 4.6.)
1.2.6. Precision (overall procedure)
The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level. The precision at the 95% confidence level for the 16-day ambient temperature storage test is ±11.6%. (Section 4.7.) This includes an additional ±5% for sampling error.
1.3. Advantages
1.3.1. The samples are stable, even when stored at room temperature for 16 days.
1.3.2. The analytical procedure is quick, sensitive, and reproducible.
1.3.3. Reanalysis of the samples is possible.
1.3.4. The analytical method is sufficient to allow the separation and quantitation of the N-nitroso and 4-C-nitroso derivatives of DFA. (Figure 4.8.)
1.4. Disadvantages
1.4.1. The use of isopropanol bubblers is both inconvenient and potentially hazardous.
1.4.2. The sampling procedure has not been field tested.
2. Sampling Procedure
2.1. Apparatus
2.1.1. An approved air sampling pump, the flow of which can be determined to within ±5% at the 1 L/min recommended air flow rate with the sampler in line.
2.1.2. Clean, dry 25-mL glass bubblers, fitted with matched ground joints and a fritted-glass inlet.
2.1.3. Clean, dry 20-mL glass scintillation vials fitted with leak-proof polyseal caps or other suitable glass containers for transporting samples.
2.1.4. Glass pipets with rubber bulbs for transferring the isopropanol collection solution.
2.2. Reagents
Isopropanol, HPLC grade.
2.3. Sampling technique
2.3.1. Place approximately 15 mL of HPLC grade isopropanol in a clean, dry glass bubbler prior to sampling. Connect the bubbler to the sampling pump with flexible tubing. Place the bubbler in an upright position. Do not allow sampled air to pass through any hose or tubing before entering the bubbler.
2.3.2. The recommended air volume is 250 L, however, with optimization of the analytical method, an air volume as low as 60 L may be sampled with satisfactory results. The recommended air sampling rate is 1 L/min.
2.3.3. It will probably be necessary to interrupt sampling to add more HPLC grade isopropanol to the bubbler because isopropanol evaporates at the rate of 6 to 7 mL/h when sampling air (30°C) at 1 L/min. Studies indicate that collected DFA is not lost in the evaporated isopropanol. (Section 2.4.1.)
2.3.4. After sampling, the isopropanol is transferred to a glass vial for shipping. Rinse the inlet tube and bubbler assembly with several 1-mL portions of isopropanol and transfer the washes to the vial containing the sample.
2.3.5. Insure that the vial containing the sample is leak-proof and then wrap each sample end to end with official OSHA seals.
2.3.6. With each batch of samples, submit at least one blank sample. The blank should be subjected to the same handling as the samples except that no air is drawn through it.
2.3.7. If bulk samples are submitted for analysis, they should be shipped in sealed glass vials. These samples must not be put in the same container used to transport air samples.
2.3.8. List possible interferences on the sample data sheet.
2.4. Breakthrough
Because of present laboratory limitations, test atmospheres of DFA could not be generated to determine collection capacity. The following data were gathered in order to approximate the 5% breakthrough air volume.
2.4.1. Retention efficiency
Two isopropanol bubblers were placed in series and 2 mg of DFA was added to the first bubbler. Air, at about 75% relative humidity and 22°C, was drawn through the train at 1 L/min. The volume of isopropanol in each bubbler was maintained at 10 to 15 mL by adding pure HPLC grade isopropanol as required. Small samples were removed from the second bubbler at various intervals and an amount of DFA equivalent to 5% of the DFA added to the first bubbler was found in the second bubbler after about 315 L of air had been sampled.
2.4.2. Vapor trapping efficiency
A short piece of silanized glass tubing containing a plug of silanized glass wool was butted to the inlet of a glass bubbler with Teflon tubing. DFA crystals were placed on the glass wool plug. A second bubbler was placed in series and then air at about 80% relative humidity and 21°C was drawn through the device at 1 L/min. The volume of isopropanol in each bubbler was maintained at 10 to 15 mL by adding pure HPLC grade isopropanol as required. Small samples were removed from both bubblers at various intervals and after 400 L of air had been sampled, breakthrough from the front to the rear bubbler was about 2%. The amount of DFA in the front bubbler was determined to be about 360 µg.
2.5. Recommended air volume and sampling rate
2.5.1. The recommended air volume is 250 L.
2.5.2. The recommended sampling rate is 1 L/min.
2.6. Interferences
It is unknown if there are interferences with the collection of DFA in isopropanol bubblers.
2.7. Safety precautions (sampling)
2.7.1. Attach the sampling equipment to the employee in such a manner that it will not interfere with work performance or safety.
2.7.2. Care must be exercised when sampling with bubblers containing isopropanol because it is a flammable solvent. Do not sample around ignition sources.
2.7.3. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
3.1. Apparatus
3.1.1. High performance liquid chromatograph equipped with pump, sample injector, UV detector, chart recorder, and necessary hardware.
3.1.2. HPLC analytical column capable of separating DFA from potential interferences. A DuPont Zorbax CN (4.6 mm H 25 cm) analytical column was used to evaluate this method.
3.1.3. An electronic integrator, or other suitable method to measure detector response.
3.1.4. Microliter syringes for sample injection.
3.1.5. Volumetric glassware for sample and analytical standard preparations.
3.2. Reagents
3.2.1. Methanol, isopropanol and water: HPLC grade.
3.2.2. Diphenylamine, analytical standard quality.
3.3. Standard preparation
3.3.1. Stock standards are prepared by diluting a weighed amount of DFA with isopropanol. The stock standard is diluted to the working range with isopropanol.
3.3.2. A solution containing 166.7 µg/mL DFA in isopropanol is equivalent to a DFA air concentration of 10 mg/m3 when 250 L of that atmosphere is sampled using a bubbler containing 15 mL (final volume) isopropanol.
3.3.3. Standards are stored in dark bottles under refrigeration.
3.4. Sample preparation
Measure to ±0.1 mL, the volume of isopropanol in the sample transport vial.
3.5. Analysis
3.5.1. HPLC conditions
| column: | DuPont Zorbax CN (4.6 mm × 25 cm) |
| mobile phase: | methanol/water 60/40 (v/v) |
| flow rate: | 1 mL/min |
| UV detector: | 280 nm (fixed wavelength) |
| injection volume: | 25 µL |
3.5.2. Chromatogram (Figure 4.5.)
3.5.3. Detector response is measured by an electronic integrator or other suitable means.
3.5.4. An external standard procedure is used to prepare a calibration curve from the analysis of at least 3 different standard solutions. The calibration curve is prepared daily. The integrator is calibrated to report results in µg/mL.
3.5.5. Bracket the samples with analytical standards.
3.6. Interferences
3.6.1. Any compound that absorbs UV light at 280 nm and has the same HPLC column retention time as DFA is an interference. Possible interferences should be listed on the sample data sheet.
3.6.2. HPLC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means when required. DFA is amenable to gas chromatographic procedures.
3.7. Calculations
3.7.1. The integrator value in µg/mL is used for reference only. More reliable results are obtained by use of a calibration curve. The detector response for each standard is compared to its concentration in µg/mL and the best straight line through the data points is determined by linear regression.
3.7.2. The concentration in µg/mL for a particular sample is determined by comparing its detector response to the calibration curve.
3.7.3. The air concentration for a sample result is calculated by the following equation:
DFA, mg/m3 = (A)(B)/C
| where | A | = | µg/mL from Section 3.7.2. |
| B | = | volume (in milliliters) of isopropanol from Section 3.4. | |
| C | = | air volume in liters |
3.8. Safety precautions (analytical)
3.8.1. Sample and standard preparations should be done in a fume hood. Avoid exposure to both samples and standards.
3.8.2. Avoid skin contact with the solvents.
3.8.3. Confine the use of solvents to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
4. Backup Data
4.1. Detection limit of the analytical procedure
The detection limit for DFA was 0.42 ng (25 µL × 0.017 µg/mL) per injection. This amount of analyte gave a peak whose height was about 5 times the amplitude of the baseline noise. (Figure 4.1.)
4.2. Detection limit of the overall procedure
The detection limit of the overall procedure was 0.26 µg (15 mL × 0.017 µg/mL) per sample.
4.3. Reliable quantitation limit
The reliable quantitation limit was the same as the detection limit of the overall procedure since the interval about the detection limit was less than ±25% at the 95% confidence level. This was determined by replicate injections from a standard solution.
Table 4.3.
Reliable Quantitation Limit Data
|
| |||
| peak height, mm | statistics | ||
|
| |||
| 32.0 | |||
| 35.1 | |||
| 33.1 | |
= | 34.23 |
| 31.0 | SD | = | 3.014 |
| 34.7 | CV | = | 8.8 |
| 39.5 | |||
|
| |||
4.4. Sensitivity
The data in Table 4.6. is presented graphically in Figure 4.4. This is the calibration curve and the slope of the regression line is a measure of the sensitivity of the analytical method.
4.5. Chromatogram
A typical chromatogram for DFA is presented in Figure 4.5.
4.6. Precision of the analytical method
This data represents multiple injections from standard solutions. The injection volume was 25 µL and the concentrations of the standards were 83.4, 166.7, and 333.4 µg/mL.
Table 4.6.
Precision of the Analytical Method
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/injection | 2.08 | 4.17 | 8.34 |
|
| |||
| area counts | 15550 | 29750 | 60580 |
| 16460 | 31980 | 58080 | |
| 15930 | 31780 | 59870 | |
| 15880 | 30210 | 59860 | |
| 15540 | 28520 | 61980 | |
| 15080 | 30370 | 62140 | |
| 15740.0 | 30435.0 | 60418.3 | |
| SD | 466.004 | 1295.171 | 1517.187 |
| CV | 0.0296 | 0.0426 | 0.0251 |
|
| |||
4.7. Storage
The data in Table 4.7. represent the results of storage tests
conducted at ambient (20 to
Table 4.7.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 | 104.5 | 98.4 | 97.7 | 101.6 | 98.4 | 99.4 | |
| 3 | 100.8 | 103.2 | 97.7 | 97.7 | 96.0 | 99.6 | |
| 6 | 99.7 | 98.9 | 99.7 | 98.0 | 95.4 | 98.3 | |
| 9 | 100.2 | 99.2 | 100.0 | 103.2 | 99.8 | 102.1 | |
| 13 | 98.1 | 96.5 | 99.7 | 98.4 | 101.5 | 98.0 | |
| 16 | 98.8 | 97.9 | 99.0 | 102.5 | 103.0 | 104.7 | |
|
| |||||||
The data in Table 4.7. are presented graphically in Figures 4.7.1. and 4.7.2.
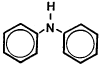
Figure 1.1.5. Molecular structure of diphenylamine.
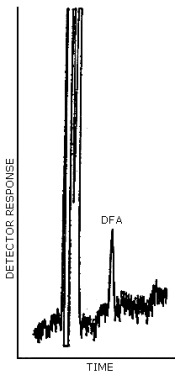
Figure 4.1. Detection limit of the analytical procedure
for diphenylamine. 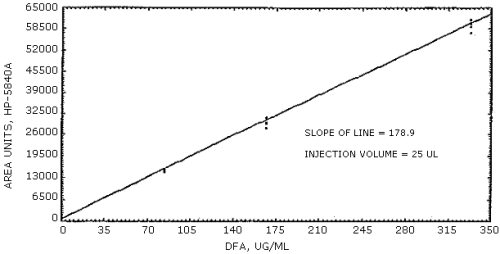
Figure 4.4. Calibration curve for diphenylamine.
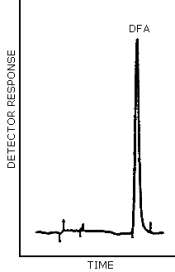
Figure 4.5. Chromatogram at the target concentration for
diphenylamine. 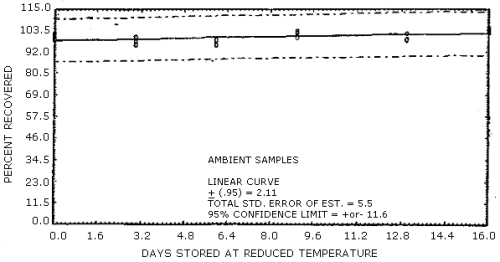
Figure 4.7.1. Ambient temperature storage test for
diphenylamine. 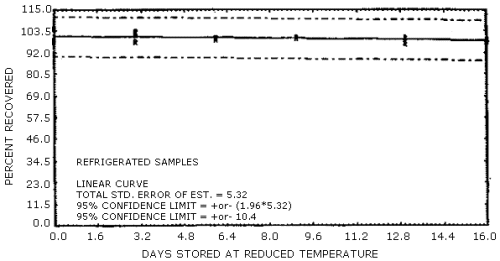
Figure 4.7.2. Refrigerated temperature storage test for
diphenylamine. 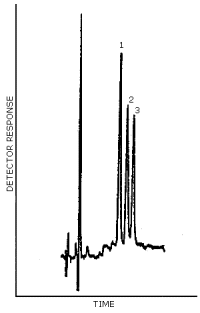
Figure 4.8. Chromatogram for a mixture containing
5. References
5.1. A. W. Archer, J. Chromatogr., 108, 401-404 (1975).
5.2. J. W. Grindlay, Explosivstoffe, 5, 177-181 (1973).
5.3. L. Vodicka, J. Kriz, J. Burda, and P. Novak, J. Chromatogr., 148, 247-254 (1978).
5.4. G. A. Jungclaus, L. M. Games, and R. A. Hites, Anal. Chem., 48, 1894-1896 (1976).
5.5. W. A. Schroeder, P. E. Wilcox, I. N. Trueblood and A. O. Dedoer, Anal. Chem., 23, 1740 (1951).
5.6. "Registry of Toxic Effects of Chemical Substances" 1978 ed., R. J. Lewis and R. L. Tatken, Eds. U.S. Department of Health, Education and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, U.S. Government Printing Office, Washington, D.C. (1978).
5.7. R. E. Gosselin, H. C. Hodge, R. P. Smith, and M. N. Gleason, "Clinical Toxicology of Commercial Products Acute Poisoning" 4th ed., The Williams and Wilkins Company: Baltimore, 140 (1976).
5.8. J. Thomas, W. Ribelin, R. Wilson, D. Keppler and F. Deeds. Toxic Appl. Pharmac., 10(2), 362-374 (1967).
5.9. "Documentation of the Threshold Limit Values for Substances in Workroom Air" 3rd ed., American Conference of Governmental Industrial Hygienists: Cincinnati, 95, 4th Printing (1977).
5.10. C. Searle, "Chemical Carcinogens" ACS Monograph 173, American Chemical Society: Washington, 467 (1976).
5.11. Kirk-Othmer, "Encyclopedia of Chemical Technology" 2nd ed., Vol. 2., John Wiley and Sons, Inc.: New York, 420-421 (1963).
5.12. G. G. Hawley, "The Condensed Chemical Dictionary", 9th ed., Van Nostrand Reinhold Company: New York, 316 (1977).
5.13. N. Sax, "Dangerous Properties of Industrial Materials" 4th ed., Van Nostrand Reinhold Company: New York, 695 (1975).
5.14. R. Weast "CRC Handbook of Chemistry and Physics" 60th ed., CRC Press, Inc.: Boca Raton, FL, C107 (1979).