| Method no.: | 21 |
| Matrix: | Air |
| Target concentration: | 0.3 mg/m3 (0.10 ppm) (OSHA PEL) |
| Procedure: | The sampling train consists of a 13-mm glass fiber filter in a Swinnex cassette (available from the laboratory), followed by a standard silica gel tube. The filter and gaskets, within the cassette used to hold the filter, are extracted in the field in exactly 1 mL of methanol. This extraction is very important so a volumetric pipette calibrated to 1 mL must be used. The silica gel tube is extracted with methanol at the laboratory. Analysis is performed by gas chromatography (GC) using a nitrogen/phosphorous detector. |
| Recommended air volume and sampling rate: |
120 L at 1 L/min |
| Detection limit of the overall procedure: |
Glass fiber filter 3.8 µg/m3 (1.3
ppb) Silica gel 3.8 µg/m3 (1.3 ppb) |
| Reliable quantitation limit: | Glass fiber filter 3.8 µg/m3 (1.3
ppb) Silica gel 3.8 µg/m3 (1.3 ppb) |
| Standard error of estimate: | 7.06% |
| Special requirements: | As soon after sampling as possible, the glass fiber filter and the gaskets holding the filter in place must be added to 1 mL of methanol. This is done to avoid sublimation of any acrylamide particulate collected on the filter. |
| Status of method: | This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: October 1980 | Chemist: Michael L. Shulsky |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History of procedure
There is little published data on the collection of acrylamide, but manufacturers have used a variety of methods due to the possibility of acrylamide being present as a vapor or aerosol. The sampling procedures used include filters, midget impingers, and adsorbent tubes.
Various analytical techniques have been employed: spectrophotometry with derivatization, refractive index measurements, titrimetry, GC with derivatization, direct GC, thin layer chromatography, polarography (Ref. 5.1.), and liquid chromatography (LC). (Ref. 5.2.)
A glass fiber filter backed up with a silica gel tube was evaluated for this procedure. It was found that solid acrylamide will vaporize from a filter rather rapidly when air is drawn through it. The filter is recommended for collection of particulate acrylamide since the collection efficiency of sorbent tubes is not known. Silica gel tubes were chosen to back up the filter because they provide good collection and resorption efficiencies. Poor desorptions were obtained from charcoal with methanol- water mixtures. Other solvents were evaluated for resorption of charcoal, but did little to increase the resorption efficiency.
Gas chromatographic analysis was selected. The variability of conditions and columns are sometimes needed to eliminate interferences. A nitrogen-phosphorus detector (NPD) was chosen because of its selectivity and sensitivity.
The plastic cassettes which hold a 37-mm filter were unsuitable for the sampling train. The cassette appeared to be responsible for low and erratic recoveries of acrylamide. This was determined by spiking a known amount of acrylamide on a glass wool plug inside a glass tube (both parts silanized), and placing the glass tube ahead of the plastic cassette in the sampling train. One hundred twenty liters of humid air, at approximately 75% relative humidity (RH), was drawn through the glass tube assembly cassette, and silica gel tube. The sampling train, including the glass tube assembly and rinses from the cassette were analyzed separately. Acrylamide was found only on the "A" portion of the silica gel tube. The recovery from numerous tests of this procedure ranged between 65% and 100% . In order to check the technique of spiking the glass wool plug, bubblers of isopropanol were used instead of a cassette and silica gel tube. These tests gave 95 to 100% recovery. Therefore, it was assumed the cassette was introducing errors in recovery. Smaller Swinnex cassettes were tried with the same spiking technique. The recovery was 95 to 100%.
1.1.2. Toxicity (This section is for information only and should not be taken as the basis for OSHA policy.)
Acrylamide may be absorbed through the skin or by inhalation. Workers exposed to the dust for 4 to 12 weeks showed symptoms of muscular weakness particularly in the legs, numbness of the limbs, absence of deep tendon reflex, fatigue, and lethargy. These problems were slow to disappear, in some cases taking months. (Ref. 5.3.)
1.1.3. Exposure
Acrylamide is used primarily in the production of polymers. Polyacrylamide is used as flocculent in water and waste treatment. The paper industry also uses polyacrylamide for strengtheners. There are approximately 20,000 workers potentially exposed to acrylamide. (Ref. 5.1.)
1.1.4. Physical properties (Ref. 5.4. unless otherwise stated)
| molecular weight: | 71.08 (Ref. 5.1.) |
| boiling point: | 125°C (25 mm Hg) |
| melting point: | 84.5°C |
| specific gravity: | 1.122 (3°C) |
| color: | colorless |
| odor: | odorless |
| vapor pressure: | 0.007 mm Hg at 25°C sublimes at room temperature (Ref. 5.1.) |
| synonyms: | propenamide, acrylic amide, akrylamide (Ref. 5.1.) |
| molecular formula: | CH2=CHCONH2 |
1.2. Limit defining parameters (All values are based on the recommended air volume.)
- 1.2.1. Analytical detection limit
The detection limit of the analytical procedure is 0.9 ng per 2-µL injection. This is the amount of acrylamide which gave a chromatographic peak 5 times the baseline noise. (Section 4.1.1.)
1.2.2. Detection limit of the overall procedure
This detection limit is 0.45 µg for each component of the sampling train (silica gel tube and glass fiber filter). The above amount, 0.45 µg, spiked on each collection media, gave a recovery equal to the analytical detection limit. (Section 4.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 0.45 µg for each collection medium which is equivalent to 3.8 µg/m3 or 1.3 ppb . This is equal to the analytical detection limit since recovery never fell below 75% and the 95% confidence limit remained within ±25%.
The reliable quantitation limit and detection limits reported in this method may be better or worse than another analyst may obtain. This is due to the factors which will effect sensitivity of an NPD such as age of the bead, the voltage applied to the bead, and the flow rate of the carrier gas and detector gases.
- 1.2.4. Sensitivity
The sensitivity for the analytical procedure over the concentration range of 0.4 to 2 times the PEL based on a 120-L air volume is 6160 area units per µg/mL. This value is the slope of the calibration curve. (Section 4.3.) The sensitivity will vary with the instrument and data system used.
1.2.5. Recovery
The recovery of acrylamide from samples used in a 15-day storage test remained above 89% for samples spiked with 40 µg of acrylamide. (Section 4.6.) The recovery of analyte from the collection medium during storage must be 75% or greater.
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate injections of analytical standards at 0.5, 1.0, and 2 times the PEL is 0.0312. (Section 4.2.)
1.2.7. Precision (overall procedure)
The precision at the 95% confidence level for the 15-day storage test is ±14.8%. (Figure 4.7.) This includes an additional ±5% for sampling error. The overall procedure must provide results that are ±25% at the 95% confidence level.
1.3. Advantages
- 1.3.1. The sampling device is small and portable.
1.3.2. Replicate injections of each sample are possible.
1.3.3. Samples are stable for at least two weeks.
1.3.4. The analytical technique is sensitive and selective.
1.4. Disadvantages
- 1.4.1. The precision of the sampling pump is dependent on the
pressure drop across the sampling train.
1.4.2. The filter portion and gaskets, which hold the filter, of each sample must be extracted with methanol as soon after sampling as possible. It may be advantageous to place exactly 1 mL of methanol in enough scintillation vials to accommodate the expected number of samples prior to going to the sampling site. After sampling, the cassette could be unscrewed and the filter and gaskets simply dropped into a vial already containing exactly 1 mL of methanol.
1.4.3. Methanol is toxic and must be handled cautiously.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump which may be calibrated within
±5% of the recommended flow rate.
2.1.2. Silica gel tubes containing a 150-mg and a 75-mg portion. The tubes used in the evaluation were obtained from SKC, Inc., and were 6-mm i.d. × 70 mm in length.
2.1.3. Scintillation vials with Teflon-lined caps, i.e., 20-mL.
2.1.4. The Swinnex cassettes with filters and gaskets can be obtained from the laboratory.
2.1.5. Tygon tubing is used to connect the cassette to the silica gel tube. This tubing should be as short as possible.
2.1.6. A 1-mL pipette is necessary for dispensing methanol into the scintillation vials.
2.2. Reagents
Methanol, reagent grade.
2.3. Sampling technique
- 2.3.1. Before going to the workplace, exactly 1.0 mL of methanol
is placed in scintillation vials and capped tightly. There should be
enough scintillation vials with 1 mL of methanol, to extract each
filter sample taken.
2.3.2. A short piece of Tygon tubing is used to connect the cassette to the silica gel tube, such that the silica gel tube is butted against the cassette.
2.3.3. Immediately before sampling, the ends of the silica gel tube are broken, the cassette and silica gel tube are connected, and the tube is connected to a sampling pump with a flexible hose.
2.3.4. The sampling device, cassette followed by a silica gel tube, is placed vertically on the employee to avoid channeling through the tube.
2.3.5. Immediately after sampling, seal the ends of the silica gel tubes with plastic caps and wrap it lengthwise with an official OSHA seal (Form 21).
2.3.6. As soon as possible, unscrew the cassette and place, the glass fiber filter and O-ring gaskets in a vial containing 1 mL of methanol. Be certain the cap is tightly screwed on the vial, then wrap the vial lengthwise with an official OSHA seal.
2.3.7. With each set of silica gel tubes send in a blank.
2.3.8. Submit a blank sample of the methanol used for extraction, containing a blank filter and gaskets.
2.3.9. If a bulk sample is submitted for analysis, it should be sent in a glass bottle sealed with a Teflon-lined cap. Bulk samples must not be shipped in the same package with air samples.
2.3.10. Transport the samples, Swinnex cassettes, and corresponding paperwork to the laboratory for analysis.
2.4. Sampler capacity
Since a controlled test atmosphere could not be produced, an exact determination of breakthrough capacity could not be obtained easily. Instead, the retention efficiency for a high concentratlon of acrylamide spiked on the silica gel tube was determined.
A study was performed by spiking a filter with a large amount of acrylamide, then attaching a silica gel tube behind it. One hundred-eighty liters of humid air (approx. 75% RH, 20°C) was pulled through the assembled sampling train. The "A" and "B" portions of the silica gel tube were analyzed. The "A" portion contained 0.644 mg of acrylamide while the "B" portion contained none. This amount (0.644 mg) is approximately 17 times the amount for a sample at the PEL.
2.5. Desorption and extraction efficiencies
- 2.5.1. Desorption efficiencies were determined by liquid
injections of methanol/acrylamide solutions onto the "A" portion of
the silica gel tubes. Studies were done over the range of 2 times
the PEL down to the detection limit of the analytical procedure
assuming a 120-L air volume. Six tubes were prepared at each
loading. The average resorption efficiency was 97.7%. (Section 4.5.)
2.5.2. Extraction efficiencies for the filters were done at 40 µg and 0.9 µg of acrylamide. These were prepared by liquid injection onto the filters and immediately extracted with 1 mL of methanol. These gave 100% recovery so there is no correction necessary for the filter portion.
2.6. Recommended air volume and sampling rate
The recommended air volume is 120 L at a sampling rate not to exceed 1 L/min.
2.7. Interferences
- 2.7.1. Other organic vapors may collect on the silica gel tubes.
Any other organic vapors present should be listed as possible
interferences.
2.7.2. Other organic particulate or aerosols may collect on the filter so they should be listed as possible interferences.
2.7.3. Large amounts of dust may plug the filter, so it should be watched if a dusty environment is encountered.
2.8. Safety precautions
- 2.8.1. Eye protection should be worn during the breaking of the
ends of the silica gel tubes.
2.8.2. Methanol is a flammable and toxic solvent, so it should be handled with care.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatography equipped with a nitrogen/phosphorus
detector.
3.1.2. Microliter syringes, such as 2-µL and 10-µL sizes.
3.1.3. Analytical GC column. For this evaluation a 3-ft glass column, 2-mm i.d. × 1/4-in. o.d., containing 10% Carbowax 20M with 2% KOH on 80/100 Chromosorb W AW, was used.
3.1.4. Pipettes and dispensers for standard preparation and sample desorption.
3.1.5. Volumetric, such as 5-mL and 10-mL sizes.
3.1.6. Small vials for desorption of silica gel.
3.1.7. A suitable method for peak area integration.
3.2. Reagents
- 3.2.1. Acrylamide, reagent grade.
3.2.2. Methanol, GC quality.
3.2.3. Helium, hydrogen, air; GC quality.
3.3. Standard preparation
Nitrogen/phosphorus detectors may not be linear over a wide range of analyte concentration. The linearity must be ascertained before a quantitative method is chosen. Standards covering the concentration range in which the samples are expected to be should be prepared by weighing acrylamide into two volumetric, diluting them to the mark with methanol, and making dilutions of these stock standards down to the desired range. Analysis of the dilutions to be used with the samples will show whether the detector is linear or not.
If the detector is linear over the desired range, then a direct correlation between area of the acrylamide peak obtained from a standard and concentration of that standard can be made.
If the detector response is not linear, then a suitable calibration curve must be used to correlate the area of a peak to the concentration.
3.4. Sample preparation
- 3.4.1. The 150-mg portion of silica gel and the glass wool plug
are placed in one vial while the 75-mg portion is placed in a second
vial. The urethane foam plugs are discarded.
3.4.2. Each portion of silica gel is desorbed with 1 mL of methanol, periodically shaken to insure all the silica gel has been wetted, and analyzed, at least 20 min later.
3.4.3. The filters and gaskets are extracted by the field personnel as per Section 2.3.6.
3.5. Instrument conditions
- 3.5.1.
| oven: | 160°C |
| detector: | 200°C |
| injector: | 200°C |
| column: | 3-ft glass 2-mm i.d. × 1/4-in. i.d. 10% Carbowax 20M/2% KOH on 80/100 Chromosorb W AW |
| carrier gas: | nitrogen - 20 mL/min |
| detector type: | Hewlett-Packard Alkali
Bead (nitrogen/phosphorus) |
| detector gases: | air - 50 mL/min hydrogen - 3 mL/min |
| detector voltage: | 19 |
3.5.2. A typical chromatogram is shown in Figure 4.8.
3.6. Interferences
- 3.6.1. Any compound which has the same retention time as
acrylamide and will respond on a NPD will be an interference. By
altering chromatographic conditions, the interfering compound may be
separated. It may be necessary to change analytical columns. A
second column to use is a 3-ft glass column 1/4-in. o.d. × 2-mm i.d.
packed with 10% SP1000 on 80/100 Supelcoport.
3.6.2. If a sample is calculated to be above the PEL, then a confirmation should be done by mass spectrometry or another suitable method. Retention time on one column is not considered proof of identity. Two different types of packing should be used to compare retention times.
3.7. Calculations
If either calibration method, linear or non-linear is used as described in Section 3.3.1., the values obtained for samples will be in concentration units. For each sample there will be results for the glass fiber filter and the silica gel tube.
Example: Air Volume = 110 L
- Quantitation method gives the following results:
Glass fiber filter = 2 µg/mL
Silica Gel = 20 µg/mL
Both collection media are extracted with 1 mL of methanol. Therefore, the total µg from the filter is a simple multiplication: 2 µg/mL × 1 mL = 2 µg while the total micrograms for the silica gel must take into account the desorption efficiency, 97.7%. The calculation is as follows:
| 20 µg/mL
0.977 |
= 20.47 µg/mL × 1 mL = 20.47 µg |
The total µg for the sample is obtained by adding the results of the GFF to the silica gel results.
- 2 µg + 20.47 µg = 22.47 µg
The air concentration for the sample is calculated by dividing the total pg for the sample by the air volume in L.
- 22.47 µg/110 L = 0.204 µg/L = 0.2 mg/m3
The result for the sample is 0.2 mg/m3.
3.8. Safety precautions
- 3.8.1. Safety glasses should be worn in all designated areas.
3.8.2. All solvents should be handled in an exhaust hood and kept away from sources of ignition such as heated injectors and detectors.
4. Backup Data
- 4.1. Detection limit data
- 4.1.1. The detection limit of the analytical procedure is 0.9 ng
per 2-µL injection. This is the amount of acrylamide which gave a
chromatographic peak five times the baseline noise. (Figure 4.1.)
4.1.2. The detection limit of the overall procedure is 0.45 µg per collection medium (3.8 µg/m3 or 1.3 ppb). The above amounts spiked on each collection medium gave a recovered amount equal to the detection limit of the analytical procedure. (Figure 4.2.)
4.1.3. The reliable quantitation limit is equal to 0.45 µg per collection medium, since neither the desorption no; extraction fell below 75% and the 95% confidence limit remained within ±25%. (Figure 4.3.)
4.2. Precision (analytical procedure)
Based on the data of Table 4.2., the precision of the analytical method at 0.5, 1, and 2 times the PEL, given as a pooled coefficient of variation, is 0.0312.
Precision of the Analytical Procedure
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/mL | 17.6 | 35.2 | 70.4 |
|
| |||
| µg/mL found | 18.45 | 36.34 | 67.95 |
| 17.91 | 34.52 | 68.80 | |
| 17.41 | 34.76 | 69.52 | |
| 18.26 | 38.17 | 68.53 | |
| 16.78 | 34.74 | 68.58 | |
| 17.29 | 35.12 | 69.39 | |
| 17.68 | 35.61 | 68.80 | |
| SD | 0.635 | 1.41 | 0.585 |
| CV | 0.0359 | 0.0395 | 0.0085 |
| ___ CV = 0.0312 |
|||
|
| |||
4.3. Sensitivity
The sensitivity of this analytical procedure is defined as the area change per µg/mL change as found from the slope of the calibration curve. (Figure 4.4.) For the instrument and data system used, the sensitivity was 6160 area counts per µg/mL.
4.4. Sampler capacity
Breakthrough studies could not be done due to instrument limitations. Therefore, a retention study was performed by spiking a glass fiber filter with a large amount of acrylamide, placing a silica gel tube after the filter, and drawing 180 L of humid air at 1 L/min through the sample train. Analysis of the "A" and "B" portions of silica gel showed 0.644 mg (644 µg) of acrylamide on the "A" portion and none on the "B" portion. The amount, 644 µg, is approximately 20 times the amount collected for a sample at the PEL with a 120-L air volume. This would give a wide safety margin for most samples.
4.5. Desorption and extraction efficiencies
- 4.5.1. Desorption efficiencies were determined at 0.4, 1.1, and
2 times the PEL (Figure 4.5.), and at 0.45 µg and 2.25 µg. (Figure
4.3. ) Silica gel tubes were spiked by liquid injection and stored
in a refrigerator overnight. Upon analysis an average desorption
efficiency of 97.7% was obtained.
Desorption Efficiency
|
| |||||
| × target concentration | 0.4× | 1.1× | 2× | ||
| µg/sample | 0.45 | 2.25 | 16 | 40 | 72 |
|
| |||||
| desorption | 98.7 | 99.8 | 96.4 | 101.0 | 95.9 |
| efficiency, % | 101.0 | 94.2 | 94.5 | 100.0 | 99.4 |
| 105.0 | 99.2 | 99.5 | 103.0 | 96.9 | |
| 87.8 | 100.0 | 105.0 | 95.3 | 97.6 | |
| 90.7 | 100.0 | 96.7 | 91.9 | 97.9 | |
| 95.3 | 104.0 | 95.3 | 91.4 | 98.4 | |
| 96.4 | 99.4 | 97.9 | 97.1 | 97.7 | |
|
| |||||
4.5.2. Extraction efficiencies for filters were done by liquid injection of acrylamide onto the filter and immediate extraction with 1 mL of methanol. The amounts spiked were 0.9 µg and 40 µg. These gave 100% recovery, so no correction is necessary for the filter portion of the samples.
4.6. Storage test
- 4.6.1. It was not possible to generate a controlled test
atmosphere of acrylamide vapors and instrument limitations prevented
the generation of an aerosol of acrylamide, so the stability samples
were prepared by liquid injection onto the "A" portion of silica gel
tubes. Thirty-six samples were prepared. Six of the samples were
analyzed after generation to be used for day zero results. Fifteen
samples were stored under refrigeration (5°C) and the remaining 15
were stored at room temperature. On every third day when possible, 3
samples from each storage set were analyzed. The results are given
below with graphic representation in Figures 4.6. and 4.7.
Storage Tests
|
| ||||||
| storage time | % recovery | |||||
| (days) | (refrigerated) | (ambient) | ||||
|
| ||||||
| 0 | 93.8 | 95.4 | 98.4 | 101.0 | 100.0 | 101.0 |
| 3 | 90.2 | 91.5 | 107.0 | 95.3 | 90.7 | 89.8 |
| 6 | 89.6 | 91.5 | 93.0 | 95.5 | 100.0 | 96.9 |
| 9 | 102.0 | 98.8 | 94.2 | 104.0 | 99.8 | 104.0 |
| 12 | 102.0 | 95.9 | 102.0 | 90.9 | 95.1 | 89.2 |
| 15 | 92.0 | 93.3 | 94.8 | 97.9 | 101.0 | 98.9 |
|
| ||||||
4.6.2. The storage stability of filters was not done since acrylamide was known to volatilize from the filters upon storage. Storage stability of filters spiked with acrylamide then extracted with methanol was done on several filters. This showed 100% recovery from the methanol solution after a 17-day storage time.
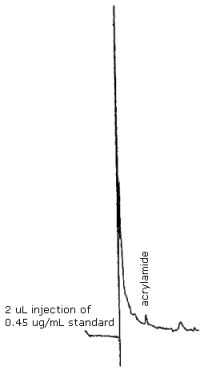
Figure 4.1. Detection limit of analytical procedure.
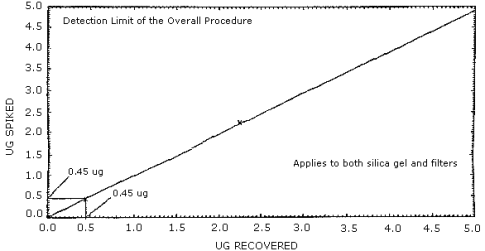
Figure 4.2. Detection limit of overall procedure. 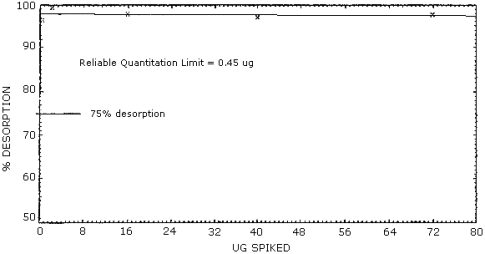
Figure 4.3. Reliable quantitation limit. 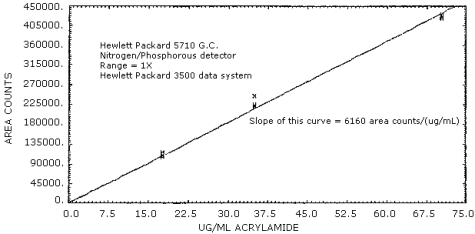
Figure 4.4. Sensitivity. 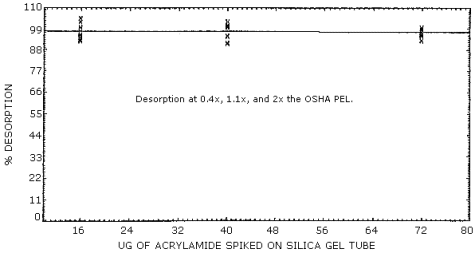
Figure 4.5. Desorption efficiencies. 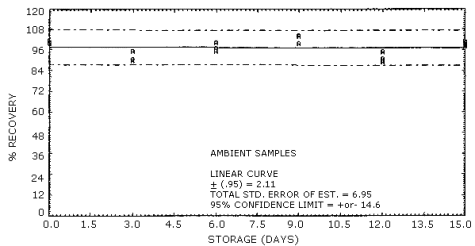
Figure 4.6. Ambient storage of acrylamide. 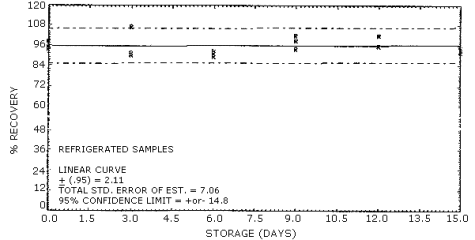
Figure 4.7. Refrigerated storage of acrylamide. 
Figure 4.8. Analytical standard (16 µg/mL).
5. References
- 5.1. "Occupational Exposure to Acrylamide." U.S. Department of
Health, Education, and Welfare. 1976, No. 17-112.
5.2. Skelly, N.E.; Husser, E.R. Analytical Chemistry 1978, 14, 1959-1962.
5.3. Proctor, N.H., Hughes, J.P. "Chemical Hazards of the Workplace" J.B. Lippincott Company: Philadelphia, 1978, p. 88.
5.4. The Condensed Chemical Dictionary" 8th Ed.; Hawley, G.G., Ed.; Van Nostrand Reinhold Company, 1971; p. 100.