DIETHYLNITROSAMINE
| Method no.: | 13 |
| Matrix: | Air |
| Target concentration: | 6 µg/m3 (1.4 ppb) |
| Procedure: | Collection on two Florisil adsorbent tubes in series,
each of which have been pretreated with 11 mg of |
| Detection limit based on recommended air volume: |
0.4 µg/m3 (96 ppt) |
| Recommended air volume and sampling rate: |
25 L at 0.2 L/min |
| Standard error of estimate at the target concentration: (Section 4.5.) |
7.4% |
| Special requirements: | The air sampler must be kept from light during and after sampling. |
| Status of method: | A sampling and analytical method which has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: August 1979 | Chemist: Warren Hendricks |
Organic Methods Evaluation Branch
OSHA Analytical
Laboratory
Salt Lake City, Utah
1. General Discussion
1.1. Background
1.1.1. History
Sample Collection: Diethylnitrosamine (DENA) vapors have been collected using the same methods used for dimethylnitrosamine. These methods include cryogenic techniques that involve the use of successive cold traps, ambient temperature KOH bubblers and Tenax GC cartridges (Ref. 5.1.).
Analytical: Analytical procedures for DENA are similar to those used for dimethylnitrosamine. The separation is usually by gas chromatography and detection by one or more of the following techniques: mass spectrometry, Coulson Electrolytic Conductivity Detector, Hall Electrolytic Conductivity Detector, nitrogen selective alkali flame-ionization detector, and Thermal Energy Analysis (Ref. 5.1.).
For several reasons, the existing sampling procedures are not adequate for use by OSHA personnel. Some of these reasons are - poor collection efficiency, low extraction efficiency and artifactual formation of DENA on the sampling device. Therefore, the primary emphasis of this work has been to develop new air sampling techniques. The analytical method utilizes separation by gas chromatography and detection with the Thermal Energy Analyzer (TEA). The TEA detector was selected because it is both sensitive and selective for nitrosamines.
1.1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Acute: The LD50 for DENA, administered by intraperitoneal injection to the rat, is 216 mg/kg. The lowest published toxic dose is 100 mg/kg also given by intraperitoneal injection to rats (Ref. 5.2.). The acute toxic effects from exposure to DENA are similar to those produced by dimethylnitrosamine with serious destruction of liver tissue as the most important result (Ref. 5.3.).
Chronic: There is no correlation between acute toxic effects and carcinogenic potential for nitrosamines. This is demonstrated by the fact that even though DENA has only about one-sixth the acute toxicity of dimethylnitrosamine (Ref. 5.2.), if administered continuously to rats, it is probably a more active liver carcinogen (Ref. 5.4.).
DENA has been shown to be carcinogenic to the mouse, the rat, the
hamster, the
A dose-response study has been conducted using rats. DENA was administered in drinking water and the daily exposure was between 0.075 and 14.2 mg/kg body weight in 9 groups of animals. The total dose, until death occurred, was between 64 and 965 mg/kg body weight. The tumor induction time was between 68 and 840 days. All daily doses higher than 0.15 mg/kg body weight gave a tumor incidence of 100%. When a dose of 0.15 mg/kg body weight per day was administered, a tumor yield of 90% was obtained. At 0.075 mg/kg body weight per day, 20 rats survived for more than 600 days and 11 of the 20 animals had tumors of the liver, esophagus, or the nasal cavity. All 4 of the animals that lived longer than 940 days at this dose level had tumors (Ref. 5.5.).
1.1.3. Worker exposure
The chemical reaction, in the condensed phase, between nitrous acid and diethylamine or triethylamine to form DENA is well known (Ref. 5.6.). Any tertiary amine that contains the diethyl moiety may react with a nitrosating agent to form DENA. Recently, it has been shown that both amines can react with oxides of nitrogen in the vapor phase to give DENA as a reaction product (Ref. 5.7.). This means that even though DENA is not used at a particular location, it may be formed from its precursors and therefore be found in the occupational environment.
Exposure to DENA can occur during operations in which diethylamine or triethylamine is utilized or produced. The exposure results because both amines are often contaminated with the nitrosamine. Further, if the amine is used as a chemical intermediate, DENA can possibly appear in the reaction product (Ref. 5.8.).
DENA is extensively used in cancer research facilities. Human exposure occurs when unchanged DENA is excreted by the laboratory animals (Ref. 5.9.).
DENA is not widely used by industry today. Uses or proposed uses
of DENA include: as a solvent in the fiber industry, as a softener
for copolymers, as an additive for lubricants, in condensers to
increase the dielectric constant and for the synthesis of
Non-occupational exposure to DENA can be a result of eating a normal western diet. It has been estimated that the weekly intake of preformed dialkyl and heterocyclic nitrosamines per person on a normal English diet is approximately 1 and 3 µg/week respectively (Ref. 5.10.). Another source of exposure to DENA is the endogenous formation of the agent in the gastrointestinal tract. Diethylamine has been shown to react with nitrite to form DENA in human gastric juice (Ref. 5.5.). DENA has been reported to be a component of tobacco and tobacco smoke (Ref. 5.11.).
1.1.4. Number of workers that face exposure - unknown
1.1.5. Physical properties (Ref. 5.5.)
| CAS no.: | 55-18-5 |
| synonyms: | diethylamine, N-nitroso; N,N-diethylnitrosamine; DEN; DENA; DANA |
| structure: | 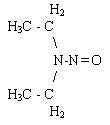 |
| mol wt: | 102.1 |
| physical appearance: | a yellow, volatile liquid |
| boiling point: | 177°C (760 mm Hg) 64-65°C (17 mm Hg) |
| density: | 0.9422 (20/4°C) |
| refractive index: | 1.4386 (20°C) |
| absorp. spec.: | max 230 nm; log e 3.86 |
| (in water) | max 332 nm; log e 1.98 |
| solubility: | about 10% in water; soluble in organic solvents and in lipids |
1.2. Detection limit, precision, sensitivity and working range
1.2.1. The detection limit for the analytical procedure is 50 pg.
The coefficient of variation is 0.14 at this level (Section 4.1.).
The detection limit was determined using
1.2.2. The pooled coefficient of variation for the analytical procedure over the range of 80 to 300 ng per sample is 0.034 (Section 4.2.). This represents an air concentration range of from 3.2 to 12.0 µg/m3 based on the recommended sampling and analytical procedures.
1.2.3. The sensitivity of the analytical procedure over a concentration range of 80 to 300 ng (3.2 to 12.0 µg/m3 based upon the recommended air sampling volume of 25 L) is 61918 area units (HP-5840A) per µg/mL. The sensitivity is determined by the slope of the calibration curve (Section 4.3.). The sensitivity will vary somewhat with the particular instrumentation used in the analysis.
1.2.4. The lower limit of the estimated working range, assuming adequate desorption efficiency, is 0.4 µg/m3. The upper limit of the working range is dependent on the capacity of the treated Florisil tubes.
1.3. Accuracy
1.3.1. The overall procedure must provide results that are within ±25% of the true value or better at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium during storage must be 75% or greater.
1.3.3. The overall procedure has met the above validation criteria (Section 4.5.).
1.4. Advantages
1.4.1. The sampling procedure is convenient.
1.4.2. The significance of artifactual formation of DENA upon the sampling device has been eliminated through pretreatment of the air sampler.
1.4.3. The analytical procedure is quick, sensitive, and reproducible.
1.4.4. Reanalysis of the samples is possible.
1.4.5. The samples are stable, even when stored at room temperature for 17 days.
1.4.6. Dimethylnitrosamine can be determined using the recommended sampling and analytical techniques. The desorption efficiency must be verified.
1.4.7. It may be possible to determine other nitrosamines simultaneously.
1.4.8. The effects of potential interferences are reduced through the use of a selective detector (the TEA) and can be further reduced by proper selection of GC parameters.
1.5. Disadvantage
The relative humidity of the sampled air affects the ability of the adsorbent to retain the analyte.
2. Sampling Procedure
2.1. Apparatus
2.1.1. An approved and calibrated personal sampling pump whose flow can be determined to ±5% at the recommended flow rate.
2.1.2. Florisil adsorbent tubes: Glass tubes, 6-mm o.d., 4-mm
i.d., 7-cm length, containing 100-mg front and 50-mg rear (separated
by a 2-mm portion of urethane foam or silylated glass wool) sections
of 20/40 mesh Florisil. SKC, Inc. Catalog No. 226-39 or equivalent.
Each tube is pretreated with 11 mg
2.2. Reagents
None required
2.3 Sampling technique
2.3.1. The air sampler is composed of two treated Florisil tubes in series. The tubes are easily connected with an end cap that has been modified by cutting off the closed portion.
2.3.2. Connect the air sampler to the sampling pump with flexible tubing. The 50-mg section of each tube should be positioned toward the sampling pump. Cover each tube of the air sampler with masking tape or other suitable material to prevent light from reaching the adsorbent.
2.3.3. The air sampler should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Sampled air should not pass through any hose or tubing before entering the sampling device.
2.3.5. Immediately after sampling, separate the air sampler into its component tubes, identify each tube as front or backup and seal each tube with plastic end caps. Also, wrap each samples end to end with official OSHA seals.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for sampling. This tube should be subjected to exactly the same handling as the samples (seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass vials with Teflon-lined caps. The samples must be kept from light. Bulk samples must not be put in the same mailing container used for the treated Florisil tubes.
2.4. Breakthrough
The relative humidity of the sampled air has a significant effect on the ability of the air sampler to retain DENA. However, laboratory studies indicate that 25 L of air containing the target concentration of DENA, at 80% relative humidity and 22°C, can be sampled with no loss of analyte. Breakthrough from the front to the rear tube, when the air sampler was challenged with vapors containing 0.15 µg DENA at these conditions, was about 30%.
No quantitative breakthrough information can be obtained from the analysis of individual tubes from a particular air sampler, but, if the amount found on the rear tube is 45% or greater of the total, then it is possible that some of the analyte may have been lost.
2.5. Desorption efficiency
2.5.1. The average desorption from
2.5.2. The desorption efficiency for DENA may vary from one lab
to another, and from one lot of
2.6. Recommended air volume and sampling rate
2.6.1. The recommended sampling rate is 0.2 L/min.
2.6.2. The recommended air volume is 25 L.
2.7. Interferences (sampling)
2.7.1 Since it is possible that the precursors of DENA - diethylamine, triethylamine, other tertiary amines with the diethyl moiety, and various nitrosating agents (oxides of nitrogen, nitrites, etc.), are present in the environment, it is conceivable that DENA may be formed upon the sampling device and not be present in the sampled air.
Laboratory studies indicate that it is possible to form DENA from
its precursors on untreated Florisil tubes. Further experiments show
that when the Florisil tube is treated with 11 mg of
2.7.2. At the present time, it is unknown if any compound would severely interfere with the collection of DENA on treated Florisil tubes. In general, the presence of other compounds will reduce the breakthrough volume for a particular compound.
2.7.3. Any compound which is suspected of interfering with the collection or analysis should be listed on the sampling data sheet.
2.7.4. Light will decompose DENA (Ref. 5.5.). The air sampler must be protected from light during and after sampling.
2.8. Safety precautions (sampling)
2.8.1. Observe due care when working with the sharp ends of the air sampler.
2.8.2. Attach the sampling equipment to the worker in such a manner that it will not interfere with work performance.
2.8.3. Follow all safety practices that apply to the work area being sampled.
3. Analytical Procedure
3.1. Apparatus
3.1.1. A gas chromatograph interfaced to a Thermal Energy Analyzer.
3.1.2. A GC column capable of resolving DENA from the desorption solvent and potential interferences. The column used in this work was a 10-ft × 1/8-in. stainless steel column containing 10% Carbowax 20M with TPA on 80/100 mesh Chromosorb W AW.
3.1.3. An electronic integrator or other suitable method to measure peak area.
3.1.4. An analytical balance capable of accurately weighing to 5 decimal places.
3.1.5. Vials. 2-mL vials with Teflon-lined caps.
3.1.6. Microliter syringes. 5-µL syringes for sample injections and other convenient sizes.
3.1.7. Pipets. Convenient sizes for diluting standards. A 1-mL dispenser pipet for desorbing solvent.
3.1.8. Volumetric flasks. Convenient sizes for diluting standards.
3.1.9. Dewar flasks. Convenient sizes for liquid nitrogen.
3.2. Reagents
3.2.1. DENA, authentic primary standard, 98% minimum.
3.2.2. Methyl alcohol, chromatographic grade.
3.2.3. Methylene chloride, chromatographic grade.
3.2.4. Isopropyl alcohol, chromatographic grade.
3.2.5. Ethyl alcohol, U.S.P., 95%.
3.2.6. Gases, purified GC grade helium and medical grade oxygen.
3.2.7. Nitrogen, liquid.
3.3. Sample preparation
3.3.1. The status of the OSHA seal on each sample is noted and recorded as intact, broken, or none.
3.3.2. The field and laboratory identification numbers on each seal are checked against those on the sample identification sheets.
3.3.3. Avoid exposure of the samples to light. DENA will photodecompose easily.
3.3.4. The front and rear tubes from each sampler are transferred to separate 2-mL vials.
3.3.5. The contents of each vial are desorbed with 1.0 mL of desorbing solution. The desorbing solution is composed of equal parts by volume methyl alcohol and methylene chloride.
3.3.6. The vials are sealed immediately with Teflon-lined caps and desorbed for 30 min with intermittent shaking.
3.4. Standard preparation
3.4.1. Stock standards are prepared by diluting a weighed amount of DENA with isopropyl alcohol. The stock standard is diluted to the working range with isopropyl alcohol (Section 3.8. Safety Precautions).
3.4.2. A solution composed of 0.15 µg/mL DENA in isopropyl alcohol is equivalent to an air concentration of 6.0 µg/m3 for a 25-L air sample desorbed with 1.0 mL of desorbing solution. This amount is not corrected for the desorption efficiency.
3.4.3. Standards are stored in dark bottles under refrigeration.
3.5. Analysis
3.5.1. GC conditions
| helium (carrier gas) flow rate: | 30 mL/min |
| injector temperature: | 200°C |
| column temperature: | 160°C |
| TEA transfer line temperature: | 205°C |
| injection volume: | 5 µL |
| elution time: | 3.5 min |
3.5.2. TEA conditions
| oxygen pressure: | 9 PSI |
| GC pyrolyzer furnace temperature: | 475°C |
| chamber vacuum: | 1.8 mm Hg |
| coarse zero: | high |
| calibrate: | 0.0 |
| attenuator: | 4 |
| cold trap temperature: | -130°C (ethyl alcohol and liquid nitrogen) |
Complete instructions for the TEA are found in its manual.
3.5.3. Chromatogram (Section 4.3.)
3.5.4. Peak areas are measured by an electronic integrator or other suitable means.
3.5.5. An external standard procedure is used to prepare a calibration curve from the analysis of at least three different standard solutions. The calibration curve is prepared daily. The integrator is calibrated to report results in micrograms per milliliter after correction for desorption efficiency.
3.5.6. Bracket the samples with analytical standards.
3.6. Interferences
3.6.1. Any compound that has the same GC retention as DENA and will elicit a response from the TEA detector is an interference. Possible interferences are listed on the sample data sheets.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single GC column is not proof of chemical identity. Samples should be confirmed by GC/Mass Spectrometry or other suitable means when required.
3.7. Calculations
3.7.1. The integrator value in micrograms per milliliter (corrected for desorption efficiency) is used for reference only. More reliable results are obtained by use of the calibration curve. The peak area, for each standard, is compared to its concentration in micrograms per milliliter (corrected for desorption efficiency) and the equation for the best straight line through the data points is determined by linear regression.
3.7.2. The concentration in micrograms per milliliter (corrected for desorption efficiency) for a particular sample is determined by comparing its area to the calibration curve.
3.7.3. Analytical results from the two tubes that compose an air sampler are added together.
3.7.4. The air concentration for a sample result is calculated by the following equation:
DENA, µg/m3 = (A)(B)(1000)/C
| where | A | = | µg/mL from 3.7.3. |
| B | = | desorption volume | |
| C | = | air volume in liters |
3.7.5. To convert micrograms per cubic meter to parts per billion (ppb), the following relationship is used:
DENA, ppb = (µg/m3)(24.46)/102.1
| where | µg/m3 | = | result from 3.7.4. |
| 24.46 | = | molar volume at 25°C and 760 mm Hg | |
| 102.1 | = | molecular weight of DENA |
3.8. Safety precautions (analytical)
3.8.1. DENA is an extremely potent carcinogen and utmost care must be exercised when working with this compound.
3.8.2. Avoid skin contact with liquid nitrogen and the solvents.
3.8.3. Confine the use of solvents to a fume hood.
3.8.4. Wear safety glasses in all laboratory areas.
3.8.5. Check to be sure that the TEA exhaust is connected to a fume hood.
4. Backup Data Section
4.1. Detection limit
The following data were generated by replicate 5-µL injections of a standard solution whose concentration was 0.01 µg/mL. The detection limit was determined to be 50 pg which is equivalent to 0.4 µg/m3 based on the recommended air volume. Peak heights were used because integrated data were unreliable at this level.
Table 4.1.
Detection Limit Data
|
| ||
| injection | peak height, mm | statistics |
|
| ||
| 1 2 3 4 5 6 |
87 82 67 62 88 80 |
SD = 10.74 CV = 0.14 |
|
| ||
4.2. Precision
These data represent multiple injections of standard solutions. The injection volume was 5 µL and the concentrations of the standards were 0.08 µg/mL, 0.15 µg/mL and 0.30 µg/mL. Peaks were integrated by a Hewlett-Packard 5840A Gas Chromatograph.
Table 4.2.
Analytical Precision
|
| |||
| × target conc. pg |
0.5× 400 |
1.0× 750 |
2.0× 1500 |
|
| |||
SD CV |
4635 4318 4184 4313 4281 4061 4298.67 191.65 0.0446 |
8714 8792 8954 8872 8982 9128 8907 147.22 0.0165 |
18090 18980 17220 17850 18250 17490 17980 619.61 0.0345 |
|
| |||
4.3. Sensitivity
A typical calibration curve is shown in Figure 4.3.1. The slope of the curve indicates the sensitivity of the method.
A representative chromatogram is shown in Figure 4.3.2.
4.4. Desorption
Samples representing 3.2, 6.0 and 12.0 µg/m3 based on 25-L air volumes were prepared by injecting liquid standards on treated Florisil tubes.
Table 4.4.
Desorption Efficiency
|
| |||
| µg/m3 | 3.2 | 6.0 | 12.0 |
|
| |||
| desorption efficiency, % |
95.0 82.5 87.5 112.5 95.0 95.0 94.6 |
100.7 90.7 89.3 92.7 92.0 94.0 93.2 |
87.0 91.3 105.7 99.7 102.0 103.0 98.1 |
| overall average = 95.3% | |||
|
| |||
4.5. Storage
Samples were generated by the liquid injection of DENA on Polar Partition tubes containing about 150 mg of adsorbent. The tubes were allowed to equilibrate overnight and then were placed in front of the treated Florisil tubes. Twenty-five liters of air, at 80% relative humidity and 22°C, were drawn through the sampling train. The DENA on the Polar Partition tube was desorbed by the humid air and was deposited on the air sampler. Studies conducted at ambient relative humidity and temperature indicated that desorption was essentially complete after 1 L of air had passed through the Polar Partition tube (Figure 4.5.1.). In these studies the spiked Polar Partition tube was connected directly to the TEA.
The data in Table 4.5. represent the effects of storage at ambient
(21-26°C) and reduced
Table 4.5.
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (refrigerated) | (ambient) | |||||
|
| |||||||
| 0 3 6 10 13 17 |
90.0 87.3 96.0 96.7 100.7 104.0 |
90.7 87.3 103.3 96.7 93.3 89.3 |
94.0 86.7 91.3 95.3 96.0 104.7 |
93.3 82.7 82.7 84.7 88.7 84.0 |
91.3 94.0 84.0 81.3 98.0 89.3 |
91.3 82.0 84.7 92.7 94.0 94.7 | |
|
| |||||||
4.6. Preparation of sampling tubes
4.6.1. Reagents
Methylene chloride, chromatographic grade.
4.6.2. Technique
Each Florisil tube is treated with 11 mg
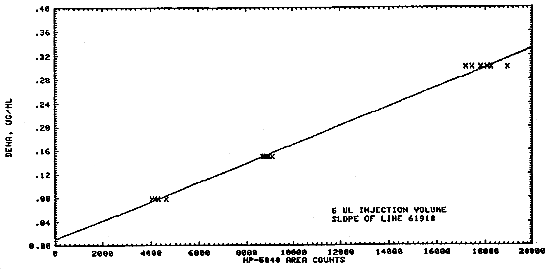
Figure 4.3.1. Calibration curve for diethylnitrosamine.
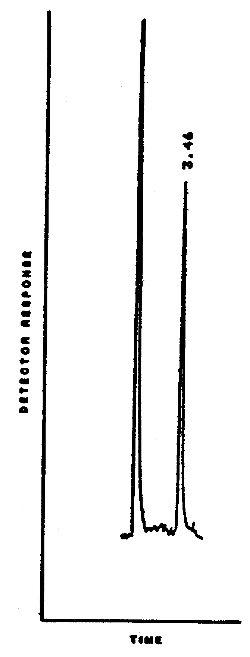
Figure 4.3.2. A typicdal chromatogram for
diethylnitrosamine. 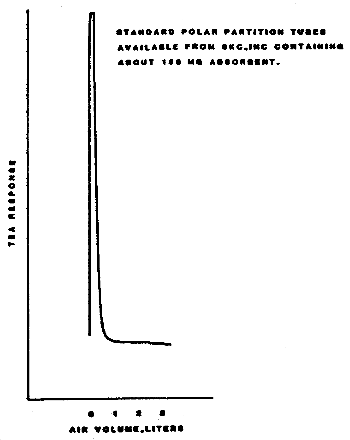
Figure 4.5.1. The rate at which diethylnitrosamine is
desorbed from Polar Partition adsorbent by air at ambient temperature
and relative humidity. 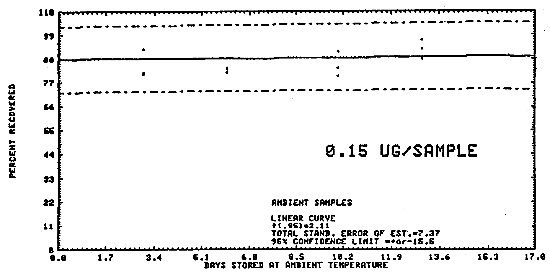
Figure 4.5.2. Ambient temperature storage test for
diethylnitrosamine. 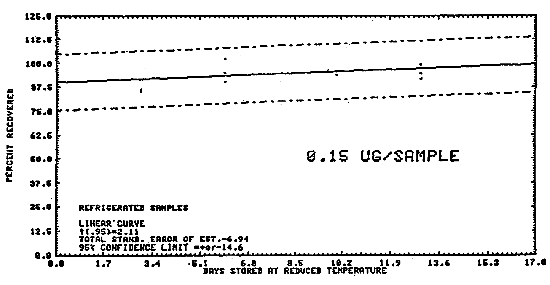
Figure 4.5.3. Refigerated temperature test for diethylnitrosamine.
5. References
5.1. W. Hendricks, Dimethylnitrosamine (Method 06, Organic Methods Evaluation Branch, OSHA Analytical Laboratory, Salt Lake City, Utah), Unpublished.
5.2. Registry of Toxic Effects of Chemical Substances, 1976 Edition. (H.E. Christensen, E.J. Fairchild, Eds., B.S. Carroll, R.J. Lewis, Project Coordinators) U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, U.S. Government Printing Office, Washington, D.C. (1976).
5.3. P.N. Magee and J.M. Barnes, Carcinogenic Nitroso Compounds, Advances in Cancer Research 10, 163-247 (1967).
5.4. D. Druckey, R. Preussmann, S. Ivankovic and D. Schmahl, Organotrope Carcinogene Wirkany bei 65 Verschiedenen N-Nitroso-ver-bindungen and BD-Ratten, Z. Krebsforsch, 69(103), 103-201 (1967).
5.5. IARC Monographs on the Evaluation of Carcinogenic Risk of
Chemicals to Man, Vol. 1, International Agency for Research on Cancer,
Lyon, France, World Health Organization,
5.6. J. March, Advanced Organic Chemistry: Reactions, Mechanisms
and Structure,
5.7. J.N. Pitts, P. Grosjean, K.V. Cauwenberghe, J.P. Schmid, and D.R. Fitz, Photooxidation of Aliphatic Amines Under Simulated Atmospheric Conditions: Formation of Nitrosamines, Nitramines, Amides and Photochemical Oxidant, Environmental Science and Technology, 12(8) 946-953 (1978).
5.8. B. Spiegelhander, G. Eisenbrand and R. Preussman,
Contamination of Amines With
5.9. P. Issenberg and H. Sornson, A Monitoring Method for Volatile Nitrosamine Levels in Laboratory Atmospheres, Environmental N-Nitroso Compounds Analysis and Formation, E.A. Walker and P. Bogovski, Eds., Lyon, France, IARC Scientific Publication No. 14 (1976).
5.10. T. Kakizoe, T.T. Wang, VW.S. Eng., R. Farrer, P. Dion and R.
Bruce, Volatile
5.11. K. D. Brunnemann, L. Ye and D. Hoffmann, Assessment of
Carcinogenic Volatile