| Method no.: | 11 |
| Matrix: | Air |
| Target concentration: | 10 ppm (55 mg/m3) OSHA PEL |
| Procedure: | Collection on charcoal adsorbent, desorption with carbon disulfide, analysis by GC using a flame ionization detector. |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min |
| Detection limit of the overall procedure: |
0.05 ppm (0.14 mg/m3) |
| Standard error of estimate at the PEL (Section 4.4.): |
6% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: February 1980 | Chemist: Duane E. Lee |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
Gas chromatography is by far the best analytical technique for determining trace amounts of 1,1,2-trichloroethane. It offers speed, sensitivity, and selectivity. Published NIOSH methodology for 1,1,2-trichloroethane proposes the collection of air samples on charcoal adsorbent and analysis by GC with an flame ionization detector. (Ref. 5.1.) This procedure was further evaluated in order to obtain additional data on storage stability of collected samples and collection capacity in humid air.
1.1.2. Toxic Effects (This section is for information only and should not be taken as a basis for OSHA policy.)
1,1,2-Trichloroethane is a mucous-membrane irritant and a central nervous system depressant in animals. It is expected that severe exposure will produce similar effects in humans. (Ref. 5.2.)
Rats which were exposed to 2000 ppm 1,1,2-trichloroethane for four hours died within 14 days. (Ref. 5.3.) Mice treated by intraperitoneal injection with anesthetic doses showed moderate hepatic dysfunction and renal dysfunction; at autopsy, findings were centrolobular necrosis of the liver and tubular necrosis of the kidneys; the LD50 for introperitoneal injection was 0.34 mL/kg. (Ref. 5.4.)
The TLV was set at a level to prevent injury to the liver and provide freedom from irritation and narcosis. (Ref. 5.5.) Recently, the National Cancer Institute (NCI) has concluded that 1,1,2-trichloroethane is carcinogenic in mice. Liver cancer was induced when 1,1,2-trichloroethane was administered with corn oil by gastric intubation (stomach tube) five days a week for 78 weeks. (Ref. 5.6.) On the basis of the NCI data, NIOSH recommends that it would be prudent to handle 1,1,2-trichloroethane in the workplace as if it were a human carcinogen. (Ref. 5.7.)
1.1.3. Worker exposure
NIOSH estimates that 112,000 workers are exposed to 1,1,2-trichloroethane. (Ref. 5.7.)
1.1.4. Use and operations where exposure occurs
1,1,2-Trichloroethane is used as a chemical intermediate and as a solvent. It is not as widely used as its isomer 1,1,1-trichloroethane. Exposure may occur in a variety of occupations, including organic chemical synthesizers and solvent makers. (Ref. 5.8.)
1.1.5. Physical properties (Ref. 5.9.)
| physical state: | colorless liquid |
| molecular weight: | 133.42 |
| specific gravity: | 1.443 (20/4°C) |
| melting point: | -36.7°C |
| boiling point: | 113.5°C |
| vapor density: | 4.6 (air = 1) |
| vapor pressure: | 25 mm Hg at 25°C |
| molecular formula: | CHCl2CH2Cl |
| synonyms: | vinyl trichloride; beta-trichloroethane |
1.2. Limit defining parameters
- 1.2.1. Detection limit of the analytical procedure
The detection limit of the analytical procedure is 1.4 ng per injection. This is the amount of analyte which will give a peak whose height is about 5 times the height of the baseline noise. (Section 4.1.1.)
1.2.2. Detection limit of the overall procedure
The detection limit of the overall procedure is 1.4 µg per sample (0.03 ppm, or 0.14 mg/m3). This is the amount of analyte spiked on the sampling device which allows recovery of an amount of analyte equivalent to the detection limit of the analytical procedure. (Section 4.1.2.)
1.2.3. Reliable quantitation limit
The reliable quantitation limit is 1.4 µg per sample (0.03 ppm, or 0.14 mg/m3). This is the smallest amount of analyte which can be quantitated within the requirements of 75% recovery and 95% confidence limits of ±25%. (Section 4.1.2.)
The reliable quantitation limit and detection limits reported in the method are based upon optimization of the instrument for the smallest possible amount of analyte. When the target concentration of an analyte is exceptionally higher than these limits, they may not be attainable at the routine operating parameters.
- 1.2.4. Sensitivity
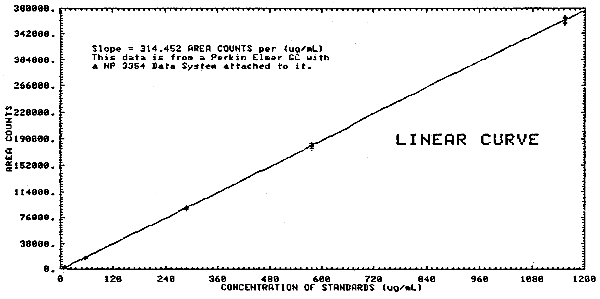
The sensitivity of the analytical procedure over a concentration range representing 0.02 to 2 times the PEL concentration based on the recommended air volume is 314 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.3.) The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.5. Recovery
The recovery of analyte from the collection medium after storage must be 75% or greater. The average recovery over the range of 0.5 to 2 times the PEL or target concentration is 91.8%. (Section 4.1.2.)
1.2.6. Precision (analytical method only)
The pooled coefficient of variation obtained from replicate determinations of analytical standards at 0.02, 0.1, 0.5, 1, and 2 times the PEL or target concentration is 0.026. (Section 4.3.)
1.2.7. Precision (overall procedure)
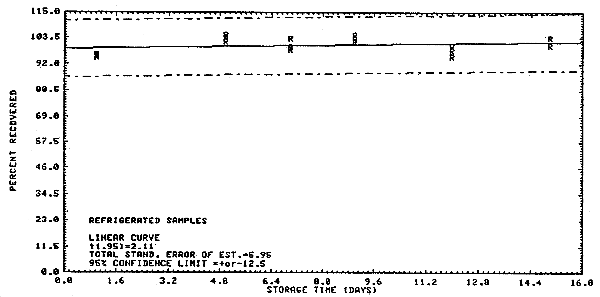
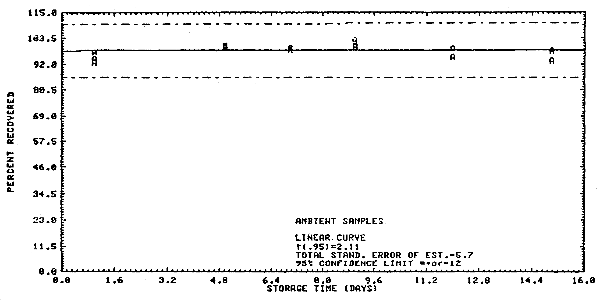
The precision at the 95% confidence level for the 15-day storage test is ±12%. (Section 4.4.) This includes an additional ±5% for sampling error. The overall procedure must provide results at the target concentration that are ±25% or better at the 95% confidence level.
1.3. Advantages
- 1.3.1. The sampling procedure is convenient.
1.3.2. The analytical procedure is quick, sensitive, and reproducible.
1.3.3. Reanalysis of the samples is possible.
1.4. Disadvantages
If other compounds are present, the GC run time must be lengthened so the late eluting peaks will not interfere with the next sample.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined with ±5% at the recommended flow.
2.1.2. Charcoal tubes: Glass tube, with both ends heat-sealed,
7.0 cm × 6-mm o.d. ×
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break open the ends of the
charcoal tube. All tubes must be from the same lot.
2.3.2. Connect the charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.5. Seal the charcoal tube with plastic caps immediately after sampling. Also, seal each sample with OSHA sealing tape lengthwise.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the charcoal tubes.
2.4. Breakthrough
A sample was taken on the primary portion of a charcoal tube (SKC Lot 107) at a sampling rate of 0.198 L/min from a controlled test atmosphere. The controlled test atmosphere was 19.8 ppm 1,1,2-trichloroethane with an average relative humidity of 80.4% at 22°C. The 5% breakthrough volume was 37.7 L.
2.5. Desorption efficiency
The desorption efficiency from liquid injections on charcoal tubes (SKC Lot 107), averaged 91.8% for 0.29 mg to 1.15 mg per tube, which covers the loading range of about 0.05 to 2 times the target concentration for a 10-L air volume. (Section 4.1.2.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences (sampling)
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of 1,1,2-trichloroethane on
charcoal. In general, the presence of other solvents will decrease
the breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering with the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
the tubes.
2.8.2. The broken ends of the tubes should be protected to avoid injury to the person being sampled.
2.8.3. When working in environments containing flammable vapors, do not provide any spark source from equipment used or pumps.
2.8.4. Observe all safety practices for working in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph equipped with a flame ionization
detector.
3.1.2. A number of GC columns are available and adequate. The column used for this study was a 10-ft × 1/8-in. stainless steel 7% Penta 100/120 Chrom P AW.
3.1.3. An electronic integrator or other suitable method of measuring peak areas.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 10-µL for preparing standards, 1-µL for sample injections.
3.1.6. Pipets for diluting standards. A 1-mL pipet for dispensing solvent for desorption, or a 1-mL dispenser.
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Carbon disulfide, chromatographic grade.
3.2.2. 1,1,2-Trichloroethane, reagent grade.
3.2.3. Purified GC grade helium, hydrogen, and air.
3.3. Standard preparation
- 3.3.1. Standards are prepared by diluting pure
1,1,2-trichloroethane with carbon disulfide.
3.3.2. Four microliters of 1,1,2-trichloroethane per 10 mL of carbon disulfide equals 10.6 ppm for a 10-L air sample desorbed with 1 mL of carbon disulfide.
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate 2-mL vials.
3.4.2. Each section is desorbed with 1.0 mL of carbon disulfide.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.5. Analysis
- 3.5.1. GC conditions
| helium (carrier gas) flow rate: | 25.1 mL/min |
| injector temperature: | 150°C |
| detector temperature: | 200°C |
| column temperature: | 120°C |
| detector: | flame ionization |
| hydrogen flow rate: | 43 mL/min |
| air flow rate: | 248 mL/min |
| injection size: | 1 µL |
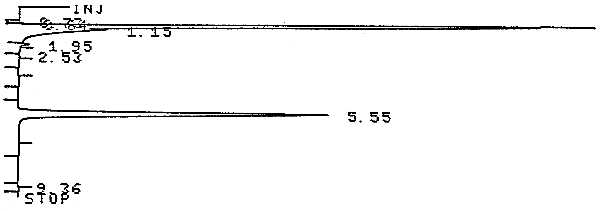
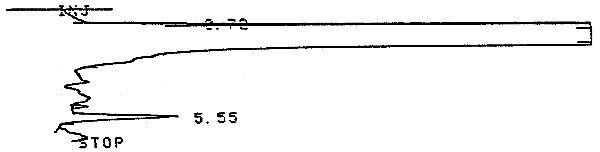
3.5.2. Chromatogram (Figure 3.5.2.)
3.5.3. Peak areas are measured by an electronic integrator or other suitable means.
3.5.4. An external standard procedure is used. The integrator is calibrated to report results in ppm for a 10-L air sample after correction for desorption efficiency.
3.6. Interferences (analytical)
- 3.6.1. Any compound having the same general retention time as
1,1,2-trichloroethane is an interference. Possible interferences are
listed on the sample data sheets. GC parameters should be chosen so
these interferences will pose no problems.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Usually the integrator is programmed to report results in ppm (corrected for desorption efficiency) for a 10-L air sample. The following calculation is used:
| ppm = A/(0.1)(B) | where | A = ppm on report B = air volume (L) |
4. Backup Data
- 4.1. Detection limit data
- 4.1.1. Analytical detection limit
The analytical detection was defined as the amount of analyte that would produce a peak whose height is about 5 times that of the baseline noise. The analytical detection limit was determined with an analytical standard that contained 0.001 µL of analyte per milliliter of carbon disulfide or 1.4 µg/mL. A chromatogram is shown in Figure 4.1.1.
Reproducibility of the peak produced by 1.4 ng injections of analyte was good. Ten injections gave an average analyte peak height of 26 mm with a coefficient of variation of 9.6%.
A sample collected from 10 L of air which contained 1.4 ng/µL after desorption with 1 mL of carbon disulfide would represent an air concentration of 0.03 ppm (0.14 mg/m3).
4.1.2. Desorption efficiency data for the determination of the overall detection limit and the reliable quantitation limit of the procedure
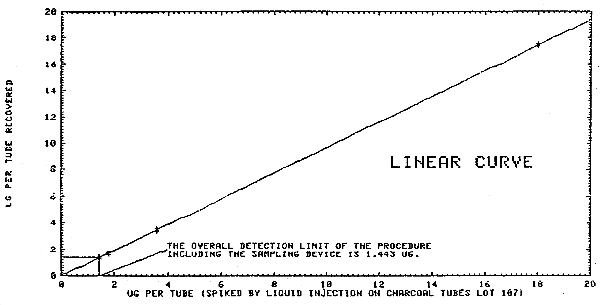
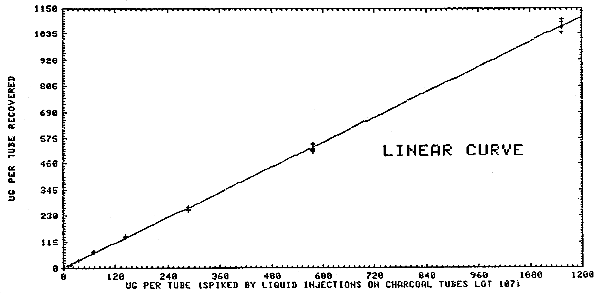
Liquid injections were made on the front portion of charcoal tubes (SKC Lot 107) at 1.44 to 1154 µg (2.0 times the target concentration). These were refrigerated overnight and desorbed and analyzed the following day. The results are given in Table 4.1.2. and and plotted as micrograms recovered in Figures 4.1.2.1. and 4.1.2.2. The overall detection limit was determined to be 1.4 µg/sample in Figure 4.1.2.1.
Desorption Efficiencies for Various Sampler Loadings
|
| ||||||||||
| µg/sample | 1154.4 | 577.2 | 288.6 | 144.3 | 72.15 | 36.08 | 18.04 | 3.608 | 1.804 | 1.443 |
|
| ||||||||||
| desorption | 92.5 | 91.8 | 89.4 | 97.1 | 97.7 | 96.3 | 96.3 | 93.9 | 89.7 | 90.6 |
| efficiency, | 92.6 | 92.1 | 90.0 | 95.8 | 96.5 | 96.2 | 96.8 | 95.1 | 99.5 | 99.8 |
| % | 90.4 | 91.3 | 90.1 | 97.3 | 100.1 | 97.7 | 97.5 | 98.8 | 97.0 | 101.1 |
| 92.4 | 89.2 | 90.7 | ||||||||
| 92.3 | 90.3 | 89.6 | ||||||||
| 93.3 | 90.6 | 91.5 | The average desorption efficiency over the range of 1154 to 289 µg (about 2 to 0.5 times the target concentration) is 91.8%. | |||||||
| 94.6 | 96.2 | 88.4 | ||||||||
| 95.5 | 95.2 | 94.3 | ||||||||
| 93.0 | 92.1 | 91.0 | ||||||||
|
| ||||||||||
4.2. Reliable quantitation limit
The reliable quantitation limit was verified to be the same as the overall detection limit by liquid spiking six samples with loadings equivalent to the overall detection limit (1.443 µg/sample). These samples were analyzed to assure that the requirements of at least 75% recovery with a precision (1.96 SD) of at least ±25% were met.
Reliable Quantitation Limit
|
| |||
| Sample no. | % recovered | sample no. | % recovered |
|
| |||
| 1 | 105.8 | 4 | 103.8 |
| 2 | 96.2 | 5 | 96.2 |
| 3 | 100.0 | 6 | 88.5 |
|
| |||
4.3. Analytical precision and sensitivity data
Multiple injections were made of standards that were prepared over a range of 0.02 to 2.1 times the target concentration OSHA standard. A standard deviation was determined at each concentration. The pooled coefficient of variation was determined for the range. The response data reported below was used to determine the calibration curve in Figure 4.3.
Analytical Precision
|
| |||||
| × target conc. µg/sample |
0.02× 11.54 |
0.1× 57.72 |
0.53× 288.6 |
1.06× 577.2 |
2.1× 1154.4 |
|
| |||||
| area
counts SD CV |
3539 3535 3572 3463 3911 3936 3659.3 207.8 0.057 |
17953 17870 17633 17704 17642 17458 17710 178.2 0.010 |
89646 88686 89798 90085 89398 89526 89523 473.9 0.005 |
180804 179293 179752 179471 182614 177122 179842 1814.1 0.010 |
367036 365116 364154 364234 358346 361497 356397 365574 362794 3730.7 0.010 |
|
| |||||
4.4. Storage test
Samples were collected on SKC Lot 107 charcoal tubes from a
generated test atmosphere containing 10.4 ppm 1,1,2-trichloroethane
with an average relative humidity of 83.3% at
Storage Tests
|
| |||||||
| storage time | % recovery | ||||||
| (days) | (-4°C to 4°C) | (17.1°C to 21°C) | |||||
|
| |||||||
| 1 5 7 9 12 15 |
95.3 102.1 99.5 102.1 100.1 100.4 |
96.3 104.8 103.3 102.9 95.4 103.9 |
95.5 103.3 98.6 105.0 98.3 100.4 |
94.2 99.4 98.5 99.8 95.3 98.1 |
97.3 100.6 99.4 102.7 98.8 93.5 |
93.0 99.8 98.3 95.0 95.0 97.7 | |
|
| |||||||
5. References
- 5.1. National Institute of Occupational Safety and Health, "NIOSH
Manual of Analytical Methods", 2nd Ed., Washington, D.C.: U.S.
Government Printing Office, DHEW (NIOSH) Publication No. 77-157-B,
Vol. 2, pp. S134-1 to S134-9, 1977.
5.2. Proctor, N.H., and Hughes, I.P., "Chemical Hazards of the Workplace", Philadelphia: J.B. Lippincott, Co., pp. 489-490, 1978.
5.3. Carpenter, C.P., Smyth, H.F., and Pozzani, U.C., J. Ind. Hyg. Toxicol., 31:343, 1949.
5.4. Klassen, C.D., and Plaa, G.L., Toxicol. Appl. Pharmacol., 9:139, 1966.
5.5. American Conference of Governmental Industrial Hygienists, "Documentation of the TLV's for Substances in Workroom Air", 3rd Ed., Cincinnati, p. 136, 1976.
5.6. National Cancer Institute, "Bioassay of 1,1,2-Trichloroethane for Possible Carcinogenicity", Carcinogenesis Technical Report Series No. 74, Washington, D.C.: U.S. Government Printing Office, DHEW (NIH) Publication No. 78-1324, 1978.
5.7. National Institute of Occupational Safety and Health, "NIOSH Current Intelligence Bulletin No. 27", Washington, D.C.: U.S. Government Printing Office, DHEW(NIOSH) Publication No. 78-181, 1978.
5.8. National Institute of Occupational Safety and Health, "Occupational Diseases, A Guide to Their Recognition", Rev. Ed., Washington, D.C., U.S. Government Printing Office, DHEW(NIOSH) Publication No. 77-181, pp. 216-217, 1977.
5.9. Patty, F.A., (editor) "Industrial Hygiene and Toxicology", 2nd Ed., New York: John Wiley and Sons, Inc., Vol. II., pp. 1290-1291, 1963.