| Method no.: | 08 |
| Matrix: | Air |
| Target concentration: | 1 ppm |
| Procedure: | Collection on charcoal, desorption with carbon disulfide, analysis by GC with a flame ionization detector. Samples should be stored in a refrigerator and analyzed as soon as possible. |
| Detection limit based on recommended air volume: |
0.2 ppm |
| Recommended air volume and sampling rate: |
5.0 L at 0.2 L/min |
| Standard error of estimate at the target concentration: (Section 4.2.) |
8.6% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: May 1979 | Chemist: Dee R. Chambers |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In September 1978, NIOSH and OSHA issued "Current Intelligent Bulletin Number 28", which recommended that vinyl bromide be considered as a potential human carcinogen in the workplace. In addition, they indicated that safe levels of exposure to carcinogens have not been demonstrated, but lowered exposure to carcinogens may, in general, decrease the probability of cancer development.
There is not an exposure standard for vinyl bromide at the present time. A target level of 1 ppm was selected because of limitations of analytical detection and because 1 ppm is the PEL for vinyl chloride (Ref. 5.1.).
1.1.2. Toxic effects
Laboratory studies have demonstrated that exposure to vinyl bromide caused angiosarcoma of the liver and other cancers in animals. At levels of 10 ppm, exposure induced lymph node angiosarcoma. Additionally, adverse health effects in animals attributed to vinyl bromide include central nervous system effects, cardiovascular effects, respiratory effects, skin effects, skeletal effects, and liver and spleen abnormalities. (Ref. 5.1.)
To date, there have been no reported cases of cancer in humans associated with exposure to vinyl bromide; however, vinyl bromide has been in commercial production in the United States only since 1971.
1.1.3. Uses
Industries which use vinyl bromide include: chemical and allied products, rubber and plastic products, leather products, fabricated metal products, and wholesale trade.
1.1.4. Approximately 360 workers are exposed to vinyl bromide and an additional 26,000 workers are potentially exposed (Ref. 5.1.).
1.1.5. Physical properties
CH2CHBr molecular weight 107
Vinyl bromide is a gas which is flammable and poses a dangerous fire risk. Its melting point is -138°C and its boiling point is 15.6°C. The specific gravity of vinyl bromide is 1.51. (Ref. 5.2.)
1.2. Detection limit, precision, sensitivity, and working range
- 1.2.1. The detection limit for the analytical procedure is 4 ng
per injection. This is based on 1-µL injections.
1.2.2. The pooled coefficient of variation of the analytical method over the range equivalent to 1.0 ppm to 10 ppm based on the recommended air volume is 1.1%. (Section 4.1.)
1.2.3. The sensitivity of the analytical procedure at 1 ppm based on a 5-L air volume is 250 area units per µg/mL. The sensitivity is determined by the slope of the calibration curve. The sensitivity will vary with the particular instrument used in the analysis. (Section 4.2.)
1.2.4. The lower limit of the estimated working range is 0.2 ppm. The upper limit is dependent on the capacity of the charcoal.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% of the true value or better at the 95% confidence level.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or better.
1.3.3. The overall procedure meets the above criteria. (Section 4.4.)
1.4. Advantages
The method of sampling is convenient. Charcoal tubes are easily transported to the laboratory through the mail. The analytical method is quick, and automation of the process is possible.
1.5. Disadvantages
It may be difficult to analyze for additional components on the same charcoal tube.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined within ±5% at 0.2 L/min.
2.1.2. Charcoal tubes: Glass tube, with both ends heat sealed,
7.0 cm ×
2.2. Reagents
None required
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break the ends of the
charcoal tubes. All tubes must be from the same lot.
2.3.2. Connect a charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup section and should be positioned nearer the pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tubes.
2.3.5. Seal the charcoal tubes with plastic caps immediately after sampling.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.4. Breakthrough
At the sampling rate of 0.2 L/min, an atmosphere at 80% relative humidity containing 1 ppm vinyl bromide was sampled for 5 L with no breakthrough from the primary portion of the charcoal tube. If the concentration is thought to be extremely high in the workplace, two charcoal tubes may be connected in series and/or the sampling rate lowered, together with a smaller air volume sampled to prevent breakthrough.
2.5. Desorption efficiency
- 2.5.1. The desorption efficiency may vary from laboratory to
laboratory and for each lot of charcoal.
2.5.2. The desorption efficiency at 8.8 µg loadings (0.4 ppm at recommended air volume) was 100%. The desorption efficiency at 17.5 µg loadings (0.8 ppm at recommended air volume) was 100%, and the desorption efficiency at 35 µg loadings (1.6 ppm at recommended air volume) was 95%. (Section 4.3.)
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 5 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences (sampling)
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of vinyl bromide on charcoal.
In general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
charcoal tubes.
2.8.2. Do not provide any spark source from pumps or other equipment when working in environments containing flammable vapors.
2.8.3. Observe all usual safety practices when sampling in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. Stainless steel column (20 ft × 0.125 in.) packed with 10% SE-30 on 80/100 mesh Chromosorb W (acid washed, silanized with dimethyl dichlorosilane). Other columns capable of performing the required separation may be used.
3.1.3. An electronic or mechanical integrator or some other method of determining peak area.
3.1.4. Vials (2-mL) that can be sealed with caps containing Teflon-lined septa.
3.1.5. Microliter syringe, 1-µL for injecting samples.
3.1.6. One-milliliter gas-tight syringe for preparing standards.
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Carbon disulfide, spectroquality or better.
3.2.2. Vinyl bromide, pure or known concentration.
3.2.3.
3.2.4. Purified helium carrier gas.
3.2.5. Purified hydrogen and air.
3.2.6. Desorbing reagent contains 0.1 mL
3.3. Standard preparation
Standards are prepared by injecting with a gas-tight syringe an
amount of vinyl bromide into a volumetric flask containing desorbing
reagent. The
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate vials.
3.4.2. Each section is desorbed with 1.0 mL desorbing reagent.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermediate shaking.
3.5. Analysis
- 3.5.1. GC conditions
| flow rates (mL/min) | temperature (°C) | ||
| helium: | 25 | injector: | 200 |
| hydrogen: | 35 | detector: | 250 |
| air: | 250 | column: | 125 |
| injection size: | 1 µL | ||
| elution time: | 1.5 min (vinyl bromide) | ||
3.5.2. Peak areas are measured by an electronic integrator or other suitable means.
3.5.3. An internal standard procedure is used. The integrator is calibrated to report results in ppm for a 1-L air volume.
3.5.4. The amount must be corrected for the desorption efficiency.
3.6. Interferences (analytical)
- 3.6.1. Any compound having the same general retention time of
vinyl bromide or
3.6.2. Retention time data on a single column is not considered proof of chemical identity. Samples over the PEL should be confirmed by GC/MS or other suitable means.
3.7. Calculations
- 3.7.1. Since the integrator is programmed to report results in
ppm (at 25°C and 760 mm Hg, and corrected for desorption efficiency)
for a 1-L air volume, the following calculation is used:
| where | A | = | ppm on report |
| B | = | air volume |
3.7.2. Add both charcoal tubes (if they were in series) before reporting the results.
3.8. Safety precautions
- 3.8.1. All work done with the solvents and with the vinyl
bromide gas should be done in a hood.
3.8.2. Avoid any skin contact with all the solvents.
3.8.3. Vinyl bromide should be considered a human carcinogen, and all work with vinyl bromide should be done using appropriate "carcinogen" safeguards.
4. Backup Data
- 4.1. Coefficient of variation of analytical technique
The coefficient of variation was determined from multiple injections of analytical standards which covered a concentration range equivalent to 1.0 ppm to 10 ppm, based on a 5-L sample.
Analytical Precision
|
| ||||
| µg/mL | 220 | 110 | 44 | 22 |
|
| ||||
| area | 59020 | 31240 | 12550 | 5547 |
| counts | 58909 | 31328 | 12530 | 5417 |
| 58673 | 30955 | 12350 | 5502 | |
| 58781 | 30266 | |||
| 58846 | 30947 | 12477 | 5489 | |
| SD | 151 | 481 | 110 | 66 |
| CV | 0.0026 | 0.016 | 0.0088 | 0.012 |
|
| ||||
4.2. Sensitivity
The sensitivity is determined from the slope of the calibration curve shown in Figure 4.2.
(5489 area counts)/(22 µg/mL) = 250 area counts/µg/mL
4.3. Desorption efficiency
Three groups of six, Lot 106, charcoal tubes were respectively spiked with 5, 10, and 20 µL of a 400 µL vinyl bromide/mL CS2 solution. The backup portion of the tubes had been removed prior to spiking. The tubes were allowed to equilibrate.
Desorption Efficiency
|
| |||
| µg/sample | 8.8 | 17.5 | 35 |
|
| |||
| desorption | 105 | 100 | 96 |
| efficiency, | 100 | 100 | 95 |
| % | 99 | 98 | 95 |
| 111 | 98 | 98 | |
| 115 | 99 | 93 | |
| 100 | 104 | ||
| 105 | 100 | 95 | |
|
| |||
4.4. Storage test
The percent recoveries are not corrected for desorption efficiency. Samples were collected at 80% relative humidity. The loadings were approximately 10 µg/sample. One group of samples were stored at ambient temperature and a second group at reduced temperature. Three samples from each group were analyzed at intervals throughout a 16-day period. These data are presented graphically in Figures 4.4.1. and 4.4.2.
Storage Tests
|
| ||||||
| storage time | % recovery | |||||
| (days) | (refrigerated) | (ambient) | ||||
|
| ||||||
| 0 | 84.1 | 81.0 | 84.0 | 81.4 | 81.8 | 86.8 |
| 3 | 88.2 | 99.9 | 100.5 | 94.9 | 87.3 | 92.5 |
| 7 | 106.7 | 95.1 | -- | 100.8 | 91.2 | 99.0 |
| 9 | 83.0 | 84.0 | 87.0 | 100.8 | 80.9 | 89.1 |
| 13 | 93.0 | 92.0 | 98.2 | 94.9 | 86.0 | 98.3 |
| 16 | 90.3 | 96.4 | 97.5 | 96.9 | 96.7 | 100.6 |
|
| ||||||
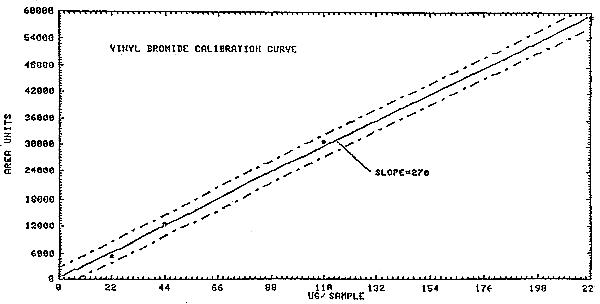
Figure 4.2. Calibration curve of instrument response to vinyl
bromide.

Figure 4.4.1. Ambient storage test of vinyl bromide.

Figure 4.4.2. Refrigerated storage test of vinyl bromide.
5. References
- 5.1. "Current Intelligence Bulletin 28" NIOSH, 1978.
5.2. "The Condensed Chemical Dictionary", 8th Edition, 1971.