| Method no.: | 04 |
| Matrix: | Air |
| Target concentration: | 1 ppm (2.5 mg/m3) (OSHA PEL) |
| Procedure: | Collection on charcoal, desorption with carbon disulfide, analysis by gas chromatography with a flame ionization detector. Stored in refrigerator and analyzed as soon as possible. |
| Detection limit based on recommended air volume: |
0.25 ppm |
| Recommended air volume and sampling rate: |
1 L at 0.05 L/min |
| Standard error of estimate at the target concentration: (Figure 4.1.) |
7.6% |
| Status of method: | Recommended by NIOSH, partially evaluated by OSHA Laboratory. |
| Date: April 1979 | Chemist: Dee R. Chambers |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In January 1974, B.F. Goodrich Chemical Company informed NIOSH of several deaths among polyvinyl chloride production workers from angiosarcoma, a rare liver cancer. In response to this and other evidence of potential hazards, OSHA lowered the workplace air standard for vinyl chloride (VC) from 500 ppm to 1 ppm 8-h time weighted average.
Since the recognition that VC was a carcinogen, a great deal of research into the sampling and analytical methodology has been conducted. Perhaps the most complete and thorough examination has been conducted by Hill, McCammon, Saalwaechter, Teass, and Woodfin for NIOSH titled "The Gas Chromatographic Determination of Vinyl Chloride in Air Samples Collected on Charcoal" (Ref. 5.1.). That paper outlines the method followed by the OSHA Laboratory.
1.1.2. Toxic effects (This section is for information only and should not be taken as a basis for OSHA policy.)
Studies of workers exposed to VC have demonstrated an excessive risk of death from cancer of the lung, brain, lymphatic system, and angiosarcoma of the liver. Cancers of the same sites were previously induced in animals following exposure to VC. In adults, untreated angiosarcoma of the liver is usually fatal within 8 months. The latency period for occupationally induced cancers is typically 15-40 years.
This evidence that VC is mutagenic has been provided by investigations showing an increase in fetal wastage among wives of male workers following exposure to VC.
Additionally, VC exposure has caused dizziness, nausea, increased blood pressure, coughing and sneezing, calf and joint pain, anemia, dermatitis, and increased perspiration. (Ref. 5.2.)
1.1.3. Industries which use VC are: chemicals and allied products, electrical equipment and supplies, and furniture and fixture. (Ref. 5.2.)
1.1.4. Approximately 27,000 workers are exposed to VC with a potential exposure to 2,200,000 workers. (Ref. 5.2.)
1.1.5. Physical properties
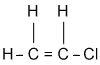
VC is a gas which is easily liquefied. Some properties are: specific gravity 0.9121, boiling point -13.9°C, freezing point -160°C, vapor pressure 2300 mm (20°C), and flash point -108°F.
VC is slightly soluble in water, soluble in alcohol and ether. (Ref. 5.3.)
1.2. Detection limit, precision, sensitivity and working range
- 1.2.1. The detection limit for the analytical procedure is 0.6
ng. This is based on a 1-µL injection.
1.2.2. The coefficient of variation is reported for the NIOSH method as 7.5%. This resulted from the analysis of two sets of sorbent tubes, one set of 27 tubes exposed to a VC concentration of 7.2 mg/m3 in air and another set of 29 tubes exposed to a concentration of 71.3 mg/m3.
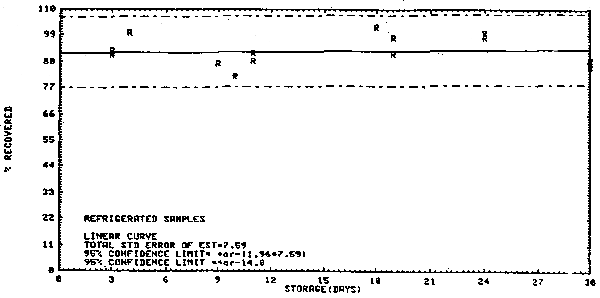
At the OSHA laboratory, 19 samples were taken from a permeation tube apparatus where the concentration was 1.1 ppm. The air volume sampled was 1 L. The average recovery was 92.5% during a 30-day storage test performed with the 19 samples and the standard error of estimate was 7.6%. (Section 4.1.)
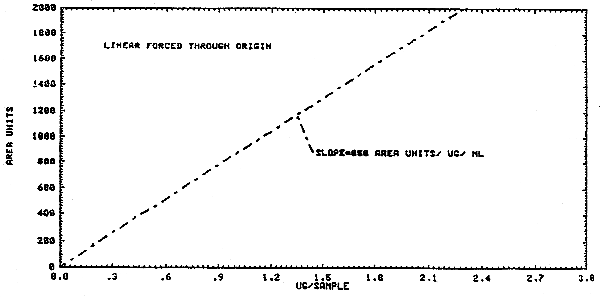
1.2.3. The sensitivity of the analytical procedure at the PEL based on a 1-L air volume is 850 area units per µg/mL. (Figure 4.3.) The sensitivity is determined by the slope of the calibration curve. The sensitivity will vary somewhat with the particular instrument used in the analysis.
1.2.4. The lower limit of the estimated working range, assuming adequate desorption, is 0.25 ppm. The upper limit of the working range is dependent on the capacity of the collection medium.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% of the true value or better at the 95% confidence
interval.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or better.
1.3.3. The overall procedure meets the above criteria. NIOSH has shown that sorbent tubes were within 6% of the average concentration of gas samples. (Ref. 5.1.)
1.4. Advantages
- 1.4.1. The method of sampling is convenient. Charcoal tubes are
easily transported to the laboratory through the mail. The
analytical method is quick; automation of the procedure is possible.
1.4.2. Possible interferences may be circumvented by altering GC parameters.
1.5. Disadvantages
It may be difficult to analyze for additional components on the same charcoal tube because of the sensitivity requirements necessitated by the low PEL.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. A personal sampling pump that can be calibrated to within
±5% of the recommended flow rate with the sampling tube in line.
2.1.2. Charcoal tubes: Glass tube, with both ends heat sealed,
7.0 cm × 6-mm o.d. ×
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break the ends of the
charcoal tubes. All tubes must be from the same lot.
2.3.2. Connect two charcoal tubes in series to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup section and should be positioned nearer the pump.
2.3.3. The tubes should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tubes.
2.3.5. Seal each charcoal tube with plastic caps immediately after sampling. Also, seal each sample with OSHA sealing tape lengthwise. Label each tube primary or backup according to its position in the series.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the charcoal tubes.
2.4. Breakthrough
- 2.4.1. At the recommended sampling flow rate of 0.05 L/min, the
total volume to be sampled should not exceed 5 L. This value is
based upon data which indicated that more than 10 L of air
containing l ppm of VC could be sampled on activated charcoal before
5% breakthrough was observed. This indicates that 5 L of air
containing no more than 2 ppm may be sampled without significant
breakthrough. If a particular atmosphere is suspected of containing
a high concentration of contaminants or high humidity is suspected,
the sampling volume should be reduced by 50%. A large safety factor
has been included in the recommended 1-L air volume and the capacity
of the first tube should be within these limits except under the
most extreme conditions. (Ref. 5.4.)
2.4.2. The second tube in the series is primarily a measure of migration rather than breakthrough. VC migrates readily upon storage, and therefore, it is necessary to provide a means to distinguish between breakthrough and migration.
2.5. Desorption efficiency
- 2.5.1. The desorption efficiency may vary from laboratory to
laboratory and for each lot of charcoal.
2.5.2. At loadings of 2.9 µg, the desorption efficiency was 93%. At loadings of 13 µg, the desorption efficiency was 86%, and at 64 µg loadings, the desorption efficiency was 89% (Ref. 5.1.) These loadings represent air concentrations of approximately 1, 5 and 25 ppm based on a 1-L air volume.
2.6. Recommended air volume and sample rate
- 2.6.1. The recommended air volume is 1 L.
2.6.2. The recommended sampling rate is 0.05 L/min.
2.7. Interferences
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of VC on charcoal. In
general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
charcoal tubes.
2.8.2. Observe all usual safety practices when sampling in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. Gas chromatograph equipped with a flame ionization
detector.
3.1.2. Stainless steel column (20 ft × 1/8 in.) packed with 10% SE-30 on 80/100 mesh Chromosorb W (acid washed, silanized with dimethyl dichlorosilane). Other columns capable of performing the required separation may be used.
3.1.3. An electronic or mechanical integrator or some other method of determining peak area.
3.1.4. Vials (2-mL) that can be sealed with caps containing Teflon septa.
3.1.5. Microliter syringe (1-µL) for injecting samples.
3.1.6. One-milliliter gas-tight syringe for preparing standards.
3.1.7. Volumetric flasks, convenient sizes for preparing standards.
3.2. Reagents
- 3.2.1. Carbon disulfide, spectrograde or better.
3.2.2. Vinyl chloride, pure or of known concentration.
3.2.3. n-Heptane, spectrograde or better.
3.2.4. Purified helium (carrier gas).
3.2.5. Purified hydrogen and air.
3.2.6. Desorbing reagent, 0.1 µL n-heptane per milliliter of CS2.
3.3. Standard preparation
Standards are prepared by injecting with a gas-tight syringe an amount of VC into a volumetric containing desorbing reagent. The ppm value in air that this standard represents depends on the concentration of VC used, the amount injected, and the size of volumetric flask. Standards should be near 1 ppm for a 1-L air sample.
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate vials.
3.4.2. Each section is desorbed with 1.0 mL of desorbing reagent.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.5. Analysis
- 3.5.1. GC Conditions
| flow rates (mL/min) | temperature (°C) | ||
| helium: | 25 | injector: | 200 |
| hydrogen: | 35 | detector: | 250 |
| air: | 250 | column: | 125 |
| injection size: | 1 µL | ||
| VC elution time: | about 1 min | ||
| chromatogram: | Section 4.2. | ||
3.5.2. Peak areas are measured by an electronic integrator or other suitable means.
3.5.3. An internal standard procedure is used. The integrator is calibrated to report results in ppm for a 1-L air volume.
3.5.4. The amount of analyte found should be corrected for the desorption efficiency.
3.6. Interferences
- 3.6.1. Any compound having the same general retention time of VC
or n-heptane is an interference. Possible interferences should be
listed on the sample identification sheets. GC parameters should be
chosen to circumvent these interferences.
3.6.2. Retention time data on a single column is not proof of chemical identity. Samples over the PEL should be confirmed by GC/MS or other suitable means.
3.7. Calculations
- 3.7.1. Since the integrator is programmed to report results in
parts per million for a 1-L air volume (corrected for desorption
efficiency), the following calculation is used:
| ppm VC = A/B | where | A | = | ppm on report |
| B | = | actual air volume | ||
3.7.2. Remember to add the analytical results from both charcoal tubes that were in series before reporting.
3.8. Safety precautions
- 3.8.1. All work done with the solvents and VC gas should be done
in a hood.
3.8.2. Avoid any skin contact with all the solvents.
3.8.3. VC should be considered a human carcinogen, and all work with VC should be done using appropriate "carcinogen" safeguards.
4. Backup Data
- 4.1. Generated samples/storage data
| VC test atmosphere: | 1.1 ppm |
| sampling rate: | 46.7 mL/min |
| air volume: | 0.91 - 1.03 L/sample |
| storage | refrigerator -5°C. |
Storage Test
|
| ||||||||
| days | days | |||||||
| stored | % recovery | stored | % recovery | |||||
|
| ||||||||
| 3 | 91.9 | 91.0 | 92.8 | 18 | 102.7 | |||
| 4 | 100.0 | 19 | 98.2 | 91.9 | ||||
| 9 | 87.4 | 87.4 | 24 | 98.2 | 100.0 | 98.2 | ||
| 10 | 81.8 | 30 | 86.4 | 89.1 | 89.1 | |||
| 11 | 91.9 | 88.3 | 91.9 | |||||
|
| ||||||||
No loss over 30 days, however, migration was observed. The storage data are presented graphically in Figure 4.1.
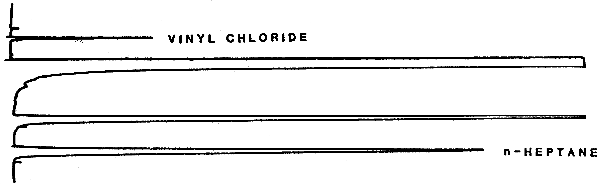
4.2. Chromatogram
A typical chromatogram of vinyl chloride is presented in Figure 4.2.
4.3. Calibration curve
A calibration curve of instrument response for vinyl chloride is
shown in Figure 4.3.,
5. References
- 5.1. "The Gas Chromatographic Determination of Vinyl Chloride in
Air Samples Collected on Charcoal", Hill, McCammon, Saalwaechter,
Teass, and Woodfin, NIOSH 1975.
5.2. "Current Intelligence Bulletin 28" NIOSH, 1978.
5.3. The Condensed Chemical Dictionary 8th Edition 1971.
5.4. NIOSH Manual of Analytical Methods, P&CAM 178.