| Method no.: | 03 |
| Matrix: | Air |
| OSHA PEL: | 50 ppm |
| Target concentration: | 5 ppm |
| Procedure: | Collection on charcoal adsorbent, desorption with
|
| Detection limit based on recommended air volume: |
0.05 ppm (for analytical procedure only) |
| Recommended air volume and sampling rate: |
10 L at 0.2 L/min |
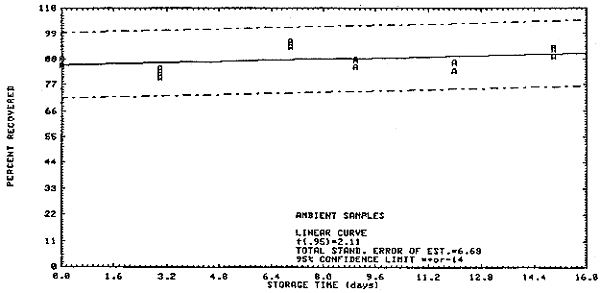
| Standard error of estimate: (Figure 4.3.1.) |
6.7% |
| Status of method: | Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. |
| Date: April 1979 | Chemist: Duane E. Lee |
OSHA Analytical Laboratory
Salt Lake City, Utah
1. General Discussion
- 1.1. Background
- 1.1.1. History
In 1946, the American Conference of Governmental Industrial Hygienists adopted a Maximum Allowable Concentration (MAC) of 100 ppm for ethylene dichloride. In 1947, they changed it to 75 ppm; and in 1948, they changed the nomenclature to Threshold Limit Value (TLV) for allowable concentrations. The TLV remained at 75 ppm until 1952 when it was changed to 100 ppm. Subsequently the TLV was changed to 50 ppm in 1962. (Ref. 5.1.)
OSHA has adopted a 50 ppm TWA and a ceiling concentration of 100 ppm with a maximum peak of 200 ppm for 5 min in any 3-h period. (Ref. 5.2.) In April 1978, NIOSH issued a "Current Intelligence Bulletin" recommending an exposure limit of 5 ppm based on adverse effects on the nervous system and being a potential carcinogen. (Ref. 5.3.) For this reason, the analytical and sampling procedures must be evaluated to assure that an adequate method exists for lower levels. In 1977 and 1978, the OSHA Analytical Laboratory analyzed approximately 50 samples each year for ethylene dichloride.
1.1.2. Toxic effects (This section is for information only and should not be used as basis for OSHA policy.)
Animal studies have evaluated the toxic effects of ethylene dichloride. The adverse effects of acute exposure include extreme lowering of blood pressure, cardiac impairment, pulmonary edema, fatty degeneration of the liver and kidney, and degeneration of the adrenal cortex. (Ref. 5.3.) Studies also indicate it causes cancer in rats and mice by producing tumors such as adenomas, adenocarcinomas, and fibromas. (Ref. 5.4.)
The effects of ethylene dichloride on humans are similar for ingestion, inhalation, and skin absorption. Acute exposures result in nausea, vomiting, dizziness, internal bleeding, bluish-purple discoloration of the mucous membranes and skin, rapid but weak pulse, and unconsciousness. (Ref. 5.3.) There is no definitive evidence that ethylene dichloride causes cancer in humans. (Ref. 5.4.)
1.1.3. Worker exposure
NIOSH estimates that as many as 2 million workers may be exposed to ethylene dichloride. Of these workers, an estimated 34,000 have been exposed to ethylene dichloride for 4 h or more per day. (Ref. 5.3.)
1.1.4. Use and operations where exposure occurs
Ethylene dichloride is the earliest known chlorinated hydrocarbon, having been produced in 1795. (Ref. 5.5.) It is one of the highest volume chemicals used in the United States with approximately 10 billion pounds produced annually. Most of the ethylene dichloride produced is used as an intermediate in the production of vinyl chloride, but it is also used in the production of other chlorinated hydrocarbons. Besides production uses, it is used as a solvent and is used in leaded fuels because it is a lead scavenger. (Ref. 5.3.)
Industries where ethylene dichloride may be used include: medical and health services, automotive dealers and service stations, machinery, printing and publishing, eating and drinking places, chemical and allied products, and miscellaneous business services. (Ref. 5.4.)
1.1.5. Physical properties (Ref. 5.5. and 5.6.)
| molecular weight: | 98.97 |
| boiling point: | 83.5°C |
| melting point: | -35.3°C |
| specific gravity: | 1.2529 at 20/4°C |
| self-ignition temp: | 449°C |
| flash point: | 18.3°C open cup 13.0°C closed cup |
| explosive limits | |
| % by volume: | 6.2 - 16.9 |
| color: | colorless liquid |
| odor: | resembles chloroform |
| molecular formula: | CH2ClCH2Cl |
| other names: | ethylene chloride; brocide; EDC; 1,2-dichloroethane; dutch liquid; s-dichloroethane; 1,2-bichloroethane |
1.2. Detection limit, precision, sensitivity, and working range
- 1.2.1. The detection limit for the analytical procedure is 2 ng
with a coefficient of variation equal to 0.05 at this level.
(Section 4.1.) The detection limit was determined using 1-µL
injections.
1.2.2. The pooled coefficient of variation of the analytical procedure over the range of 100 to 402 µg per sample is 0.017. (Section 4.2.) This represents an air concentration of 2.5 to 10 ppm based on the recommended sampling and analytical procedures.
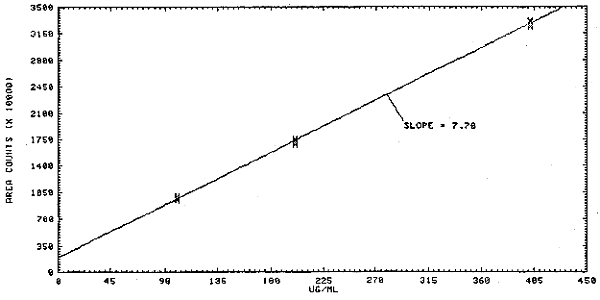
1.2.3. The sensitivity of the analytical procedure over a concentration range representing 0.5 to 2 times the target concentration based on the recommended air volume is 7.69946 × 107 area units per mg/mL. The sensitivity is determined by the slope of the calibration curve. (Section 4.2.) The sensitivity will vary somewhat with the particular instrumentation used in the analysis.
1.2.4. The lower limit of the estimated working range, assuming adequate desorption efficiency, is 0.05 ppm. The upper limit of the working range is dependent on the capacity of the collection medium.
1.3. Accuracy
- 1.3.1. The overall procedure must provide results that are
within 25% or better at the 95% confidence interval.
1.3.2. The recovery of analyte from the collection medium after storage must be 75% or greater.
1.3.3. The overall procedure has met the above validation criteria for a 15-day period. (Section 4.3.)
1.4. Advantages
- 1.4.1. The sampling procedure is convenient.
1.4.2. The analytical procedure is quick, sensitive, and reproducible.
1.4.3. Reanalysis of samples is possible.
1.4.4. Automation of the procedure is possible.
1.4.5. The electron capture detector is sensitive to ethylene chloride and not very sensitive to hydrocarbons, therefore making the method somewhat selective.
1.5. Disadvantages
- 1.5.1. It may be difficult to analyze for hydrocarbons because
of the solvent used and the electron capture detector.
1.5.2. The analysis time is lengthened because a temperature program is required to get the o-xylene off the GC column.
2. Sampling Procedure
- 2.1. Apparatus
- 2.1.1. An approved and calibrated personal sampling pump whose
flow can be determined within ±5% at the recommended flow.
2.1.2. Charcoal tubes: Glass tube, with both ends heat sealed,
7.0 cm × 6-mm o.d. ×
2.2. Reagents
None required.
2.3. Sampling technique
- 2.3.1. Immediately before sampling, break the ends of the
charcoal tube. All tubes must be from the same lot.
2.3.2. Connect the charcoal tube to the sampling pump with flexible tubing. The short section of the charcoal tube is used as a backup and should be positioned nearer the sampling pump.
2.3.3. The tube should be placed in a vertical position during sampling to minimize channeling.
2.3.4. Air being sampled should not pass through any hose or tubing before entering the charcoal tube.
2.3.5. Seal the charcoal tube with plastic caps immediately after sampling. Also, seal each sample lengthwise with an OSHA sealing tape.
2.3.6. With each batch of samples, submit at least one blank tube from the same lot used for samples. This tube should be subjected to exactly the same handling as the samples (break, seal, transport) except that no air is drawn through it.
2.3.7. Transport the samples (and corresponding paperwork) to the lab for analysis.
2.3.8. If bulk samples are submitted for analysis, they should be transported in glass containers with Teflon-lined caps. These samples must not be put in the same container used for the charcoal tubes.
2.4. Breakthrough
Three separate samples were drawn at 0.195 L/min from an atmosphere containing 10 ppm ethylene dichloride and varying relative humidity. The breakthrough (5% breakthrough) volume was: 15.8 L for 75% relative humidity, 14.3 L for 82% relative humidity, and 11.9 L for 85% relative humidity.
2.5. Desorption efficiency
- 2.5.1. The desorption efficiency, from liquid injections on
charcoal tubes SKC Lot 107, averaged 87.9% for 0.1 to 0.4 mg per
tube, which is 2.5 to 10 ppm for a 10-L air volume. (Section 4.4.)
2.5.2. The desorption efficiency may vary from laboratory to laboratory and for each lot of charcoal.
2.6. Recommended air volume and sampling rate
- 2.6.1. The recommended air volume is 10 L.
2.6.2. The recommended sampling rate is 0.2 L/min.
2.7. Interferences
- 2.7.1. At the present time, it is unknown if any compound would
severely interfere with the collection of EDC on charcoal. In
general, the presence of other solvents will decrease the
breakthrough volume for a particular solvent.
2.7.2. Any compound which is suspected of interfering in the collection or analysis should be listed on the sampling data sheet.
2.8. Safety precautions
- 2.8.1. Safety glasses should be worn when breaking the ends of
the tubes.
2.8.2. The broken ends of the tubes should be protected to avoid injury to the person being sampled.
2.8.3. When working in environments containing flammable vapors, do not provide any spark source from equipment used or pumps.
2.8.4. Observe all safety practices for working in hazardous areas.
3. Analytical Procedure
- 3.1. Apparatus
- 3.1.1. A gas chromatograph equipped with an electron capture
detector.
3.1.2. GC column capable of separating 1,1-dichloroethane, 1,2-dichloroethane, and 1,1,2-trichloroethane. The column used for this study was a 10-f t × 1/8-in. stainless steel 0.1% SP-1000 coated on 80/100 mesh Carbopack C.
3.1.3. An electronic integrator or other suitable method of measuring detector response.
3.1.4. Two-milliliter vials with Teflon-lined caps.
3.1.5. Microliter syringes, 10-µL for preparing standards, 1-µL for sample injections.
3.1.6. Pipets for diluting standards. A 1-mL pipet for dispensing solvent for desorption, or a 1-mL dispenser pipet.
3.1.7. Volumetric flasks, for preparing standards.
3.2. Reagents
- 3.2.1. o-Xylene, chromatographic grade.
3.2.2. Ethylene dichloride, reagent grade.
3.2.3. Argon/methane, 95/5; purified GC grade carrier gas.
3.3. Standard preparation
- 3.3.1. Standards are prepared by diluting pure ethylene
dichloride with o-xylene.
3.3.2. Eight microliters of ethylene dichloride per 50 mL of o-xylene equals 4.95 ppm for a 10-L air sample desorbed with 1 mL of o-xylene.
3.4. Sample preparation
- 3.4.1. The front and back sections of each sample are
transferred to separate 2-mL vials.
3.4.2. Each section is desorbed with 1.0 mL of o-xylene.
3.4.3. The vials are sealed immediately and allowed to desorb for 30 min with intermittent shaking.
3.5. Analysis
- 3.5.1. GC conditions
| argon/methane flow rate: | 25 mL/min |
| injector temperature: | 200°C |
| detector temperature: | 275°C |
| initial column temperature: | 80°C for 15 min |
| temperature program rate: | 10°C/min |
| final column temperature: | 210°C for 5 min |
| EC detector: | Ni-63 |
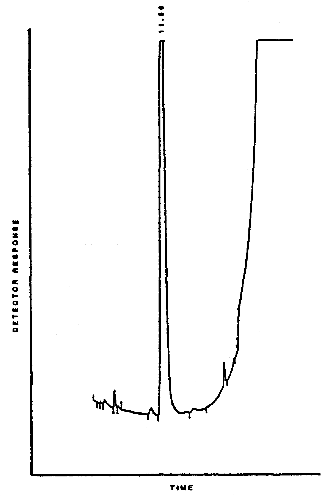
3.5.2. Chromatogram (Figure 4.5.)
3.5.3. Peak areas are measured by an electronic integrator or other suitable means.
3.5.4. Because an external standard method is used, duplicate injections of all standards and samples are made.
3.6. Interferences
- 3.6.1. Any substance having the same general retention time as
ethylene dichloride is an interference.
3.6.2. GC parameters may be changed to circumvent most interferences.
3.6.3. Retention time on a single column is not considered proof of chemical identity. Samples should be confirmed by GC/MS or other suitable means.
3.7. Calculations
Usually the integrator is programmed to report results in ppm (corrected for desorption efficiency) for a 10-L air volume. The following calculation is used:
| ppm = A/(0.1)(B) | where | A | = | ppm on report |
| B | = | air volume (L) | ||
3.8. Safety precautions
- 3.8.1. All work using solvents should be done in a hood.
3.8.2. Avoid any skin contact with all of the solvents.
3.8.3. Safety glasses should be worn throughout the procedure.
4. Backup Data
- 4.1. Detection limit
Standard used was 0.0016 µL ethylene dichloride per milliliter of o-xylene (2.0 µg/mL).
Detection Limit Data
|
| |||
| injection | area | injection | area |
|
| |||
| 1 | 200600 | 7 | 203900 |
| 2 | 189400 | 8 | 200300 |
| 3 | 191300 | 9 | 193900 |
| 4 | 198000 | 10 | 194600 |
| 5 | 189800 | 11 | 200600 |
| 6 | 194800 | 12 | 230200 |
| |
SD
=
10889.236 CV = 0.0547 | ||
|
| |||
Detection limit is (2.0 ng/µL)(1 µL) = 2.0 ng per injection, or 0.05 ppm for a 10-L air volume desorbed with 1.0 mL of o-xylene.
4.2. Instrument response to the analyte and analytical precision
Precision of the Analytical Procedure
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/mL | 100.6 | 201.1 | 402.2 |
|
| |||
| area | 9932000 | 17330000 | 33150000 |
| counts | 10000000 | 16840000 | 33130000 |
| 9468000 | 17100000 | 32440000 | |
| 9450000 | 17090000 | 32690000 | |
| 9760000 | 17470000 | 33250000 | |
| 9668000 | 17590000 | 32690000 | |
| 9713000 | 17236667 | 32891667 | |
| SD | 229677.16 | 277680.87 | 327805.84 |
| CV | 0.0236 | 0.0161 | 0.00997 |
|
| |||
The response data from above are used to determine the calibration curve in Figure 4.2.
4.3. Storage tests
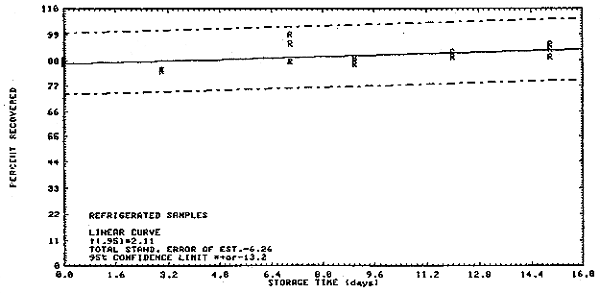
Thirty-six samples were collected on charcoal tubes (SKC Lot 107) from a dynamic test atmosphere containing 5 ppm ethylene dichloride and 80% relative humidity. The recommended sampling conditions were used. Six samples were analyzed immediately. The remainder were divided into two equal groups, one stored at room temperature (20 to 23°C) and the other under refrigeration (-5 to 0°C). Three samples from each group were analyzed at 2 to 4 day intervals over 15 days. The results shown below are also shown in Figures 4.3.1. and 4.3.2.
Storage Tests
|
| |||||||
| storage time | % recovery | % recovery | |||||
| (refrigerated) | (ambient) | ||||||
|
| |||||||
| 0 | 88.7 | 86.9 | 88.6 | 85.1 | 88.5 | 85.5 | |
| 3 | 83.8 | 84.6 | 83.6 | 82.6 | 80.0 | 84.0 | |
| 7 | 99.4 | 87.9 | 95.2 | 95.8 | 95.8 | 93.3 | |
| 9 | 86.5 | 88.0 | 89.1 | 84.9 | 88.0 | 87.9 | |
| 12 | 91.8 | 89.6 | 90.8 | 86.7 | 82.9 | 86.5 | |
| 15 | 89.4 | 92.7 | 95.1 | 92.2 | 93.2 | 89.3 | |
|
| |||||||
4.4. Desorption efficiency
Desorption Efficiency of Ethylene
Dichloride from Charcoal with o-Xylene
|
| |||
| × target conc. | 0.5× | 1× | 2× |
| µg/sample | 100.6 | 201.1 | 402.2 |
|
| |||
| desorption | 88.9 | 87.2 | 90.7 |
| efficiency, | 87.4 | 84.5 | 91.7 |
| % | 77.1 | 87.4 | 90.5 |
| 87.6 | 87.8 | 89.4 | |
| 87.7 | 87.6 | 91.2 | |
| 86.8 | 88.2 | 90.5 | |
| 85.9 | 87.1 | 90.7 | |
|
| |||
4.5. Chromatogram
A typical chromatogram of ethylene dichloride under the GC conditions used in the analysis is shown in Figure 4.5.
5. References
- 5.1. "Criteria for a Recommended standard. . .Occupational
Exposure to Ethylene Dichloride" NIOSH April, 1976.
5.2. General Industrial Safety and Health standards, OSHA 2206 (29 CFR 1910), Revised January, 1976.
5.3. "Current intelligence Bulletin 25" NIOSH, April 19,1978.
5.4. "Current Intelligence Bulletin 27" NIOSH, August 21, 1978.
5.5. Encyclopedia of Chemical Technology, Second Edition, Vol. 5, 1967.
5.6. Industrial Hygiene and Toxicology, 2nd Revised Edition, Vol. 11, Frank A. Patty, editor, page 1280.