(IMPREGNATED ACTIVATED BEADED CARBON)
| Method Number: | ID-212 (This method supersedes ID-177) |
| Matrix: | Air |
| OSHA Permissible Exposure Limits Final Rule Limit*: |
0.1 ppm (Ceiling) |
| Transitional Limit: | 0.1 ppm (Ceiling) |
| Collection Device: | An air sample is collected using a calibrated
sampling pump and a glass tube containing impregnated activated
beaded carbon (IABC). A modified sampling tube (MST) can be used to
preclude any |
| Recommended Sampling Rate: | 0.5 liter per minute (L/min) |
| Recommended Minimum Sampling Time: | 5 min |
| Analytical Procedure: | The sampling medium is desorbed using an aqueous solution containing 1.5 mM sodium carbonate (Na2CO3) and 1.5 mM sodium bicarbonate (NaHCO3). An aliquot of this solution is analyzed for iodine (as iodide, I-) by an ion chromatograph equipped with a pulsed electrochemical detector (PED). |
| Detection
Limit Qualitative: Quantitative: |
0.0004 ppm as I2 (2.5-L air sample) 0.0010 ppm as I2 (2.5-L air sample) |
| Precision and Accuracy Validation Range: CVT(pooled): Bias: Overall Error: |
0.05 to 0.20 ppm 0.045 -0.025 ±11.5% |
| Method Classification: | Interim Method (See Special Precautions below) |
| Special Precautions: | 1) Loss of iodine has been noted when sampling in
>50% RH. See Sections 2 and 4.6. for details. The loss in
relation to humidity is concentration-dependent. Due to the
dependence on humidity, this method is considered an interim
procedure until alternate sampling can be explored further.
2) Samples should be shipped for laboratory analysis as soon as possible. |
| Chemist: | James C. Ku |
| Date: | May, 1994 |
* The U.S. Court of Appeals, Eleventh Circuit, has ruled that Final
Rule Limits of 29 CFR 1910.1000 be vacated. The Transitional Rule Limits
are currently in effect and in the case of iodine the limits are identical
in value. The Final Rule definition of a "Ceiling" has been retained (See
Federal Register, Vol. 58, No. 124, Wednesday June 30, 1993 p.35339 for
further details regarding the ceiling definition).
for descriptive use only and do not constitute endorsements by
OSHA Salt Lake Technical Center
Salt Lake City, Utah
1. Introduction
This method describes the sample collection and analysis of airborne iodine (I2). Samples are taken in the breathing zone of workplace personnel, and analysis is performed by ion chromatography (IC) equipped with a pulsed electrochemical detector (PED).
- 1.1. History
Previously, iodine vapor in the workplace atmosphere was collected in a 1% sodium bisulfite solution and analyzed by an ion specific electrode (ISE) technique (5.1.). This method was considered inadequate due to the instability of I2 in bisulfite solution. Two stopgap methods were later developed, in which any iodine present in the air was collected in 0.01 N sodium hydroxide solution contained in a midget fritted glass bubbler (MFGB). These samples were then analyzed by ISE (5.2.) or IC equipped with an electrochemical detector (ED) (5.3.). Because bubblers are inconvenient to use as personal samplers due to spillage or breakage, it was desirable to develop a solid-sorbent sampling method. A method was developed which collected I2 in the air using activated charcoal impregnated with an alkali metal hydroxide. The base-impregnated charcoal converted I2 to iodide (I-) and iodate (IO3-) which was then desorbed using a weakly basic solution. An aliquot of the solution was analyzed for I- by IC or ISE. Unfortunately, background levels of I- (up to 5 µg) found in the impregnated charcoal were considered unacceptable (5.4.).
Impregnated activated beaded carbon (IABC), previously developed (5.5., 5.6.) at the OSHA Salt Lake Technical Center (OSHA-SLTC), was used to replace impregnated charcoal sampling tubes (ICST) used for the collection of I2. The IABC has a significantly lower background level of I- (< 0.008 µg). This current method was evaluated using IABC as the collection medium.
1.2. Principle
Iodine is collected using IABC sorbent contained in a glass tube. The collected I2 is converted to I- and IO3- by the treated sorbent based on the following chemical reaction:
The resultant I- is analyzed by IC-PED. A stoichiometric conversion factor is used to calculate the amount of I2 collected. The same mechanism is used for iodine collection in impingers containing 1.5 mM sodium carbonate and 1.5 mM sodium bicarbonate solutions. These solutions are used for sampling when the relative humidity >50%.
1.3. Advantages and Disadvantages
- 1.3.1. This method has adequate sensitivity for determining
compliance with the OSHA Ceiling Permissible Exposure Limit (PEL)
for I2 exposure.
1.3.2. The method is simple, rapid, and easily automated.
1.3.3. The method is specific and can determine the I2 (as I-) in the presence of other halogen-containing substances.
1.3.4. The solid sorbent sampling tube used to collect I2 is small, portable, and contains no liquid. The tube used to collect only iodine vapor is identical to that used to collect sulfur dioxide in OSHA Method No. ID-200. A modified sampling tube is also available for collecting particulates. The modified tube is similar to the one also recommended in OSHA Method No. ID-200; however, the foam used in the modified SO2 sampling device must be removed before sampling iodine. See Section 2 for further details.
1.3.5. Desorption and preparation of samples for analyses involve simple procedures and equipment.
1.3.6. The amount of I2 (as I-) can also be analyzed and confirmed by an ISE technique. This ISE technique is listed as an Appendix to this method.
1.3.7. The I- contaminant (background) levels of the IABC sorbent are extremely low, especially when compared to the sampling media previously used in OSHA Method No. ID-177.
1.3.8. One major disadvantage of the method is that lower recoveries are noted when samples are taken at high humidities. A 30% loss in recovery was noted at 80% RH when the I2 concentration was near 0.5 × PEL (see Section 4.6.). Due to this humidity problem, the method is considered an interim method until more suitable sampling media can be developed or the humidity problem rectified.
1.3.9. Another disadvantage is the skilled maintenance required for the analytical detector. As specified in Section 3.2.1., the detector is equipped with two electrodes, a silver working electrode and a silver/silver chloride reference electrode. The silver working electrode must be polished before it is installed in the amperometric cell. It is also necessary to polish the working electrode whenever a high background current, decreased sensitivity, or other degradation in detector output is observed. Polishing is a time-consuming process requiring skill and care. Recently developed silver/silver chloride reference electrodes require little, if any, maintenance.
1.4. Method Performance
A synopsis of the method performance is presented below. Further information can be found in Section 4.
- 1.4.1. This method was validated over the concentration range of
0.05 to 0.20 ppm. An air volume of 2.5 L and a flow rate of 0.5
L/min were used.
1.4.2. The qualitative detection limit was 0.0027 µg/mL or 0.008 µg (as I-) when using a 3-mL solution volume. This corresponds to 0.0004 ppm I2 for a 2.5-L air volume.
1.4.3. The quantitative detection limit was 0.009 µg/mL or
0.03 µg (as I-) when using a 3-mL solution volume.
This corresponds to 0.001 ppm I2 for a
2.5-L air volume. A
1.4.4. The sensitivity of the analytical method, when using the instrumental parameters listed in Section 3.6.3., was calculated from the slope of a linear working range curve (0.1 to 5.0 µg/mL I-). The sensitivity was 2.5 × 106 area units per 1 µg/mL. A Dionex Series 4500i ion chromatograph with AI450 computer software was used (Dionex, Sunnyvale, CA).
1.4.5. The performance of this method was significantly better than OSHA Method No. ID-177 for I2 (5.4.).
1.4.6. The total pooled coefficients of variation (CVT), bias, and total overall error (OE) are as follows:
CVT (pooled) = 0.045; bias = -0.025; OET = ±11.5%
1.4.7. The collection efficiency at 2 × PEL was 100%. Samples were collected from a generated test atmosphere of 0.20 ppm I2 for 5 min.
1.4.8. Breakthrough tests were performed at concentrations of 0.75 ppm I2. No breakthrough was found for a sampling time of 30 min and an average sample flow rate of 0.5 L/min.
1.4.9. Samples can be stored at ambient (20 to 25°C) temperature on a lab bench for a period of 29 days. Results show the mean sample recovery after 29 days of storage was within ±10% of results at Day 0; however, a loss over time was noted and samples should be analyzed as soon as possible. There was no significant difference in results for samples stored at room temperature, in a refrigerator, or freezer.
1.5. Interferences
- 1.5.1. Other gaseous or particulate iodide compounds may
interfere in the analysis of I2 if
collected on the IABC. A modified sampling tube (MST) should be used
to collect any iodide-containing particulate if suspected to be a
problem.
1.5.2. Any substance that has the same retention time as I-, when using the ion chromatographic operating conditions described in this method, is an interference. Changing the chromatographic separation conditions (detector settings, column, eluent flow rate, and strength, etc.) may circumvent the interference.
1.6. Uses
Industrial uses and products of iodine (5.7.):
| unsaturation indicator | x-ray contrast media |
| dyes | catalysts |
| production of iodides and iodates | pharmaceuticals |
| process engraving and lithography | iodized salt |
| preservatives | photography |
| disinfectants | food and feed additive |
| special lubricants for titanium and stainless steel parts | |
1.7. Physical and Chemical Properties (5.8.)
Iodine exists as dense crystalline grayish-black plates or granules and has a metallic luster and characteristic odor. Iodine readily sublimes, producing a violet vapor.
- Iodine (CAS No. 7553-56-2)
| Chemical formula | I2 |
| Formula weight | 253.8 |
| Melting point | 113.5°C |
| Boiling point | 184.4°C |
| Vapor pressure | 0.0410 kPa (0.309 mmHg) at 25°C |
| Vapor density | 4.98 times that of air |
| Solubility | Soluble in alcohol, carbon disulfide, chloroform, toluene, ether, carbon tetrachloride, glycerol, and alkaline iodide solutions. Slightly soluble in water. |
| Combustibility | Noncombustible |
1.8. Toxicology
Information listed within this section is a synopsis of current knowledge of the physiological effects of I2 and is not intended to be used as a basis for OSHA policy.
- 1.8.1. Iodine is considered a greater physiological irritant and
more corrosive than bromine or chlorine (5.9.). Occupational routes
of exposure for I2 are inhalation, skin
contact, and eye contact.
1.8.2. Symptoms of I2 exposure (5.10., 5.11., 5.12.)
| Inhalation: | Nasal passage irritation, headache, tightness in chest, chronic pharyngitis, rhinitis, possible pulmonary edema. |
| Skin: | Rash, cutaneous hypersensitivity, skin burns. |
| Eyes: | Burning, stinging sensations, blepharitis, lacrimation. |
1.8.3. Excretion:
Iodine absorbed by the lungs is physiologically converted to iodide and eliminated primarily in the urine.
2. Sampling (See Interferences, Section 1.5., and Notes in Section 2.1.2. before sampling.)
Wipe samples are normally not taken for iodine. If wipe sampling is necessary, the ability to perform this will have to be conducted on a case-by-case basis. Contact the OSHA-SLTC for further sampling information.
- 2.1. Equipment - Air Samples
- 2.1.1. Calibrated personal sampling pumps capable of sampling
within ±5% of the recommended flow rate of 0.5 L/min are used.
2.1.2. Solid sorbent sampling tubes are prepared using glass
tubes, glass wool plugs, and IABC. Commercially available sampling
tubes consist of glass tubes packed with
Notes:
a) IODIDE PARTICULATE:
If there is reason to
suspect the sampled air could contain iodide particulate, a
modified sampling tube (MST) should be used to exclude the
particulate. See Section 2.1.6. for more details. Most
industrial operations that use iodine in their processes do not
normally also use iodide salts.
b) RELATIVE HUMIDITY:
If the relative humidity is
>50%, impinger samplers should be used. A 20% loss of
iodine at 80% RH (25°C) and 1 × PEL has been noted when using SKC
Cat. no. 226-80 tubes. It is unknown what loss of iodine occurs
above 80% RH. Please note the following equipment is used:
- 1) Midget impingers, (order no. 7531, Ace Glass Co.,
Vineland, NJ).
2) Sample shipment vials: Scintillation vials, 20-mL (part no. 74515 or 58515, Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with polypropylene or Teflon® cap liners.
3) Collection solution: 1.5 mM Na2
CO3 + 1.5 mM
NaHCO3
Dissolve 0.159 g sodium
carbonate (Na2
CO3) and 0.126 g sodium bicarbonate
(NaHCO3) in 1-L deionized water. Use
at least reagent grade chemicals.
2.1.3. A stopwatch and bubble tube or meter are used to calibrate pumps.
2.1.4. A battery-operated or sling psychrometer, or a water vapor detector tube is used to measure the relative humidity prior to sampling.
2.1.5. Various lengths of polyvinyl chloride (PVC) tubing are used to connect sampling media to pumps.
2.1.6. Sampling in presence of iodide-containing particulates. Filter media normally used for collecting or precluding particulate (i.e. polyvinyl chloride, mixed-cellulose ester, etc.) should not be used as prefilters due to the possibility of reaction with iodine. If the workplace air being sampled is suspected of containing particulate which could interfere (i.e. iodides), a MST device containing a Teflon® filter as shown below should be used to exclude particulate. (Note: If impingers are used, the IABC and glass wool are removed from the rear of the MST and only the glass jacket/ Teflon® filter and retaining rings are connected in front of the impinger.) The sampled air should always travel in the direction shown below:
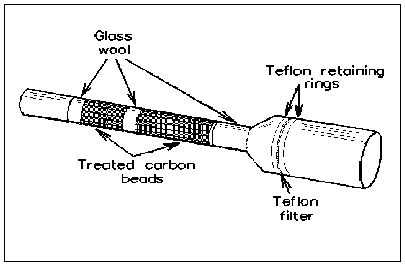
Note: This MST (Forrest Biomedical, Salt Lake City, UT) is the same sampling device (Type II) discussed in OSHA Method ID-200 (5.6.) for sulfur dioxide with one exception: The foam normally used as spacers in the tube are replaced with glass wool. Foam can absorb the iodine.
2.2. Equipment - Bulk Samples
Scintillation vials, 20-mL (part no. 74515 or 58515, Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with polypropylene or Teflon® cap liners. If possible, submit bulk samples in these vials. Tin or other metal cap liners should not be used because the metal and iodine may react.
2.3. Sampling Procedure - Air Samples
The following flow chart is an overview of the sampling protocol for iodine.
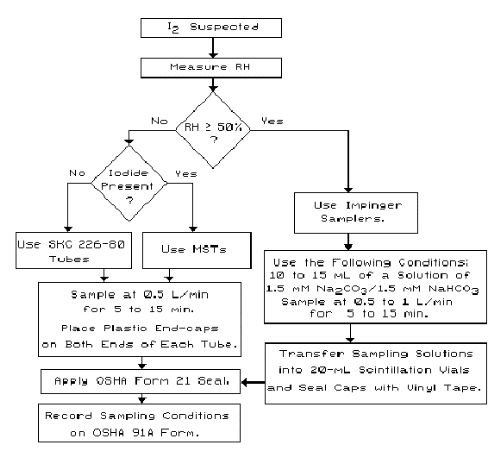 Overview of I2 Sampling Procedures |
- 2.3.1. Determine the humidity at the sampling site using a
psychrometer, detector tube, or other suitable device. Loss of
iodine has been noted at high humidities. This loss is
concentration-dependent, and at iodine levels near the PEL the loss
in recovery is approximately 20%. An alternate method employing
impinger solutions should be used when sampling in high humidities
(>50% RH) as described above. See Section 4.6. for further
details regarding the humidity- and concentration-dependent loss of
iodine.
2.3.2. Connect the sampling tube (or impinger) to the calibrated pump. If sampling tubes are used, make sure sampled air enters the large section (100 mg) of IABC first. Place the sampling device on the employee such that air is sampled from the breathing zone.
2.3.3. Use a flow rate of 0.5 L/min and a sampling time between 5 to 15 min. Take additional samples as necessary.
2.3.4. After sampling, place plastic end caps tightly on both ends of the tube and apply OSHA Form 21 seals in such a way as to secure the end caps. If impingers were used, transfer solutions to 20-mL scintillation vials and apply seals. Record the sampling conditions such as sampling time, air volume, etc. on the OSHA 91A form. When other compounds are known or suspected to be present in the air, record such information and transmit with the samples.
2.3.5. Use the same lot of IABC tubes for blank and collected samples. Handle the blank sorbent tube (or solution if impingers are used) in exactly the same manner as the sample tubes except that no air is drawn through it. Submit at least one blank tube for each batch of ten samples.
2.4. Sampling Procedure -Bulk Samples
Bulk samples can be taken to assist in resolving any potential interferences or to confirm the presence of free iodine in the workplace.
- 2.4.1. Take a representative sample of the bulk material in the
workplace. Transfer the bulk material into a 20-mL scintillation
vial and seal the cap with vinyl or electrical tape. Securely wrap
an OSHA-21 seal length-wise from vial top to bottom.
2.4.2. The type of bulk sample should be stated on the OSHA 91A and cross-referenced to the appropriate air sample(s).
2.5. Shipment
- 2.5.1. Immediately send the samples to the laboratory with the
OSHA 91A paperwork requesting iodine analysis.
2.5.2. Ship any bulk samples separately from air samples. Enclose Material Safety Data Sheets if available. Check current shipping restrictions and ship to the laboratory by the appropriate method.
3. Analysis
Note: If the instrumentation in Section 3.2.1. is unavailable, samples can be prepared and analyzed using an ion specific electrode (ISE) technique. Directions are given in the Appendix of this method. Due to the greater specificity of an IC-PED, this ISE procedure is only recommended if an IC-PED system is unavailable.
- 3.1. Safety Precautions
- 3.1.1. Refer to appropriate IC instrument manuals, PED
maintenance manual (especially for electrode maintenance), and any
Standard Operating Procedures (SOP) for proper instrument operation
(5.13.).
3.1.2. Observe laboratory safety regulations and practices.
3.1.3. Some chemicals are corrosive. Use appropriate personal protective equipment such as safety glasses, goggles, face shields, gloves, and lab coat when handling corrosive chemicals.
3.2. Equipment
- 3.2.1. Ion chromatograph (Model 4000i or 4500i Dionex,
Sunnyvale, CA) equipped with a pulsed electrochemical detector (PED)
containing a silver working electrode and silver/silver chloride
reference electrode.
[Note: A combination PED/conductivity detector (Dionex, part no. 42039) was used to validate this method. The conductivity detector is not used for this method; however, having both detectors present in one package is advantageous because of increased applications.]
3.2.2. Automatic sampler (Dionex Model AS-1) and sample vials (0.5 mL).
3.2.3. Laboratory automation system: Ion chromatograph interfaced with a data reduction system (AI450, Dionex).
3.2.4. Separator and guard columns, anion (Model HPIC-AS5 and AG5, Dionex).
3.2.5. Disposable syringes (1 mL).
3.2.6. Syringe prefilters, 0.5-µm pore size (part no. SLSR 025 NS, Millipore Corp., Bedford, MA).
Note: Some syringe prefilters are not cation- or anion-free. Tests should be performed with blank solutions first to determine contamination and suitability with the analyte.
3.2.7. Miscellaneous volumetric glassware: Micropipettes, pipettes, volumetric flasks, Erlenmeyer flasks, graduated cylinders, and beakers.
3.2.8. Scintillation vials, glass, 20-mL.
3.2.9. Equipment for eluent degassing (vacuum pump, ultrasonic bath).
3.2.10. Analytical balance (0.01 mg).
3.3. Reagents - All chemicals should be at least reagent grade.
- 3.3.1. Principal reagents:
| Sodium carbonate
(Na2CO3) Sodium bicarbonate (NaHCO3) Sodium nitrate (NaNO3) Potassium iodide (KI) Potassium iodate (KIO3) Deionized water (DI H2O) |
3.3.2. Desorbing solution (1.5 mM Na2CO3 + 1.5 mM NaHCO3):
Dissolve 0.318 g Na2CO3 and 0.252 g NaHCO3 in 2.0 L DI H2O.
3.3.3. Eluent (0.02 M NaNO3):
Dissolve 3.40 g NaNO3 in 2.0 L DI H2O. Sonicate this solution and degas under vacuum for 15 min before use.
3.3.4. Iodide (I-) stock standard (100 µg/mL):
Dissolve and dilute 0.1308 g of KI to 1.0 L with DI H2O. Prepare monthly.
3.3.5. Iodide (I-) standard solutions, 10, 1, and 0.1 µg/mL:
Pipette appropriate volumes of the 100 µg/mL I- stock standard into volumetric flasks and dilute to the mark with desorbing solution. Prepare monthly.
3.3.6. Iodate (IO3-) stock standard (100 µg/mL):
Dissolve and dilute 0.1224 g of KIO3 to 1.0 L with DI H2O. Prepare monthly (Note: This standard is only used if confirmation of iodine is necessary in addition to the iodide analysis. Dilute to concentrations in Section 4.12. with desorbing solution. See Sections 4.11. and 4.12. for more details.).
3.4. Working Standard Preparation - Prepare monthly.
Prepare I- working standards in desorbing solution. A suggested scheme for preparing a series of working standards using 10-mL final solution volumes is shown below:
| Working Std | Std Solution | Aliquot | Eluent Added |
| (µg/mL) | (µg/mL) | (mL) | (mL) |
| 0.1 | 0.1 | * | * |
| 0.2 | 1.0 | 2.0 | 8.0 |
| 0.5 | 1.0 | 5.0 | 5.0 |
| 1.0 | 1.0 | * | * |
| 2.0 | 10.0 | 2.0 | 8.0 |
| 5.0 | 10.0 | 5.0 | 5.0 |
| * Already prepared in Section 3.3.5. | |||
3.5. Sample Preparation
IABC samples: Follow Sections 3.5.1. to 3.5.3. below.
Impinger samples: Follow Section 3.5.4. only.
Note: If the MST device discussed in Section 2.1.6. is used, the prefilter is not analyzed for iodine.
- 3.5.1. Carefully remove and discard the rear glass wool plug
from each glass tube without losing any IABC sorbent.
Note: The sorbent should always be removed from the glass tube via the opposite end of collection (i.e. 50-mg IABC backup section is removed first). This will minimize the possibility of contamination from any collected particulate.
3.5.2. Carefully transfer each IABC section from a sample tube into a separate 25-mL Erlenmeyer flask or 20-mL scintillation vial.
3.5.3. Pipette 3.0 mL of desorbing solution into each flask.
Note: Alternate sample volumes can be used and are dependent on the analytical sensitivity desired. For most industrial hygiene samples, 3-mL volumes will allow for detection of iodine within the range of standards specified. Cap each flask or vial tightly and allow each solution to sit for at least 60 min. Occasionally swirl each solution.
3.5.4. Impinger samples: Measure and record the solution volume of each sample using individual graduated cylinders.
3.6. Analysis
- 3.6.1. Pipette or pour a 0.5- to 0.6-mL portion of each standard
or sample solution into separate automatic sampler vials. Place a
filtercap into each vial. The large filter portion of the cap should
face the solution.
3.6.2. Load the automatic sampler with labeled samples, standards, and blanks.
3.6.3. Set up the ion chromatograph in accordance with the SOP (5.13.).
Note: An SOP is a written procedure for a specific instrument. It is suggested that SOPs be prepared for each type of instrument used in a lab to enhance safe and effective operation.
Typical operating conditions for a Dionex 4000i or 4500i with a PED and an automated sampler are listed below:
| Ion Chromatograph | |
| Eluent: | 0.02 M NaNO3 |
| Column temperature: | ambient |
| Anion precolumn: | AG5 |
| Anion separator column: | AS5 |
| Amperometry Output range: | 0.1 µA |
| Sample injection loop: | 50 µL |
| Pump | |
| Pump pressure: | |
| Flow rate: | 2 mL/min |
| Chromatogram | |
| Run time: | 5 min |
| Peak retention time: | |
3.6.4. Follow the SOP for further instructions regarding analysis (5.13.).
3.7. Calculations
- 3.7.1. After the analysis is completed, retrieve the peak areas
or heights. Obtain hard copies of chromatograms from a printer. An
example chromatogram of a solid sorbent sample collected at an
iodine concentration of approximately 2 × PEL for 5 min is shown
below:

3.7.2. Prepare a concentration-response curve by plotting the peak areas or peak heights versus the concentration of the I- standards in µg/mL.
3.7.3. Perform a blank correction for each IABC front and backup section. Subtract the µg/mL I- blank value (if any) from each sample reading if blank and sample solution volumes are the same. If a different solution volume is used, subtract the total µg blank value from each total µg sample value.
3.7.4. Calculate the air concentration of I2 (in ppm) for each air sample:
| ppm I2 = | (A) × (mol vol)
(AV) × (mol wt) |
Where:
| A | = µg I2 |
| µg/mL I- | = Amount found (from calibration curve) |
| Sol Vol | = Solution volume (mL) from Section 3.5.3. (or 3.5.4.) |
| GF | = Gravimetric factor = 3I2/5I- = 6/5 = 1.2 |
| Mol Vol | = Molar volume (L/mol) = 24.45 (25°C and 760 mmHg) |
| AV | = Air volume (L) |
| Mol Wt | = Molecular weight for I2 = 253.8 (g/mol) |
3.8. Reporting Results
Add the backup section ppm I2 result (if any) to the front section result for each IABC sample. Report results to the industrial hygienist as ppm I2.
4. Backup Data
This method has been validated for a 2.5-L, 5-min sample taken at a flow rate of 0.5 L/min. The method validation was conducted near the OSHA Ceiling PEL of 0.1 ppm I2. The sampling media used during the validation consisted of two-section tubes packed with 100-mg of IABC for the front and 50 mg for the backup sections. Tubes were obtained commercially from SKC (Lot no. 810, Cat. no. 226-80, SKC Inc., Eighty Four, PA).
The validation consisted of the following experiments and discussion:
- 1. An analysis of 20 spiked samples was performed (7 samples each
at 2 × and 1 ×, and 6 samples at 0.5 × the Ceiling PEL) to evaluate
desorption efficiency (DE).
2. A sampling and analysis of 18 samples (6 samples each at 2 ×, 1 ×, and 0.5 × Ceiling PEL) collected from dynamically generated test atmospheres at 50% RH to determine bias and overall error.
3. A determination of the sampling media collection efficiency at approximately 2 × Ceiling PEL).
4. A determination of potential breakthrough.
5. An evaluation of storage stability at room (20 to 25°C),
refrigerator (0 to 4°C), and freezer
6. A determination of any significant effects on results when sampling at different humidities.
7. A determination of the qualitative and quantitative detection limits for I-.
8. Comparison of sampling methods: IABC versus Impinger.
9. Evaluation of a prefilter/cassette assembly and a modified sampling tube for use during sampling.
10. Evaluation of the previous OSHA sampling device, an impregnated charcoal sampling tube (SKC, Lot 411, Cat. No. 226-67).
11. Confirmation of iodine using the disproportionation product iodate (IO3-).
12. A determination of the qualitative and quantitative detection limits for IO3-.
13. Summary.
A generation system was assembled, as shown in Figure 1, and used for all experiments except the analysis and detection limit determinations. All samples were analyzed by IC-PED. All known concentrations of generated test atmospheres were calculated from the I2 diffusion rate.
All results were calculated from concentration-response curves and statistically examined for outliers. In addition, the analysis (Section 4.1.) and sampling and analysis results (Section 4.2.) were tested for homogeneity of variance. Possible outliers were determined using the Treatment of Outliers Test (5.14.). Homogeneity of variance was determined using Bartlett's test (5.15.). Statistical evaluation was conducted according to the Inorganic Methods Evaluation Protocol (5.16.). The overall error (OE) (5.16.) was calculated using the equation:
OEi% = ±(|biasi| + 2CVi) × 100% (at the 95% confidence level)
Where i is the respective sample pool being examined.

- 4.1. Spiked Sample Analysis
Twenty samples were prepared by adding known amounts of I- (as KI) stock solution to the IABC tubes to determine desorption efficiencies (DEs) for the analytical portion of the method.
- 4.1.1. Procedure: Sampling tubes containing IABC were
spiked using a 25-µL syringe (Hamilton
Microliter®/Gastight®
Syringe, Hamilton Co., Reno, NV). Spikes were 2.25, 4.50, and 9.00
µg I-. These levels correspond approximately to
0.5, 1, and 2 × the Ceiling PEL for a 5-L air sample collected at a
0.5-L/min flow rate. [Note: 5 L (10-min sample) was used as an
intermittent point between a 5 to 15-min sample. The spiked sample
analysis test is conducted first to determine analytical merits of
the method. It was unknown whether this method could collect iodine
as a 5-min sample when this test was conducted and 10-min was chosen
from past experience.]
4.1.2. Results: Desorption efficiencies are presented in Table 1. As shown, the average DE is very close to 1.0. No DE corrections are necessary for I2 collection using IABC tubes.
Iodine (as I-) Analysis - Desorption Efficiency (DE)
| Level | N | Mean DE | SD | CV1 |
| 0.5 × PEL | 6 | 1.048 | 0.072 | 0.069 |
| 1 × PEL | 7 | 0.997 | 0.017 | 0.017 |
| 2 × PEL | 7 | 1.001 | 0.023 | 0.023 |
| All Levels | 20 | 1.015 | 0.042 | 0.041* |
4.2. Sampling and Analysis
To determine the precision and accuracy of the method, known concentrations of I2 were generated, samples were collected and then analyzed.
- 4.2.1. Procedure:
- 1) Test atmospheres of I2 were
generated using a permeation/diffusion chamber (Model 450
Dynacalibrator, Metronics, Santa Clara, CA) and a dynamic
generation system. To generate I2 vapor,
I2 resublimed crystal was placed in a
diffusion tube, and heated to 70 ± 0.1°C. The
I2 vapor was diluted with filtered,
humidified air using the system shown in Figure 1 and as discussed
below.
2) Dynamic generation system
A Miller-Nelson Research Inc.
flow, temperature, and humidity control system (Model HCS-301,
Monterey, CA) was used to control and condition the dilution
airstream. All generation system fittings and connections were
Teflon®. The
I2 concentrations were varied by
adjusting the dilution airstream volume. The dilution airstream
was adjusted using the mass flow controller of the
3) The total flow rate of the generation system was measured using a dry test meter.
4) Solid-sorbent samples were taken from the sampling manifold
using
4.2.2. Results: The results are shown in Table 2. The spiked sample (Table 1) and test atmosphere sample (Table 2) results each passed the Bartlett's test and were pooled to determine a CVT for the sampling and analytical method.
Iodine Sampling and Analysis - Ceiling PEL Determination
| Level | N | Mean | SD | CV2 | OE (±%) |
| 0.5 × PEL | 6 | 0.987 | 0.045 | 0.046 | 10.5 |
| 1 × PEL | 6 | 0.942 | 0.033 | 0.035 | 12.8 |
| 2 × PEL | 6 | 0.997 | 0.043 | 0.043 | 8.90 |
| All Levels | 18 | 0.975 | - | 0.042* | 10.9** |
** = OE2
The total pooled coefficients of variation (CVT), bias, and total overall error (OET) are as follows:
CVT (pooled) = 0.045; bias = -0.025; OET = ±11.5%
(Note: "T" values include data from Section 4.1. and are calculated using equations specified in references 5.15. and 5.16.)
4.3. Collection Efficiency
Procedure: Six commercially-prepared sampling tubes were used for collection at a concentration of approximately 2 × the OSHA Ceiling PEL for 5 min at 0.5 L/min (50% RH and 25°C). The amounts of I2 vapor collected in the first section (100 mg of sorbent) and second section (50 mg) were determined. The collection efficiency (CE) was calculated by dividing the amount of I2 collected in the first section by the total amount of I2 collected in the first and second sections.
Results: The results in Table 3 show a CE of 100%. No I2 was found in the second sorbent section for the CE experiment and indicates the sorbent can adequately collect iodine near the PEL.
Collection Efficiency
(2 × PEL, 25°C & 50% RH)
|
| |||||||||
| -----------ppm I2----------- | |||||||||
| Sample No. | First Section | Second Section | % Collection Efficiency | ||||||
|
| |||||||||
| 1 | 0.201 | ND | 100.0 | ||||||
| 2 | 0.185 | ND | 100.0 | ||||||
| 3 | 0.194 | ND | 100.0 | ||||||
| 4 | 0.210 | ND | 100.0 | ||||||
| 5 | 0.192 | ND | 100.0 | ||||||
| 6 | 0.196 | ND | 100.0 | ||||||
| |||||||||
4.4. Breakthrough
(Note: Breakthrough is defined as >5% loss of analyte through the sampling media at 50% RH)
Procedure: The same procedure as the CE experiment (Section 4.3.) was used with two exceptions: The generation concentration was increased to a level approximately 7 × the Ceiling PEL, and samples were taken at approximately 0.5 L/min for 30 min.
The amount of breakthrough for each sampling tube was calculated by dividing the amount collected in the second section by the total amount of I2 collected in the first and second sections.
Results: No breakthrough of I2 into the second section was found, and indicates the sorbent has adequate retention of iodine at concentrations higher than expected in industrial workplaces. Results are shown in Table 4.
Breakthrough Study
(25°C and 50% RH)
|
| ||||||||||||
| --------ppm I2 Found---------- | ||||||||||||
| Sample No. | 1st Section | 2nd Section | % Breakthrough | |||||||||
|
| ||||||||||||
| 1 | 0.766 | ND | 0 | |||||||||
| 2 | 0.762 | ND | 0 | |||||||||
| 3 | 0.775 | ND | 0 | |||||||||
| 4 | 0.765 | ND | 0 | |||||||||
| 5 | 0.735 | ND | 0 | |||||||||
| 6 | 0.747 | ND | 0 | |||||||||
| ||||||||||||
4.5. Storage Stability
Procedure: Two tests were conducted to assess storage stability. The first was a preliminary study of room temperature storage (20 to 25°C) after I2 collection. Twenty-four samples were taken near the OSHA Ceiling PEL of 0.1 ppm. After collection, all samples were stored under normal laboratory conditions (20 to 25°C) on a lab bench and were not protected from light. Six samples were initially desorbed and analyzed, then six samples were desorbed and analyzed after various periods of storage (5, 15, and 30 days).
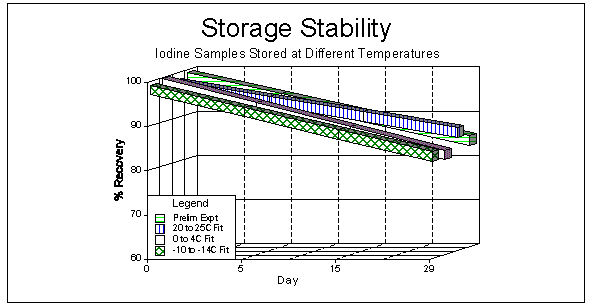
In order to assess if sample storage is temperature-dependent for iodine samples, an additional test was conducted to determine if refrigeration could improve recoveries. This test was conducted by generating 56 samples, including 6 control samples (used for day 0). The samples were separated into 3 groups, consisting of 5 or 6 samples per storage period. A group was stored at either room (20 to 25°C), refrigerator (0 to 4°C), or freezer (-10 to -14°C) temperatures. The same analytical procedure as the previous storage test was used. Samples were analyzed after 0, 5, 15, and 29 days.
Results: As shown in Table 5a, the results of the preliminary test conducted at room temperature show the mean of samples analyzed after 30 days displayed a 12% loss when compared to the value of day 0.
Storage Stability - Iodine (1 × PEL, 25°C, and 50% RH)
| Day | N | Mean | SD | CV | Recovery (%) |
| 0 | 5 | 0.114 | 0.005 | 0.045 | 99.1 |
| 5 | 6 | 0.109 | 0.004 | 0.037 | 94.8 |
| 15 | 6 | 0.096 | 0.007 | 0.073 | 83.5 |
| 30 | 6 | 0.100 | 0.003 | 0.027 | 87.0 |
Table 5b shows the results of the second study at different temperatures. The recovery shows 9.5% less of the value of day 0 after a 29-day storage at room temperature, and losses of 15.5% and 14.7% at refrigerator and freezer temperatures, respectively. Therefore, an improvement in recoveries was not noted when samples are stored at reduced temperatures. A significant reduction in recovery over 30 days was not noted using the previous charcoal treated sampling media in OSHA Method No. ID-177 (5.4.) or in the bicarbonate/carbonate solutions used as impinger collection or as analytical standards. The carbon bead used for this collection procedure appears to be responsible for the loss and further work will have to be performed to determine the extent and mechanism.
Room, Refrigerator, and Freezer Temperatures
(Known I2 Concentration = 0.116 ppm at 50% RH)
| Day | Temperature | N | Mean | SD | CV | Recovery (%) |
| 0 | Room | 6 | 0.114 | 0.003 | 0.025 | 98.3 |
| 5 | Room | 5 | 0.109 | 0.009 | 0.081 | 94.0 |
| Refrigerator | 5 | 0.109 | 0.008 | 0.075 | 94.0 | |
| Freezer | 5 | 0.108 | 0.007 | 0.063 | 93.1 | |
| 15 | Room | 6 | 0.101 | 0.008 | 0.074 | 87.1 |
| Refrigerator | 5 | 0.102 | 0.004 | 0.042 | 87.9 | |
| Freezer | 5 | 0.101 | 0.003 | 0.034 | 87.1 | |
| 29 | Room | 6 | 0.103 | 0.004 | 0.035 | 88.8 |
| Refrigerator | 6 | 0.096 | 0.004 | 0.047 | 82.8 | |
| Freezer | 6 | 0.097 | 0.008 | 0.086 | 83.6 |
Figure 2 shows results from both studies and linear curve fits of the recovery data over time. The loss over time shows that samples should be analyzed as soon as possible, preferably within 1 or 2 weeks after sampling.
- 4.6. Humidity Study
Procedure: A study was conducted to determine any effect on results when samples are collected at different humidities. Samples were taken using the generation system and procedure described in Section 4.2. Test atmospheres were generated at 25°C and at approximately 0.5, 1, and 2 × the OSHA Ceiling PEL. Relative humidities of 25%, 50%, and 80% were used at each concentration level tested. All tests were conducted by taking IABC samples side-by-side with impinger samples containing an aqueous collecting solution mixture of 1.5 mM Na2CO3 and 1.5 mM NaHCO3. Impingers were used at first instead of bubblers to minimize any back pressure which could result from sampling rates of approximately 1 L/min and dual sampling trains. Two impingers in series were thought to be necessary for iodine collection; however, after a few experiments it was determined iodine collection efficiency using one impinger was adequate.
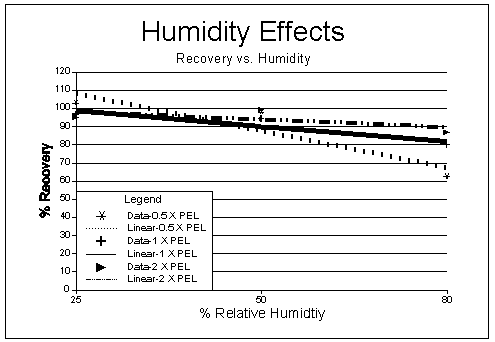
Figure 3
Results: Results of the humidity tests are listed in Table 6 (results of impinger samples taken at 80% RH are also shown for comparison) and shown in Figures 3 through 5. An F test was used to determine if any significant effect occurred when sampling at different RHs. As shown, at the 99% confidence level, the calculated F values are larger than critical F values (5.16.) for all the concentrations tested, and a significant difference in results occurred across the RH ranges tested. Figures 3 and 5 show a dependence on RH occurs and samples collected at higher RHs tend to display lower recoveries.
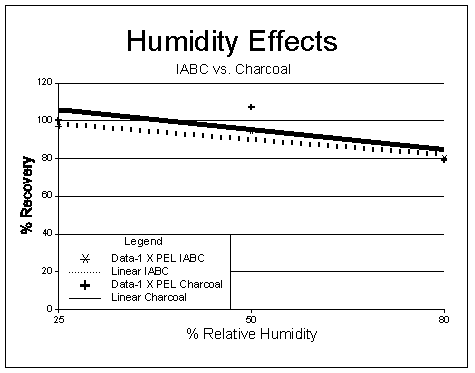
Figure 4
The loss over humidity levels appears to follow a linear trend as shown in Figures 3 and 4. Figure 4 shows an almost identical loss occurs when using the treated charcoal sampler listed in OSHA Method No. ID-177 (5.4). A dependence on concentration is also readily apparent when examining the recoveries for Figure 5. This loss versus concentration appears to follow a logarithmic function (concentrations above 2 × PEL are extrapolated). Loss of iodine is more readily evident at low concentration and high RHs than higher concentrations and high RHs. The lower recoveries were not readily evident at low concentrations when samples were collected in impingers containing weakly basic solutions. Tests were not conducted above 80% RH due to difficulties associated with generating higher humidity levels in the laboratory. It is unknown what losses will occur at RH levels above 80%. If low concentration and high humidity situations are anticipated (>50% RH) prior to sampling, sample collection using impingers will give increased recoveries; however, the inconvenience of collection using these devices is a serious disadvantage.
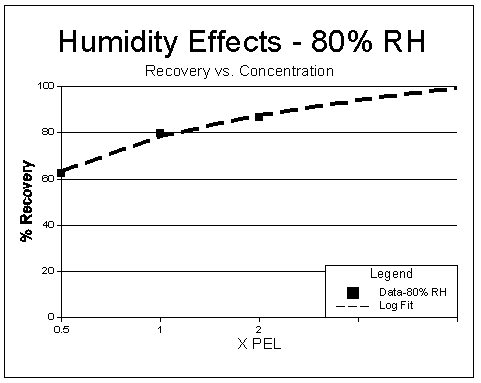
Figure 5
Although impingers were used for this test, bubblers will most likely give better results than impingers due to increased dispersion of the sample into the bubbler solution. The increased collection efficiency of bubblers over impingers for the same substance and collection solution has been demonstrated in previous studies of formaldehyde (5.18.). As previously discussed in Section 1, two stopgap methods were developed using bubblers containing basic collection solutions. Adequate recoveries were noted using this approach. Further testing of bubblers may be necessary to demonstrate improved collection of iodine over impingers.
Humidity Test - Iodine - 25°C
| Level | % RH | N | Mean | SD | CV | Taken | Recovery (%) | Fcrit | Fcalc |
| 0.5 × PEL | 25 | 6 | 0.063 | 0.008 | 0.134 | 0.061 | 103 | 6.36 | 33.5 |
| 50 | 6 | 0.049 | 0.002 | 0.046 | 0.050 | 98.0 | |||
| 80 | 6 | 0.037 | 0.005 | 0.129 | 0.059 | 62.7 | |||
| 80* | 3 | 0.058 | 0.005 | 0.086 | 0.061 | 95.1 | |||
| 1 × PEL | 25 | 6 | 0.116 | 0.006 | 0.050 | 0.120 | 96.7 | 6.52 | 10.5 |
| 50 | 5 | 0.113 | 0.004 | 0.039 | 0.120 | 94.2 | |||
| 80 | 6 | 0.092 | 0.014 | 0.152 | 0.115 | 80.0 | |||
| 80* | 3 | 0.109 | 0.002 | 0.018 | 0.115 | 94.8 | |||
| 2 × PEL | 25 | 6 | 0.210 | 0.014 | 0.068 | 0.221 | 95.0 | 6.36 | 14.0 |
| 50 | 6 | 0.196 | 0.009 | 0.043 | 0.197 | 99.4 | |||
| 80 | 6 | 0.184 | 0.008 | 0.042 | 0.212 | 86.8 | |||
| 80* | 3 | 0.183 | 0.004 | 0.022 | 0.212 | 86.3 |
- *Samples were taken with impingers containing collecting solution
(1.5 mM
Na2CO3/NaHCO3),
which was same as desorbing solution used in preparing working
standards (see Section 3.4.).
4.7. Qualitative and Quantitative Detection Limit Study
A modification of the National Institute for Occupational Safety and Health (NIOSH) detection limit calculations (5.19., 5.20.) was used to calculate detection limits.
Note: For this experiment, a comparison of samples with and without the sampling media present was used to determine limits, and a separate recovery study was not conducted as mentioned in the NIOSH protocol. The recovery study was not conducted so as not to confound the difficulty of generating very low concentration samples with the comparison of spiked samples with and without the sampling media.
Procedure: Two sets of low concentration samples were prepared by spiking desorbing solutions (Section 3.3.2.) with aliquots of aqueous standards prepared from KI (Section 3.3.4.) at five different concentrations. One set of samples was spiked at five different levels without the IABC sorbent present while the other set included IABC in each solution prior to spiking. Both sets of samples were analyzed using a 50-µL sample injection loop and a PED setting of 0.1 µA.
Results: The IABC spiked sample results are shown in Table 7 for qualitative and quantitative detection limits, respectively. Both sets of samples gave virtually identical detection limits, indicating in this case, sampling media did not have a significant effect on the detection limit. The qualitative detection limit is 0.003 µg/mL as I- at the 99.8% confidence level. The quantitative detection limit is 0.009 µg/mL as I-. Using a 2.5-L air volume and a 3-mL sample solution volume, the qualitative and quantitative detection limits are 0.0004 ppm and 0.001 ppm, respectively as I2.
Qualitative and Quantitative Detection Limits
|
| ||||
| --------------------I2 (as µg/mL, I-) Level------------------- | ||||
| 0.002 | 0.005 | 0.010 | 0.020 | |
| Sample No. | PA | PA | PA | PA |
| 1 | 3,244 | 5,918 | 18,190 | 36,081 |
| 2 | 3,501 | 9,682 | 18,624 | 37,305 |
| 3 | 2,598 | 6,929 | 20,428 | 35,622 |
| 4 | 3,002 | 10,842 | 20,398 | 38,262 |
| PA = Integrated
Peak Area (as
I-) The blank and 0.001 µg/mL integrated peak areas were all equal to zero. | ||||
|
| ||||
- The response of the low-level calibration samples were plotted
to obtain the linear regression equation (Y = mX + b), and the
predicted responses (
| Using the equations: | Sy | = | [S( |
| Q1 | = | (3Sy + B - b)/m | |
| Q2 | = | 3.33 Q1 |
Where:
| B | = | the mean blank response |
| b | = | intercept of the regression |
| m | = | analytical sensitivity or slope as calculated by linear regression |
| Sy | = | the standard error of the regression |
| N | = | the number of data points |
| Q1 | = | qualitative detection limit |
| Q2 | = | quantitative detection limit |
B = 0; b = -546.3833; m = 1,887,623;
Sy = 1,496; N = 16 and
Y = b + mX =
-546.3833 + 1,887,623X
| Therefore, | Q1 = (3Sy + B - b)/m = 2.667 × 10-3 µg/mL as I- | |
| 8.00 × 10-3 µg as I- (3-mL sample volume). | ||
| 3.70 × 10-4 ppm I2 (2.5-L air volume). |
Using the International Union of Pure and Applied Chemistry (IUPAC) approach as discussed in prior methods (5.20.) gave a detection limit (Q2) of 5.5 × 10-4 ppm I2 when using the data presented above.
4.8. Comparison of Sampling Methods
Procedure: In order to compare the performance of this method, the IABC and impinger samples were collected side-by-side from the generation system at approximately 1 × PEL. The impinger collection solution is specified in Section 4.6. All samples were analyzed by IC-PED.
Results: Table 8 shows the results of comparison study. As shown, the IABC and impinger results are in good agreement.
Comparison Study - IABC vs Impinger
(Known Concentration = 0.115 ppm I2)
(25°C, and 50% RH)
| N | Mean | SD | CV | Recovery, % | |
| IABC | 6 | 0.113 | 0.004 | 0.035 | 98.3 |
| Impinger | 6 | 0.111 | 0.002 | 0.019 | 96.5 |
| Notes: | (a) | Sampling Time = 5 min |
| (b) | Flow Rate = 0.525 L/min for IABC
samples. = 0.942 L/min for impinger samples. | |
| (c) | Sample solution volume for desorbing IABC samples
= 3.0 mL Sample solution volume for impinger samples = 10.0 mL |
4.9. Evaluation of a Prefilter and a Modified Sampling Tube
As previously discussed, particulate iodide compounds may interfere with the determination of iodine in workplace atmospheres. Past research regarding aerosols (5.21.) has indicated that particulate in the air sampled may penetrate any glass wool plugs and the sorbent when using conventional sampling tubes. Different types of prefilters have been be used to assist in capturing particulate before entry into the sampling tube. A study was conducted to evaluate the possibility of I2 reacting with a prefilter/cassette or a MST device to capture iodides or other particulate.
Procedure: Evaluations were performed using either IABC sampling tubes with prefilter sampling assemblies (5-F pore size, 25-mm diameter PVC filter/carbon-filled polypropylene cassette), or MSTs with Teflon® filter [similar to Type II tubes for SO2 (OSHA Method No. ID-200) without polyurethane foam] for particulate and vapor collection.
A test was conducted by taking six IABC samples without prefilters side-by-side with six IABC samples connected to prefilters. Another test was performed by taking six IABC samples side-by-side with six MSTs. All samples were taken at a flow rate of about 0.5 L/min for 5 min. The generation system concentration was approximately at the Ceiling PEL for iodine.
Results: The results of the comparison of IABC samples taken, with and without prefilters, and MSTs are displayed in Table 9. As shown, a difference in the amount of I2 collected was noted between the prefilter/IABC and IABC, and not noted between MST and IABC. The PVC prefilter/cassette does appear to slightly react with I2 when using the sampling conditions stated in Section 2. The PVC filter/cassette assembly is not recommended for particulate sample collection when also sampling I2. The MST device may be used for collection of I2. Glass wool is used for separation in the sampling tube rather than foam. Some foams will absorb or react with iodine and subsequently contribute to lower recoveries.
Comparison Study - Known Concentration = 0.105 ppm I2
With/Without Prefilter and Modified Sampling Tube (25°C and 50% RH)
| N | Mean | SD | CV2 | Recovery, % | |
| IABC (WOPF) | 6 | 0.103 | 0.004 | 0.036 | 98.1 |
| IABC (WPF) | 6 | 0.077 | 0.004 | 0.050 | 73.3 |
| MST | 6 | 0.100 | 0.005 | 0.054 | 95.2 |
| Notes: | ||||||||||
| (a) |
| |||||||||
| (b) | The MST device: The dimensions of the front
portion of the glass jacket are 12-mm o.d., 10-mm i.d., and
25-mm long and is used to retain the
Teflon®
| |||||||||
| (c) | Teflon® prefilter analyzed after sampling contained 0.008 ± 0.001 ppm as I2. | |||||||||
| (d) | Sampling Time = 5 min Flow Rate = 0.526 to 0.544 L/min | |||||||||
| (e) | Sample Solution Volume for Desorption = 3.0 mL |
4.10. Evaluation of Impregnated Charcoal Sampling Tube (OSHA Method No. ID-177) vs. the IABC tube
Procedure: A side-by-side test using the previous OSHA sampling device for I2 was conducted. The prior device was a glass tube containing base-impregnated charcoal (SKC Inc., Cat. No. 226-67) and glass wool plugs. Lot number 411 was chosen for the test. The I2 vapor was collected by taking six impregnated charcoal sampling tubes (ICST) side-by-side with six IABC tubes using the generation system and sampling conditions are stated as follows for both sampling media:
| (a) | Sampling Time | = | 15 min |
| (b) | Flow Rate | = | 0.526 to 0.544 L/min |
| (c) | Desorption Vol | = | 3.0 mL |
Results: Results are listed in Table 10. As shown, the average blank value for SKC lot no. 411 contains 5.50 µg of I2. This is equivalent to 0.21 ppm of I2 if a 2.5-L air volume is used in calculations, and indicates serious contamination of this lot. Table 10 also shows results of I2 collected in these tubes with/without blank correction.
Evaluation of Impregnated Charcoal Sampling Tube
(Known Concentration = 0.105 ppm I2)
(25°C and 50% RH)
| N | Mean | SD | CV | Recovery, % | |
| ICST (BT) | 6 | 5.50 µg | 0.37 µg | 0.067 | - |
| ICST (WOBC) | 6 | 0.162 ppm | 0.010 ppm | 0.060 | 154.3 |
| ICST (WBC) | 6 | 0.096 ppm | 0.010 ppm | 0.107 | 91.4 |
| IABC (BT) | 6 | ND | - | - | - |
| IABC | 6 | 0.103 ppm | 0.004 ppm | 0.036 | 98.1 |
| Notes: | (a) | ICST (BT) | = | Blank Impregnated Charcoal Sampling Tube |
| (b) | ICST (WOBC) | = | Impregnated Charcoal Sampling Tube without blank correction. | |
| (c) | ICST (WBC) | = | Impregnated Charcoal Sampling Tube with blank correction. | |
| (d) | IABC (BT) | = | Blank Impregnated Activated Beaded Carbon tube | |
| (e) | ICSTs were taken from SKC lot 411. | |||
| (f) | ND | = | None detectable (<0.01 µg I2) | |
As shown, the IABC and impregnated charcoal recoveries are similar, provided blank corrections are made to the impregnated charcoal results.
4.11. Confirmation of I2 from IO3- Peak
The collected I2 is converted to I- and IO3- by the IABC sorbent as shown by the chemical reaction discussed in Section 1.2. If IO3- can also be analyzed, additional evidence of the presence of I2 can be obtained.
Procedure: A previous analysis using IC has demonstrated that the IO3- peak had interferences, presumably from the KOH used to impregnate the sorbent, and from other anions, such as fluoride (F-), and chlorite (ClO2-). To analyze IO3-, an alternate analytical procedure was used to minimize interference problems. In order to demonstrate the disproportionation product IO3-, I2 samples were prepared by dissolving small amounts of pure I2 (U.S.P., J.T. Baker Chemical Co., NJ) in the collecting solution (1.5 mM Na2CO3 and 1.5 mM NaHCO3). Two different detectors (conductivity or UV) were tried for analysis of IO3-. Samples were analyzed for IO3- using each detector separately.
Typical operating conditions for a Dionex 4000i or 4500i with a conductivity or UV detector and an automated sampler are listed below:
- Ion Chromatograph - IO3-
Analysis
| Eluent: | 1.0 mM Na2CO3/1.0 mM NaHCO3 |
| Column temperature: | ambient |
| Anion precolumn: | AG5 |
| Anion separator column: | AS5 |
| Anion self-regeneration suppressor: | ASRS-1 (4 mm)* |
| Conductivity output range: | 1 µS* |
| UV output range: | 0.1 AU** |
| Wavelength: | 200 nm** |
| Rise time: | 5.0 s** |
| Sample injection loop: | 50 µL |
*Conductivity detector only
**UV detector only
Pump - IO3- Analysis
| Pump pressure: | |
| Flow rate: | 2 mL/min |
Chromatogram - IO3- Analysis
| Run time: | 4 min |
| Peak retention time: |
Results: A 10 µg/mL IO3- standard and an I2 sample using conductivity and UV detectors are presented in Figure 3.

uS = MicroSiemens
AU = Absorbance Unit
The preliminary determination of IO3- using either detector appears satisfactory; however low sensitivity for IO3- was evident. A further study to determine detection limits was also conducted as discussed in Section 4.12.
4.12. Qualitative and Quantitative Detection Limit Study for Iodate
Procedure: Low concentration samples of IO3- were prepared by spiking desorbing solutions (Section 3.3.2.) with aliquots of aqueous standards prepared from KIO3 (Section 3.3.6.). These samples were analyzed using a 50-µL sample injection loop and a conductivity detector setting of 0.1 MicroSiemens (FS). The modified NIOSH method previously discussed in Section 4.7. was used.
Results: The results are shown in Table 11 for qualitative
and quantitative detection limits, respectively. The qualitative
detection limit is 1.01 µg/mL as IO3-
at the 99.8% confidence level. The quantitative detection limit is
3.36 µg/mL as IO3-. Using a 2.5-L air
volume and a
Qualitative and Quantitative Detection Limits
|
| |||
| ----------------------IO3- Level------------------ | |||
| 1.0 µg/mL | 2.0 µg/mL | 3.0 µg/mL | |
| Sample No. | PA | PA | PA |
| 1 | 7.73 | 20.36 | 34.02 |
| 2 | 7.65 | 23.77 | 31.51 |
| 3 | 7.42 | 16.63 | 32.85 |
| 4 | 11.74 | 20.68 | 27.65 |
| 5 | 6.21 | 17.09 | 30.25 |
| 6 | 10.68 | 18.02 | 37.92 |
| PA = Integrated Peak Area
(IO3-)/10,000 The blank and 0.1, 0.2 and 0.5 µg/mL integrated peak areas, and their standard deviations (Std Dev) were all equal to zero. | |||
|
| |||
The response of the low-level calibration samples were plotted to
obtain the linear regression equation (Y = mX + b), and the predicted
responses (![]() i) at each
X.
i) at each
X.
| Using the equations: | Sy | = | [S( |
| Q1 | = | (3Sy + B - b)/m | |
| Q2 | = | 3.33 Q1 | |
| Therefore, | Q1 | = | (3Sy + B - b)/m = 1.01 µg/mL as IO3- |
| 3.04 µg as IO3- (3-mL sample volume). | |||
| 0.17 ppm I2 (2.5-L air volume). | |||
| Q2 | = | 3.33 Q1 = 0.57 ppm I2 (2.5-L air volume). | |
The ability to confirm the presence of I2 using IO3- is compromised by the low sensitivity noted here. If there is sufficient quantity of I2 in the collected air samples, the IO3- analysis using the conditions specified in Section 4.11. can be used. Samples could be taken for longer periods of time to collect sufficient amounts of IO3- for detection; however, this would defeat the monitoring criteria that iodine is regulated as a Ceiling unless separate short- and long-term samples are taken. Also, if a bulk material is available, the I2 content could also be examined by assessing both I- and IO3- results, provided there is sufficiently high concentration of I2 in the bulk.
4.13. Summary
The validation results indicate the method meets both the NIOSH and
OSHA criteria for accuracy and precision (5.15., 5.16.). Performance
during collection efficiency, breakthrough, and storage stability
tests is adequate. Unfortunately, it appears that recovery is
5. References
- 5.1. Occupational Safety and Health Administration Salt Lake
Technical Center: Internal Document. Salt Lake City, UT,
1979.
5.2. Occupational Safety and Health Administration Salt Lake Technical Center: Determination of Iodine in Workplaces as Iodide using an Ion Specific Electrode (USDOL/OSHA Method No. ID-170-SG). Salt Lake City, UT, 1985.
5.3. Occupational Safety and Health Administration Salt Lake Technical Center: Determination of Iodine in Workplaces as Iodide using an Ion Chromatograph (USDOL/OSHA-SLCAL Method No. ID-169-SG). Salt Lake City, UT, 1985.
5.4. Occupational Safety and Health Administration Salt Lake Technical Center: Iodine in Workplace Atmospheres (Solid Sorbent) (USDOL/OSHA-SLCAL Method No. ID-177). revised, 1992.
5.5. Occupational Safety and Health Administration Salt Lake
Technical Center: Phosphine in Workplace Atmospheres and Backup
Report (USDOL/OSHA Method No.
5.6. Occupational Safety and Health Administration Salt Lake Technical Center: Sulfur Dioxide in Workplace Atmospheres (USDOL/OSHA Method No. ID-200). In OSHA Analytical Methods Manual. 2nd ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1991.
5.7. Lewis, R.J., ed.: Hawley's Condensed Chemical Dictionary. 12th ed. New York: Van Nostrand Reinhold Co., 1993.
5.8. Lide, D.R., ed: CRC Handbook of Chemistry and Physics. 73rd ed. Boca Raton, FL: CRC Press, Inc., 1992.
5.9. American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values and Biological Exposure Indices. 5th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1986.
5.10. Flury, F. and F. Zernik: Schadliche Gase. Berlin: Julius Springer, 1931.
5.11. Clayton, G.D. and F.E. Clayton, ed.: Patty's Industrial Hygiene and Toxicology. 3rd ed. New York: John Wiley and Sons, 1981.
5.12. Sittig, M.: Handbook of Toxic and Hazardous Chemicals. Park Ridge, NJ: Noyes Publications, 1981.
5.13. Occupational Safety and Health Administration Salt Lake Technical Center: Ion Chromatography Standard Operating Procedure (Ion Chromatographic Committee). Salt Lake City, UT. In progress.
5.14. Mandel, J.: Accuracy and Precision, Evaluation and Interpretation of Analytical Results, The Treatment of Outliers. In Treatise On Analytical Chemistry. 2nd ed., Vol. 1, edited by I. M. Kolthoff and P. J. Elving. New York: John Wiley and Sons, 1978. pp. 282-285.
5.15. National Institute for Occupational Safety and Health: Documentation of the NIOSH Validation Tests by D. Taylor, R. Kupel, and J. Bryant (DHEW/NIOSH Pub. No. 77-185). Cincinnati, OH: National Institute for Occupational Safety and Health, 1977. pp. 1-12.
5.16. Occupational Safety and Health Administration Salt Lake Technical Center: Evaluation Guidelines of the Inorganic Methods Branch. In OSHA Analytical Methods Manual. 2nd ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 1991. pp. 1-18.
5.17. Dowdy, S. and S. Wearden: Statistics for Research. New York: John Wiley and Sons, 1983. Chapter 8.
5.18. Septon, J.C. and J.C. Ku: Workplace Air Sampling and Polarographic Determination of Formaldehyde. Am. Ind. Hyg. Assoc. J. 43:845-852 (1982).
5.19. Burkart, J.A.: General Procedures for Limit of Detection Calculations in the Industrial Hygiene Chemistry Laboratory. Appl. Ind. Hyg. 1:153-155 (1986).
5.20. National Institute for Occupational Safety and Health: Standard Operating Procedures for Industrial Hygiene Sampling and Chemical Analysis, SOP 018, Cincinnati, OH: National Institute for Occupational Safety and Health, Revised Sept., 1992.
5.21. Long, G.L. and J.D. Winefordner: Limit of Detection -- A Closer Look at the IUPAC Definition. Anal. Chem. 55:712A-724A (1983).
5.22. Fairchild, C.I. and M.I. Tillery: The Filtration Efficiency of Organic Vapor Sampling Tubes against Particulates. Am. Ind. Hyg. Assoc. J. 38:277-283 (1977).
Samples may also be analyzed by ISE or in place of IC; however, interferences particular to ISE may have to be considered. The following procedure addresses the analysis of IABC or impinger samples by ISE.
Detection Limit and Range (ISE)
- 1. The quantitative detection limit is 0.25 µg of iodide in
a 25-mL sample solution. This corresponds to 0.004 ppm iodine for a
7.5-L air volume.
2. The range is from 0.25 µg to 0.25 g iodide based on a 25-mL solution volume. This corresponds to either 0.3 µg to 0.3 g iodine or 0.004 to 4,000 ppm iodine for a 7.5-L air volume.
Interferences [ISE - also see Iodide Ion Specific Electrode (Model 94-53a) Instruction Manual, Orion Research Inc., Boston, MA or other manufacturer instruction manuals if a different iodide electrode is used.]
- 1. Either sulfide and cyanide, if present in the sampled
atmosphere, will give a negative interference; however, this can be
resolved by adding a nickel (Ni2+) solution
to the samples before analysis.
2. The maximum allowable ratios of interfering ions to iodide are 1 × 106, 5 × 103, and 1 × 105 moles/L for chloride, bromide, and thiosulfate, respectively. These are extremely high concentrations and normally should not be seen when sampling industrial environments.
3. Iodide ions may form complexes with some metal ions which will ultimately result in lower iodide recoveries. This problem can be resolved by using a "known (standard) additions" method.
Equipment (ISE)
- 1. Ion Specific Electrode (ISE) and filling solution, iodide
(Model 94-53a, Orion Research Inc., Cambridge, MA).
2. Single junction reference electrode and filling solution (Model 90-01, Orion Research Inc.).
3. Millivolt/pH meter, capable of relative mV, pH, standard addition or concentration measurements (Model EA 940 Expandable Ionanalyzer, Orion Research Inc.).
4. Stirrer, electronic, or magnetic with Teflon® stirring bars.
5. Miscellaneous laboratory glassware: Volumetric flasks, pipettes, beakers, disposable beakers, 25-mL Erlenmeyer flasks, etc.
Reagents (ISE - All chemicals should be at least reagent grade)
- Sodium nitrate (NaNO3),
Sodium hydroxide (NaOH),
Potassium iodide (KI),
Sodium iodide (NaI)
1. Deionized water (DI H2O)
2. Sodium hydroxide, 0.01 N: Dissolve 0.4 g NaOH and dilute to 1-L with DI H2O.
3. Ionic Strength Adjuster (ISA) - Sodium nitrate, 5 M: Dissolve 42.5 g NaNO3 in 100 mL DI H2O.
4. Nickel (Ni2+) solution, 1,000 µg/mL: A commercially prepared 1,000 µg/mL solution of nickel common for atomic absorption purposes can be used.
5. Standard stock solution, 1,000 µg/mL iodide: Place 1.31 g KI or 1.18 g NaI in a 1-L volumetric flask. Add about 500 mL DI H2O, swirl to dissolve, and dilute to the mark with DI H2O.
6. Working iodide standard solutions, 100, 10, 1, and 0.1 µg/mL: Pipette appropriate volumes of 1,000 µg/mL stock solution into volumetric flasks and dilute to volume with 0.01 N NaOH. The 100 µg/mL standard is used for known additions as discussed below.
Analysis (ISE - Known Additions)
- Known Additions Spike Preparation:
1. Standard Spike, 100 µg/mL is added to all samples and standards.
Note: The concentration of this spike should normally be at least
10 times the expected sample concentration. If concentrations of
iodine are expected to be large (> 10 µg/mL) or small
Sample Preparation: For IABC samples follow Steps 1 to 3 below. For impinger samples, follow Steps 4 and 5 only.
IABC Samples:
1. Carefully remove and discard the rear glass wool plug from each glass tube without losing any IABC sorbent.
Note: The sorbent should always be removed from the glass tube via the opposite end of collection (i.e. 50-mg IABC backup section is removed first). This will minimize the possibility of contamination from any collected particulate.
Carefully transfer each IABC section from a sample tube and place into a separate 25-mL Erlenmeyer flask or 20-mL scintillation vial.
2. Add 1 mL of 1,000 µg/mL Ni2+ solution and pipette 24 mL of 0.01 N NaOH into each flask.
3. Cap tightly, shake vigorously, and allow the solution to sit for at least 1 h.
Impinger Samples Only:
4. Transfer impinger sample solutions to separate 25-mL volumetric flasks.
5. Add 1 mL of 1,000 µg/mL Ni2+ solution to each flask and dilute to volume with 0.01 N NaOH.
Electrode Check:
1. Set up the mV meter and electrodes according to the manufacturers instruction manuals or Standard Operating Procedure (SOP).
2. Place 100 mL DI H2O and 2 mL ISA (5 M NaNO3) into a 250-mL beaker. Use the relative mV scale setting on the mV meter and then place electrodes (reference and iodide) in the solution.
3. Pipet 1.0 mL of the 1,000 µg/mL stock I- standard into the solution while stirring. Allow reading to stabilize, then record the mV of the solution.
4. Add 10 mL of the 1,000 µg/mL iodide stock standard while stirring. Read the mV of the solution when stable. Correct electrode operation is indicated by a difference of 55 to 59 mV (assuming the solution temperature is between 20 to 25°C).
Analytical Procedure
1. If available, use a standard additions program intrinsic within the instrument to calibrate and convert readings directly to concentration values. If an automated program is not available, record the mV reading prior to standard addition (Eo) and after addition (Es). The "standard addition" normally is a 2.5-mL aliquot of 100 µg/mL iodide working standard (see note on previous page for information).
2. Rinse the electrodes, blot dry, and place in a 25 mL sample or working standard solution. Add 0.5 mL of ISA. Allow time for the reading (Eo) to stabilize.
3. Using a glass or automatic pipet, add 2.5 mL of the 100 µg/mL Standard Spike, and allow time for the reading to stabilize. Take a final reading (Es or concentration for automated programs).
4. Analyze a standard in the concentration range of the samples after every fifth or sixth sample and at the end of the analysis.
Note: The ISE can be affected by changes in temperature. Standards and samples should be at the same temperature before analyzing. Fluctuations in the ambient temperature during analysis can sometimes be compensated for by slope adjustment. Refer to manufacturer instruction manuals for further details.
- 1. Determine the total µg iodine content of each sample and
blank using a
2. The concentration of iodine in the air sample is expressed in ppm:
| ppm I2 = | (24.45) (µg/mL Iodide) (sample
volume, mL) (mole ratio)
(MW) (air volume, L) |
Where:
| Sample volume | 25.0 mL |
| Mole ratio of I2 to Iodide | 1.2 |
| Molecular weight (MW) of I2 | 253.8 |
Therefore, for a 25 mL sample volume:
| ppm I2 = | (2.89) (µg/mL Iodide)
(MW) (air volume, L) |